 Open Access Article
Open Access ArticleAldol reactions mediated by a tetrahedral boronate†
Tobias
Müller
,
Kristina
Djanashvili
,
Isabel W. C. E.
Arends
,
Joop A.
Peters
and
Ulf
Hanefeld
*
Biokatalyse & Organische Chemie, Gebouw voor Scheikunde, Afdeling Biotechnologie, Technische Universiteit Delft, Julianalaan 136, 2628BL Delft, The Netherlands. E-mail: u.hanefeld@tudelft.nl; Fax: +31 15 2781415
First published on 16th November 2012
Abstract
The base is a key factor in aldol reactions in organic media, determining the selectivity. Here, we describe a tetrahedral phenylboronate salt as a mild non-nucleophilic base that is able to catalyse the aldol reaction and significantly decrease the formation of undesired elimination products.
The aldol addition is an important C–C bond forming reaction.1 A key step in the mechanism is nucleophilic attack of a deprotonated ketone (enolate) on an aldehyde to form a β-hydroxyketone. Various inorganic bases, including LiOH, NaOH, Na2CO3 and Ca(OH)2, have been applied for the initial deprotonation of the ketone.2–5 However, the base catalyst is often also active in the elimination reaction, the dehydration of the β-hydroxyketone. Suppression of the aldol elimination is of great interest, as β-hydroxyketones are versatile building blocks in, for instance, the synthesis of diols, amino alcohols, lactones and polyketides.6–10
Boronates are known to mediate aldol reactions by acting both as an activator and as a template for the reactants, the ketone and the aldehyde.11–14 An interesting modification is the addition of a highly reactive trimethyl silyl enol ether to aldehydes in water with sodium dodecyl sulfate as a surfactant and diarylborinic acid 1 as the catalyst (Fig. 1).15 More recently, it was shown that the activation of the ketone as silyl enol ether is unnecessary when salt 2 as the catalyst is applied (Fig. 1). In this system, which is limited to aqueous solutions, intramolecular interaction of the neighbouring nitrogen and boron atoms stabilizes the tetrahedral boronate. Side reactions, such as dehydration, remain a problem; particularly when the reaction is favoured by stabilization of the elimination product as a result of mesomerism.
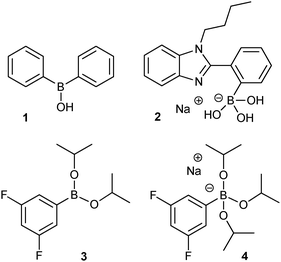 | ||
| Fig. 1 Molecular structures of the boron-based catalysts discussed. | ||
Here, we report the results of an investigation on the feasibility of the application of phenylboronate 4 as a catalyst in aldol reactions in organic media (Scheme 1). For comparison, the corresponding boronic acid 3 and the classic base sodium isopropoxide were included in this study. Compounds 3 and 4 were synthesized from 3,5-difluorophenylboronic acid by reaction with the appropriate amount of isopropanol and sodium isopropoxide (see Scheme S1, ESI†) and were isolated as stable solids with a purity greater than 99%. Both new compounds show good solubility and thermal stability in organic solvents; they did not show any dissociation as demonstrated by their characteristic 11B NMR chemical shifts for trigonal and tetragonal B-atoms, respectively (Fig. S1C and S2C, ESI;† related to H3BO3 (0.1 M) at 0 ppm). The high stability of these B-compounds may be ascribed to the electron-withdrawing fluoro-substituents in the aromatic ring. These substituents also contribute to the high solubility of these complexes in organic solvents, which allows us to perform the aldol reaction with one of the reactants acting as solvent. In the present investigation, acetone was used as the solvent and the reactions were monitored by NMR and GC-MS. To rule out the possible deactivation of the catalyst (4) by water, the reaction was performed in the presence of equimolar amounts of it (relative to 4). Neither product formation nor selectivity was influenced by water.
 | ||
| Scheme 1 The aldol reaction with a series of aldehydes and acetone. | ||
In the presence of NaOiPr as the catalyst and at 30 °C, benzaldehyde (5a) and an excess of acetone (6) reacted very fast to give aldol 7, which was subsequently dehydrated to α,β-unsaturated ketone 8 with a lower reaction rate (Fig. 2B). The rates of both the aldol reaction and the elimination reaction appeared to be strongly dependent on the concentration of the catalyst (Table 1, entries 1–4). In addition to the elimination side product, higher molecular weight side product 9 resulting from dimerization was observed as well. Decreasing the temperature to 5 °C did not improve the selectivity towards the desired aldol product (Fig. 2B). Decreasing temperature and catalyst concentration at the same time did not inhibit the elimination reaction either (Fig. S3, ESI†).
