 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium catalyzed cyclizations of oximeesters with 1,1-disubstituted alkenes: synthesis of α,α-disubstituted dihydropyrroles and studies towards an asymmetric protocol†
Adele
Faulkner
a,
James S.
Scott
b and
John F.
Bower
*a
aSchool of Chemistry, University of Bristol, Bristol, BS8 1TS, UK. E-mail: john.bower@bris.ac.uk; Fax: +44 (0)117 927 7985
bAstraZeneca, Alderley Park, Macclesfield, Cheshire, SK10 4TG, UK
First published on 4th January 2013
Abstract
We report efficient Pd-catalyzed cyclizations of oximeesters with 1,1-disubstituted alkenes as the basis of a general entry to α,α-disubstituted pyrrolidine derivatives. We also demonstrate that catalytic asymmetric variants of this chemistry are feasible by employing a suitable chiral ligand.
As part of a programme directed towards the development of synthetic entries to chiral N-heterocyclic scaffolds,1 we recently reported efficient conditions for the “Narasaka–Heck” cyclization2,3 of pentafluorobenzoyl oxime esters with cyclic alkenes (Scheme 1A).4,5 Here, following oxidative addition of Pd(0) into the oxime ester N–O bond,6 C(sp3)–N bond formation is enforced by the mechanistic requirements of syn-imino-palladation and syn-β-hydride elimination.4,5 The oxime ester starting materials 1 are easily accessed in enantioenriched form and cyclization of diastereomeric mixtures at C-2 provides stereoconvergent access to chiral heterocyclic targets 2 that retain synthetically flexible alkene and imine moieties.4 Key to the success of these diastereoselective processes was the identification of P(3,5-(CF3)2C6H3)3 as a privileged ligand.4,7
 | ||
| Scheme 1 Synthesis of chiral N-heterocycles by Pd-catalyzed cyclization of oximeesters with alkenes. | ||
We reasoned that our catalytic system might also facilitate a direct entry to α,α-disubstituted dihydropyrroles 4 by 5-exo cyclizations involving 1,1-disubstituted alkenes3 (Scheme 1B).8 Here, an exciting possibility resides in utilizing appropriate chiral ligands to control the absolute stereochemistry of the newly formed quaternary amino-stereocentre of 4. The synthesis of α,α-disubstituted pyrrolidine derivatives is synthetically challenging and highly flexible asymmetric methods have not been reported. Conventional strategies employ chirality transfer protocols to modify a pre-established enantioenriched core structure.9 Auxiliary controlled trapping of iminium ions is also effective in certain cases.10 More recently, approaches based upon enantioretentive [1,2]- or [1,3]-rearrangement have emerged.11 Enantiopure N-Boc-2-phenylpyrrolidine has been converted to α,α-disubstituted derivatives by a stereoretentive lithiation-electrophile trapping sequence.12 Spirocyclic systems can be accessed by intramolecular alkylidene carbene 1,5-C–H insertion reactions involving enantioenriched precursors.13 Strategies based upon asymmetric catalysis, which do not rely on chiral starting materials, have also been reported but do not offer general substrate scope. In specific cases, catalytic enantioselective intra- or inter-molecular trapping of transiently generated iminium ions is effective.14 Catalytic asymmetric phase transfer alkylation provides products where one of the substituents is limited to an ester.15 An alternative and very attractive approach involves enantioselective intramolecular amination of 1,1-disubstituted alkenes. Although efficient hydroamination protocols that achieve this have remained elusive,16 aminooxygenation,17 bromocyclization18 and multicomponent coupling processes19 are effective in certain cases.
