Neural differentiation on synthetic scaffold materials
Busra Mammadov, Melike Sever, Mustafa O. Guler* and Ayse B. Tekinay*
Institute of Materials Science and Nanotechnology, National Nanotechnology Research Center (UNAM), Bilkent University, Ankara, Turkey 06800. E-mail: atekinay@unam.bilkent.edu.tr; moguler@unam.bilkent.edu.tr
First published on 20th September 2013
Abstract
The potential of stem cells to differentiate into a variety of subgroups of neural cells makes stem cell differentiation and transplantation a promising candidate for neurodegenerative disorder therapies. However, selective differentiation of stem cells to neurons while preventing glial scar formation is a complex process. Mimicking the natural environment of neural tissue is pivotal, thus various synthetic materials have been developed for this purpose. The synthetic scaffolds can direct stem cells into a neural lineage by including extracellular factors that act on cell fate, which are mainly soluble signals, extracellular matrix proteins and physical factors (e.g. elasticity and topography). This article reviews synthetic materials developed for neural regeneration in terms of their extracellular matrix mimicking properties. Functionalization of synthetic materials by addition of bioactive chemical groups and adjustment of physical properties such as topography, electroactivity and elasticity are discussed.
 Busra Mammadov | Busra Mammadov graduated from Department of Molecular Biology and Genetics at Middle East Technical University (METU) in 2006 and obtained her MS degree in Biology at Fatih University in 2008. Currently she is a PhD candidate at Institute of Materials Science and Nanotechnology, National Nanotechnology Research Center (UNAM), Bilkent University. She is working on development of synthetic scaffolds for neural regeneration and testing these scaffolds in vitro for induction of neural differentiation and in vivo for neural regeneration. |
 Melike Sever | Melike Sever graduated from Department of Molecular Biology and Genetics at Middle East Technical University (METU), Turkey in 2011. She obtained her MSc degree in Biochemistry from the same university in 2012. Currently, she is pursuing her doctoral research in Materials Science and Nanotechnology at National Nanotechnology Research Center (UNAM), Bilkent University, Turkey. She is continuing her research on neural tissue regeneration at Nanobiotechnology Laboratory. |
 Mustafa O. Guler | Mustafa O. Guler is a professor of Materials Science and Nanotechnology at Bilkent University. He received his PhD degree in chemistry from Northwestern University in Evanston, IL, USA in 2006. After receiving his PhD, he had worked at the Institute for Bionanotechnology in Medicine at Northwestern University and Nanotope Inc. in Chicago, IL, USA until 2008. His research is based on discoveries of nanostructures at the interface of chemistry, biology, and materials science. |
 Ayse B. Tekinay | Ayse B. Tekinay is a professor of Materials Science and Nanotechnology at Bilkent University. She received her PhD degree in molecular biology at the Rockefeller University, New York, USA in 2006. After receiving her PhD, she continued her post-doctoral studies at the Rockefeller University until 2009. Her research focus is nanobiotechnology, cell-extracellular matrix interactions, and use of extracellular matrix platforms for tissue regeneration and regenerative medicine. |
1. Introduction
Neurodegeneration is the progressive loss of structure and function of nerve cells. Degeneration is a common phenomenon in many neurodegenerative diseases and nerve injury. Neurodegeneration generally occurs due to the accumulation of misfolded aggregated proteins in some parts of the aging brain and, as a result, cell death and inflammatory damage occur in those areas in the brain.1 Clinically, there are different types of neurodegeneration in different neurodegenerative diseases. Therefore, there are some differences in the proximal triggers and pathological markers such as Lewy bodies in Parkinson's disease, plaques and tangles in Alzheimer's disease, demyelination in multiple sclerosis and motor-neuron death in ALS. On the other hand, despite different triggering factors, these diseases share some overlapping downstream and secondary pathways such as neuroinflammation. Adult central nervous system cells have poor regeneration capacity, so any damage to the central nervous system might be permanent. Lost cells cannot be replaced with new functional ones, and remaining nerve cells cannot make new connections after injury due to the inhibitory properties of the extracellular matrix.2 Besides neurodegenerative disorders, traumatic injuries such as spinal cord injuries have destructive effects on motor, sensory, and autonomic functions. It generally causes a permanent loss of sensation below the site of the injury. In the case of peripheral nerve injuries, cells in the peripheral nervous system have a regeneration capacity unlike the ones in the central nervous system.Fully restoring the functional capacities of neurons after damage due to traumatic injuries or neurodegenerative disorders is still not possible. Although conventional therapies provide neural regeneration up to a certain level, they are not as efficient as desired. When the neural regeneration term is used, it refers to several different mechanisms to restore the functions of the degenerated neural tissue. It either occurs by the generation of new neurons from the progenitor cells residing nearby the damaged area, the generation of new synapses by the neurons that survive after the damage or by the repair of the axons/myelin sheets around the axons to prevent secondary cell loss after injury. It is more effective to combine these strategies in order to achieve functional regeneration of the nervous system. However, due to some intrinsic characteristics of the nervous system such as the unproliferative nature of neurons, the presence of progenitors in very localized areas in low numbers along with the upregulation of inhibitory elements upon injury leading to glial scar formation, the regeneration capacity of the central nervous system (CNS) is quite limited. This limited capacity of regeneration is aimed to be improved by materials designed for stem cell culture, differentiation and transplantation, which are called ‘scaffolds’.