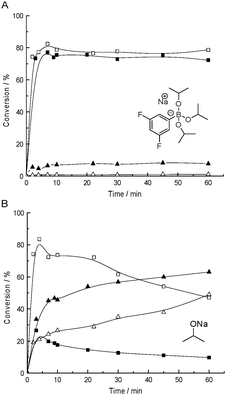 | ||
| Fig. 2 Reaction between benzaldehyde and acetone, catalysed by 20 mol% of boronate salt 4 (A) and sodium 2-propanolate (B), monitored by 1H NMR at 30 °C (solid data points) and 5 °C (empty data points). The aldol and the elimination product formation is presented by squares and triangles, respectively. | ||
Trigonal B-compound 3 (up to 20 mol%) was completely inactive in the aldol reaction of 5a and 6, which suggests that a base is essential for the reaction to proceed. By contrast, application of tetragonal boronate 4 resulted in high conversion to the aldol within a few minutes (Table 1, entries 5–7); the reaction rate towards the aldol was about the same as that with NaOiPr as the catalyst, but now the subsequent elimination reaction was much slower. With 20 mol% 4, only 8% elimination product was obtained. By decreasing the reaction temperature to 5 °C, similar results were obtained (Fig. 2A). Combining 10 mol% 4 and 10 mol% of NaOiPr resulted in full conversion of the benzaldehyde. The aldol product was formed which was dehydrated by NaOiPr to the condensation product and some higher molecular weight products resulting from dimerization (Table 1, entry 8).
A series of aldehydes was tested in the aldol reaction in the presence of catalyst 4 (Scheme 1 and Table 2). The reactions proceeded within a few minutes with high conversions. Aromatic aldehydes and furfural, a bio-based platform chemical, all gave rise to both aldol adduct 7 and the corresponding dehydration product 8 with a high selectivity towards 7, compared to the results obtained with the reported boron catalysts 1 and 2.15,16 The highest selectivity was obtained with aliphatic aldehyde 5e: 66% of the β-hydroxyketone 7e and less than 1% of the dehydration product 8e were formed (Table 2, entry 5). For the aromatic aldehydes conversion, selectivity to the β-hydroxyketone seems to be related to the nature of substituents and the bond delocalization. Similar lower yields and selectivities for 5b and c were observed earlier.15,16
We suggest a reaction mechanism as depicted schematically in Scheme 2. Under the reaction conditions applied, the dissociation of compound 4 towards −OiPr and corresponding 3 is negligible in acetone. This is confirmed by its 11B NMR spectrum, which shows exclusively a resonance for tetrahedral boron at around −15.49 ppm (Fig. S4, ESI;† as a standard 0.1 M boric acid solution in D2O at 0 ppm was used). No resonance related to compound 3 was observed. Since the reaction rate of the aldol reaction, in the presence of 2 mol% NaOiPr, is much lower than with 20 mol% 4, it is likely that undissociated 4 rather than isopropoxide is the actual catalyst in this system (Table 1, entries 4 and 5). After deprotonation of acetone by 4, the resulting enolate exchanges with iPrOH. The resulting tetrahedral boron-enolate 10 reacts with the aldehyde to form the aldol product. In the presence of iPrOH the aldol product 7 is released and the tetrahedral boronate 4 returns into the catalytic cycle.
 | ||
| Scheme 2 Proposed mechanism for the aldol reaction catalysed by tetrahedral boronate salt 4, including the elimination reaction in the presence of a strong nucleophilic base, NaOiPr. | ||
The occurrence of an intermediate tetrahedral boronate with both aldehyde and acetone coordinated to the B-atom, as suggested by Evans et al. for other B-activated aldol reactions,11 is unlikely in the present case. 11B NMR spectra of a sample of 4 and benzaldehyde showed a single resonance at −16 ppm, thus no coordination of benzaldehyde to 4 (Fig. S5, ESI;† related to 0.1 M boric acid solution in D2O at 0 ppm). This also rules out the mechanism, similar to Evans, suggested by Shibasaki et al.11
In contrast, acetone coordinates to tetrahedral boronate 4, which could be confirmed by both 11B NMR and Raman spectroscopy (Fig. S6, ESI†). The latter spectra demonstrate the appearance of an additional boron ester peak as a result of the interaction between 4 and acetone (solid line) compared to the spectra of 4 measured in its solid form (dashed line). Furthermore, the 11B NMR spectra (inset) show an additional resonance at around −17.1 ppm, confirming the interaction of 4 with acetone.
In contrast 11B NMR experiments with 3 show that no tetrahedral boronate was formed due to coordination of acetone (6) or benzaldehyde (5a) to phenyl boronic ester 3 (Fig. S7, ESI†). In both cases, the only observable resonance was at 8 ppm (related to H3BO3 (0.1 M) at 0 ppm) corresponding to the trigonal boronic acid ester 3. This is in agreement with its catalytic inertness in the aldol reaction. The advantage of the designed catalytic tetrahedral boronate 4 is its steric bulkiness, which results in a reduced rate of water elimination reaction in the aldol formation compared to that when isopropoxide is applied as the base catalyst.