Here, we report that using P(3,5-(CF3)2C6H3)3 as ligand enables highly efficient Pd-catalyzed 5-exo cyclizations of oximeesters with a wide range of representative 1,1-disubstituted alkenes. Additionally, we report studies towards an asymmetric protocol that provide the very same α,α-disubstituted derivatives with moderate enantioselectivity. To the best of our knowledge, these studies encompass the first asymmetric Narasaka–Heck cyclisations and thereby validate the potential of this reaction manifold for catalytic asymmetric C(sp3)–N bond construction.2,3,8
Our initial studies focused on evaluating the cyclization of aryl oxime ester 3a to imine4a using our previously established achiral catalysis system (Table 1). Here, we found that a slightly modified variant (7.5 mol% [Pd], 15 mol% P(3,5-(CF3)2C6H3)3, 200 mol% Et3N) of our earlier conditions was effective at generating the target compound in 80% yield. This protocol tolerates a wide range of ketoximeesters3b–3f20 and products 4b–4f were isolated in moderate to excellent yield.21 In the case of 3b a higher catalyst loading (10 mol% [Pd]) was required for efficient cyclization. For C–N bond formation to occur, the N–Pd(II) bond of the imino–Pd(II) intermediate must be oriented towards the alkene. Presumably, due to steric factors, smaller R1 groups (e.g. n-Bu as in 3b) are less effective at enforcing this configuration and, as such, substrates of this type cyclize with lower efficiency.
| a Pd2(dba)3 (5 mol%) and P(3,5-(CF3)2C6H3)3 (20 mol%) were employed. |
|---|

|
Variation of the alkene partner provides direct access to a wide range of representative scaffolds (Table 2). Cyclizations of 3g–i, which contain sterically encumbered alkenes (cf.3a) were all efficient. In the case of 4g, the formation of a challenging quaternary amino-substituted benzylic stereocentre is particularly noteworthy. Processes that generate terminal alkenes are also effective and the potentially vulnerable olefinic moiety of 4j was stable to the reaction conditions. Cyclization of 3k, which involves a 1,1-disubstituted cyclic alkene, generated spirocycle 4k in 84% yield. Here, small amounts of the corresponding alkene regioisomer (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioisomeric ratio) were also formed, presumably via Pd-hydride mediated isomerization of the initial adduct 4k.22 Electron deficient alkenes participate using this protocol but cyclize less efficiently. Substrate 3l, which requires 5-exocyclization onto the α-position of a pendant acrylate, generated dihydropyrrole 4l in 31% yield.23 This represents a very direct and flexible entry to complex proline derivatives24 and studies to optimize this class of cyclization are ongoing.
1 regioisomeric ratio) were also formed, presumably via Pd-hydride mediated isomerization of the initial adduct 4k.22 Electron deficient alkenes participate using this protocol but cyclize less efficiently. Substrate 3l, which requires 5-exocyclization onto the α-position of a pendant acrylate, generated dihydropyrrole 4l in 31% yield.23 This represents a very direct and flexible entry to complex proline derivatives24 and studies to optimize this class of cyclization are ongoing.
More complex pyrrolidine derivatives are accessible by employing C-2 di- or tri-substituted oximeesters (Scheme 2). Cyclizations involving both 3m and 3n were efficient and the target heterocycles 4m and 4n were isolated in good yield. In the former case, relative stereochemistry was not readily controlled (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dr); this is either reflective of non-diastereoselective cyclization or the lability of the C-2 stereocentre of the product.25
3 dr); this is either reflective of non-diastereoselective cyclization or the lability of the C-2 stereocentre of the product.25
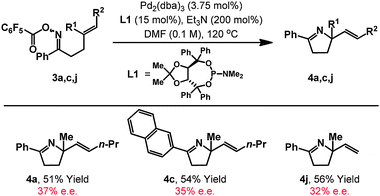
Having established an achiral catalyst system, we sought effective chiralligands capable of mimicing the beneficial steric and electronic effects of P(3,5-(CF3)2C6H3)3. Accordingly, we evaluated an extensive range of commercial electron neutral/poor systems and established that TADDOL-derived phosphoramidite L1 is unique at providing appreciable levels of asymmetry (Table 3).26 Using this ligand, cyclizations of 3a, 3c and 3j proceeded in moderate yield to provide adducts 4a, 4c and 4j in 32–37% ee.27 These results are significant because they provide compelling evidence for the close association of Pd during the C–N bond forming event28,29 and, at the same time, establish the feasibility of asymmetric Narasaka–Heck cyclizations. Importantly, the TADDOL scaffold of L1 is readily modified30 and, in the longer term, fine tuning of steric and electronic properties should facilitate provision of a more effective chiral ligand.
