Recent progress in research on anion-responsive pyrrole-based π-conjugated acyclic molecules†
Abstract
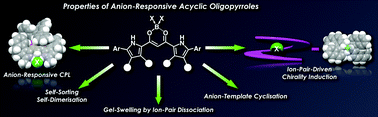
This article summarises recent progress in the study of acyclic oligopyrrole molecules that show anion-binding behaviours. Compared to cyclic oligopyrroles, acyclic systems have shown fascinating properties in terms of their linear geometries, as seen in the examples of biotic oligopyrroles and related analogues, which have shown the ability to transport anions across membranes. Another example of anion-responsive acyclic oligopyrroles is a dipyrrolyldiketone boron complex reported by our group. Modifications of the pyrrole rings and the boron unit of the anion-responsive molecule have resulted in the preparation of various derivatives, which showed efficient anion-binding behaviours and unique electronic and optical properties and formed assemblies in a variety of states. In this article, we mainly focus on anion-driven discrete assemblies, such as the helical structures of covalently linked oligomers and their resulting properties in the solution state.
- This article is part of the themed collection: Emerging Investigators 2013

 Please wait while we load your content...
Please wait while we load your content...