In conclusion, we present tetrahedral sodium triisopropyl 3,5-difluorophenylboronate (4) as a base, which can catalyse the aldol reaction. It is soluble in organic media, allowing the use of a substrate, acetone, as solvent. The reaction proceeds rapidly, with high conversion and good selectivity towards β-hydroxyketone. The tetrahedral boronate acts as a base, suitable to form boron-enolate with acetone, but is not able to dehydrate the formed aldol.
This research has been performed within the framework of the CatchBio program. The authors gratefully acknowledge the support of the Smart Mix Program of the Dutch Ministry of Economic Affairs and the Dutch Ministry of Education, Culture and Science. The authors are thankful to L. Panella (DSM), P. Alsters (DSM), J. G. de Vries (DSM), B. Kaptein (DSM), G. Kemperman (MSD) for fruitful discussions, and S.A. Kulkarni (TU Delft, P&E/IRS) for the Raman measurements.
Notes and references
- J. Podlech, Angew. Chem., Int. Ed., 2010, 49, 6490–6495 CrossRef CAS.
- P. S. Gradeff, US Patent, 1974, 3840601; AN 1975:43622 Search PubMed.
- P. W. D. Mitchell, US Patent, 1989, 4874900; AN 1989:574454 Search PubMed.
- K. Weissermel and H.-J. Arpe, Industrial Organic Chemistry, Wiley-VCH, Weinheim, 4th edn, 2003 Search PubMed.
- Z. Zhang, Y. W. Dong and G. W. Wang, Chem. Lett., 2003, 32, 966–967 CrossRef CAS.
- (a) S. K. Karmee and U. Hanefeld, ChemSusChem, 2011, 4, 1118–1123 CrossRef CAS; (b) A. M. Frey, S. K. Karmee, K. P. de Jong, J. H. Bitter and U. Hanefeld, ChemCatChem DOI:10.1002/cctc.201200282.
- R. Mestres, Green Chem., 2004, 6, 583–603 RSC.
- J. Sukumaran and U. Hanefeld, Chem. Soc. Rev., 2005, 34, 530–542 RSC.
- P. Clapes, W.-D. Fessner, G. A. Sprenger and A. K. Samland, Curr. Opin. Chem. Biol., 2010, 14, 154–167 CrossRef CAS.
- C. Hertweck, Angew. Chem., Int. Ed., 2009, 48, 4688–4716 CrossRef CAS.
- (a) D. A. Evans, E. Vogel and J. V. Nelson, J. Am. Chem. Soc., 1979, 101, 6120–6123 CrossRef CAS; (b) T. Nitabaru, N. Kumagai and M. Shibasaki, Tetrahedron Lett., 2008, 49, 272–276 CrossRef CAS; (c) T. Nitabaru, A. Nojiri, M. Kobayashi, N. Kumagai and M. Shibasaki, J. Am. Chem. Soc., 2009, 131, 13860–13869 CrossRef CAS.
- D. A. Evans, J. Bartroli and T. L. Shih, J. Am. Chem. Soc., 1981, 103, 2127–2129 CrossRef CAS.
- D. A. Evans, J. V. Nelson, E. Vogel and T. R. Taber, J. Am. Chem. Soc., 1981, 103, 3099–3111 CrossRef CAS.
- (a) G. Li, H.-X. Wei, B. S. Phelps, D. W. Purkiss and S. H. Kim, Org. Lett., 2001, 3, 823–826 CrossRef CAS; (b) U. Hanefeld, A. M. Hooper and J. Staunton, Synthesis, 1999, 401–403 CrossRef CAS; (c) A. Koskinen, Asymmetric Synthesis of Natural Products, John Wiley & Sons, Chichester, 1993, pp. 69–75 Search PubMed; (d) R. C. Harris, A. L. Cutter, K. J. Weissman, U. Hanefeld, M. C. Timoney and J. Staunton, J. Chem. Res., 1998, 283 RSC; (e) A. L. Wilkinson, U. Hanefeld, B. Wilkinson, P. F. Leadlay and J. Staunton, Tetrahedron Lett., 1998, 39, 9827–9830 CrossRef CAS; (f) K. J. Weissman, M. Bycroft, A. L. Cutter, U. Hanefeld, E. J. Frost, M. C. Timoney, R. Harris, S. Handa, M. Roddis, J. Staunton and P. F. Leadlay, Chem. Biol., 1998, 5, 743–754 CrossRef CAS; (g) C. Körner, P. Starkov and T. D. Sheppard, J. Am. Chem. Soc., 2010, 132, 5968–5969 CrossRef.
- Y. Mori, J. Kobayashi, K. Manabe and S. Kobayashi, Tetrahedron, 2002, 58, 8263–8268 CrossRef CAS.
- K. Aelvoet, A. S. Batsanov, A. J. Blatch, C. Grosjean, L. G. F. Patrick, C. A. Smethurst and A. Whiting, Angew. Chem., 2008, 120, 780–782 ( Angew. Chem., Int. Ed. , 2008 , 47 , 768–770 ) CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Material and methods; NMR and Raman spectroscopy data. See DOI: 10.1039/c2cc37047f |
| This journal is © The Royal Society of Chemistry 2013 |

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C
C(C