|
In summary, efficient conditions for the Pd-catalyzed cyclization of oximeesters with 1,1-disubstituted alkenes are described. These are the first examples of this class of cyclization, and this provides an approach to synthetically challenging α,α-disubstituted pyrrolidine derivatives. The method is operationally simple and has wide scope with respect to both the oxime ester and alkene. Additionally, we have established for the first time the feasibility of asymmetric cyclizations based upon the use of a chiral ligand system. This has important ramifications for the further development and utility of this type of process. More efficient chiral ligands and other classes of cyclization are currently being developed.
We thank the EPSRC (EP/J007455/1) and AstraZeneca for supporting this work. J.F.B. is indebted to the Royal Society for a University Research Fellowship.
Notes and references
- There is a pressing demand for the development of efficient methodologies that target low molecular weight (200–350 Da), 3D (sp3-rich) scaffolds: A. Nadin, C. Hattotuwagama and I. Churcher, Angew. Chem., Int. Ed., 2012, 51, 1114 CrossRef CAS . Efficient chirality generating methods should facilitate an “escape from flatland”: F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752 CrossRef.
- (a) H. Tsutsui and K. Narasaka, Chem. Lett., 1999, 45 CrossRef CAS; (b) H. Tsutsui, M. Kitamura and K. Narasaka, Bull. Chem. Soc. Jpn., 2002, 75, 1451 CrossRef CAS ; For reviews, see:; (c) M. Kitamura and K. Narasaka, Chem. Rec., 2002, 2, 268 CrossRef CAS; (d) K. Narasaka and M. Kitamura, Eur. J. Org. Chem., 2005, 4505 CrossRef CAS.
- The Narasaka–Heck process has been used extensively for C(sp2)–N bond formation in the context of heteroaromatic synthesis. Leading references: Imidazoles: (a) S. Zaman, K. Mitsuru and A. D. Abell, Org. Lett., 2005, 7, 609 CrossRef CAS ; Azaazulenes:; (b) M. Kitamura, S. Chiba, O. Saku and K. Narasaka, Chem. Lett., 2002, 606 CrossRef CAS; (c) S. Chiba, M. Kitamura, O. Saku and K. Narasaka, Bull. Chem. Soc. Jpn., 2004, 77, 785 CrossRef CAS ; Isoquinolines and pyridines:; (d) H. Tsutsui and K. Narasaka, Chem. Lett., 2001, 526 CrossRef CAS; (e) M. Kitamura, D. Kudo and K. Narasaka, ARKIVOC, 2006, iii, 148 Search PubMed; (f) J.-L. Zhu, Y.-L. Su, Y.-H. Chan, I.-C. Chen and C.-C. Liao, Heterocycles, 2009, 78, 369 CrossRef CAS ; Phenanthridines:; (g) T. Gerfaud, L. Neuville and J. Zhu, Angew. Chem., Int. Ed., 2009, 48, 572 CrossRef CAS; (h) Pyrroles: J. Ichikawa, R. Nadano and N. Ito, Chem. Commun., 2006, 4425 RSC , see also ref. 2.
- A. Faulkner and J. F. Bower, Angew. Chem., Int. Ed., 2012, 51, 1675 CrossRef CAS.
- For an earlier example of a Narasaka–Heck cyclization involving a cyclic alkene, see: (a) A. Fürstner, K. Radkowski and H. Peters, Angew. Chem., Int. Ed., 2005, 44, 2777 CrossRef; (b) A. Fürstner, K. Radkowski, H. Peters, G. Seidel, C. Wirtz, R. Mynott and C. W. Lehmann, Chem.–Eur. J., 2007, 13, 1929 CrossRef.
- The oxidative addition event has been confirmed by X-ray crystallographic analysis of an isolable imino-Pd(II) intermediate: Y. Tan and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 3676 CrossRef CAS.
- A similar catalyst system is effective for decarboxylative nitrene generation from oximeesters: K. Okamoto, T. Oda, S. Kohigashi and K. Ohe, Angew. Chem., Int. Ed., 2011, 50, 11470 CrossRef CAS.
- Cyclizations involving 1,1-disubstituted alkenes have been reported previously but only in the context of cascade processes: (a) M. Kitamura, S. Zaman and K. Narasaka, Synlett, 2001, 974 CrossRef CAS; (b) S. Zaman, M. Kitamura and K. Narasaka, Bull. Chem. Soc. Jpn., 2003, 76, 1055 CrossRef CAS . These processes represent rare examples of the use of this reaction manifold for C(sp3)–N bond formation.
- Leading references: (a) D. Seebach, M. Boes, R. Naef and W. B. Schweizer, J. Am. Chem. Soc., 1983, 105, 5390 CrossRef CAS; (b) L. E. Burgess and A. I. Meyers, J. Am. Chem. Soc., 1991, 113, 9858 CrossRef CAS.
- See: J. C. Killen, J. Leonard and V. K. Aggarwal, Synlett, 2010, 579 CAS and references cited therein.
- Stereoretentive [1,2] Stevens rearrangement: (a) K. W. Glaeske and F. G. West, Org. Lett., 1999, 1, 31 CrossRef CAS; (b) E. Tayama, S. Nanbara and T. Nakai, Chem. Lett., 2006, 35, 478 CrossRef CAS; (c) P. Tuzina and P. Somfai, Org. Lett., 2009, 11, 919 CrossRef CAS; (d) Stereoretentive [2,3] Sommelet–Hauser rearrangement: E. Tayam and H. Kimura, Angew. Chem., Int. Ed., 2007, 46, 8869 CrossRef; (e) For a related approach involving SO2extrusion from N-sulfonyl proline derivatives: F. Foschi, D. Landini, V. Lupi, V. Mihali, M. Penso, T. Pilati and A. Tagliabue, Chem.–Eur. J., 2010, 16, 10667 CrossRef CAS; (f) For a mechanistically distinct process that proceeds via an ammomiun ylide, see: T. Igarashi, E. Tayama, H. Iwamoto and E. Hasegawa, Tetrahedron Lett., 2011, 52, 1819 CrossRef CAS.
- N. S. Sheikh, D. Leonori, G. Barker, J. D. Firth, K. R. Campos, A. J. H. M. Meijer, P. O'Brien and I. Coldham, J. Am. Chem. Soc., 2012, 134, 5300 CrossRef CAS.
- W. R. Esmieu, S. M. Worden, D. Catterick, C. Wilson and C. J. Hayes, Org. Lett., 2008, 10, 3045 CrossRef CAS and references cited therein.
- Chiral hydrogen-bond donor catalysts and chiral Brønsted acids promote enantioselective Pictet–Spengler type cyclizations to provide structures containing embedded α,α-disubstituted pyrrolidine motifs: (a) I. T. Raheem, P. S. Thiara, E. A. Peterson and E. N. Jacobsen, J. Am. Chem. Soc., 2007, 129, 13404 CrossRef CAS; (b) M. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt and D. J. Dixon, J. Am. Chem. Soc., 2009, 131, 10796 CrossRef CAS; (c) C. A. Holloway, M. E. Muratore, R. I. Storer and D. J. Dixon, Org. Lett., 2010, 12, 4720 CrossRef CAS; (d) A. Gómez-SanJuan, N. Sotomayor and E. Lete, Tetrahedron Lett., 2012, 53, 2157 CrossRef . Related intermolecular processes: ; (e) X. Yu, Y. Wang, G. Wu, H. Song, Z. Zhou and C. Tang, Eur. J. Org. Chem., 2011, 3060 CrossRef CAS; (f) E. Aranzamendi, N. Sotomayor and E. Lete, J. Org. Chem., 2012, 77, 2986 CrossRef CAS.
- T. Kano, R. Sakamoto, H. Mii, Y.-G. Wang and K. Maruoka, Tetrahedron, 2010, 66, 4900 CrossRef CAS.
- Intramolecular asymmetric hydroamination involving 1,1-dialkylated alkenes generates the corresponding pyrrolidines in up to 55% ee.: Y. Chapurina, H. Ibrahim, R. Guillot, E. Kolodziej, J. Collin, A. Trifonov, E. Schulz and J. Hannedouche, J. Org. Chem., 2011, 76, 10163 CrossRef CAS.
- (a) Chiral hypervalent iodine reagents promote enantioselective intramolecular aminooxygenation of 1,1-disubstituted styrene derivatives: U. Farid and T. Wirth, Angew. Chem., Int. Ed., 2012, 51, 3462 CrossRef CAS; (b) Copper catalyzed intramolecular aminooxygenation encompassing 1,1-dialkylated alkenes: P. H. Fuller, J.-W. Kim and S. R. Chemler, J. Am. Chem. Soc., 2008, 130, 17638 CrossRef CAS.
- Amino-thiocarbamate catalyzed bromoaminocyclization: L. Zhou, J. Chen, C. K. Tan and Y.-Y. Yeung, J. Am. Chem. Soc., 2011, 133, 9164 CrossRef CAS.
- Catalytic asymmetric coupling of alkenyl isocyanates and alkynes provides substrates containing embedded α,α-disubstituted pyrrolidines: (a) E. E. Lee and T. Rovis, Org. Lett., 2008, 10, 1231 CrossRef CAS; (b) R. K. Friedman and T. Rovis, J. Am. Chem. Soc., 2009, 131, 10775 CrossRef.
- Previous studies have established that oxime ester geometry does not affect the efficiency of cyclization and facile interconversion likely occurs at the stage of the putative imino-Pd(II) intermediate (see ref. 2).
- Aldoxime esters are challenging substrates due to competing Beckmann rearrangement (for example, see ref. 8b).
- Efforts to suppress isomerization are ongoing. This is a particularly problematic side reaction in Heck-type processes involving cycloalkenes: C. G. Hartung, K. Köhler and M. Beller, Org. Lett., 1999, 1, 709 CrossRef CAS.
- 5-exocyclization requires imino-palladation to occur with the opposite regioselectivity to that normally favoured for Heck-type reactions involving acrylates. For related examples in the conventional Heck reaction that involve cyclization onto the α-position of acrylamides, see: A. B. Dounay, K. Hatanaka, J. J. Kodanko, M. Oestreich, L. E. Overman, L. A. Pfeifer and M. M. Weiss, J. Am. Chem. Soc., 2003, 125, 6261 CrossRef CAS.
- For a review on the synthesis of quaternary proline analogues, see: M. I. Calaza and C. Cativiela, Eur. J. Org. Chem., 2008, 3427 CrossRef CAS.
- In our previous studies (see ref. 4) equilibration of the C-2 stereocentre of the product was observed under the cyclization conditions.
- This ligand has been used in conventional asymmetric intramolecular Heck reactions: R. Imbos, A. J. Minnaard and B. L. Feringa, J. Am. Chem. Soc., 2002, 124, 184 CrossRef CAS.
- The corresponding cis-olefinic isomers cyclize with lower enantioselectivity but with the same sense of stereoinduction.
- For studies on the insertion of alkenes into N–Pd bonds see: (a) P. S. Hanley and J. F. Hartwig, J. Am. Chem. Soc., 2011, 133, 15661 CrossRef CAS; (b) J. D. Neukom, N. S. Perch and J. P. Wolfe, Organometallics, 2011, 30, 1269 CrossRef CAS and references cited therein.
- Alkene insertion into the N–Pd bond of Pd(II)-sulfonamidates is reversible: P. B. White and S. S. Stahl, J. Am. Chem. Soc., 2011, 133, 18594 CrossRef CAS . Product e.e. is constant over the course of the reactions outlined in Table 3.
- (a) B. L. Feringa, Acc. Chem. Res., 2000, 33, 346 CrossRef CAS; (b) J. F. Teichert and B. L. Feringa, Angew. Chem., Int. Ed., 2010, 49, 2486 CrossRef CAS; (c) H. W. Lam, Synthesis, 2011, 2011 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures for all compounds. See DOI: 10.1039/c2cc38944d |
| This journal is © The Royal Society of Chemistry 2013 |