Stem cells are promising candidates for the treatment of neurological disorders since they can differentiate into neural cells when induced appropriately. Their differentiation can be enhanced by using bioactive materials, which can also be used as vehicles for cell transplantation to the damage site. In previous studies, various synthetic materials have been analyzed as scaffolds in vitro and in vivo for their potential to induce neural differentiation and nerve regeneration.3–6 This review is focused on synthetic materials developed for neural regeneration including polymeric materials and self-assembled systems, and their modifications to support neural differentiation.
Natural cues for directing cell fate consist of different chemical, physical and biological signals in the extracellular matrix (ECM), neighboring cell interactions, soluble factors such as chemokines, growth factors and hormones, and the inherited potency of the cell.7 Combination of these factors and their interactions affects the ultimate cellular differentiation mechanisms (Fig. 1). Understanding this complex environment and mimicking it as efficiently as possible will enable the directing of cell differentiation in a desired manner. One way of mimicking the natural environment of cells in vitro is to supply soluble factors in the culture media. This approach is relatively simple but costly since it requires a high concentration of soluble factors. Also it is not very efficient since it does not mimic the mechanical properties of the cells’ natural environment. Cells are embedded in tissues in a three-dimensional (3D) manner and they can migrate or grow extensions such as axons and dendrites of neural cells. Physical properties of the 3D network including stiffness, roughness and pore size are also important and should be considered while designing a synthetic system that mimics the native tissue. As conventional in vitro cell culture surfaces cannot provide an appropriate environment to cells, 3D scaffolds combined with soluble signals or bioactive signals tethered to the surface are used to mimic the natural neural niche.
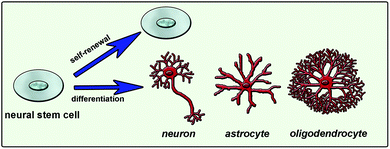 | ||
| Fig. 1 Neural stem cell fate determination is mainly guided by extracellular matrix molecules, soluble factors and cell-to-cell interactions. | ||
Natural extracellular matrix proteins or synthetic scaffolds have been used for developing 3D scaffolds to mimic the biological, chemical and mechanical properties of natural matrix of the cells. Although natural proteins are biocompatible, it is not easy to functionalize these scaffolds with desired bioactive groups, or to manipulate their mechanical properties such as stiffness. Thus, these scaffolds might not be sufficient to direct the differentiation of cells especially into complex cells like neurons.8–12 On the other hand, synthetic materials can be synthesized with desired functionalities such as specific bioactive groups, stiffness and roughness in order to mimic the natural environment of cells.
2. Approaches in the design of synthetic materials for neural differentiation
A variety of different methods can be used to develop scaffold materials with desired properties, including the support of cell survival and the induction of differentiation into desired cell types. Besides changing the material type, nerve regeneration studies have also focused on designing scaffolds with incorporated bioactivity for the induction of neurogenesis. One approach to induce differentiation is by adding soluble inducers while culturing cells on non-bioactive scaffolds. In this approach, a scaffold is a better environment for cell survival compared to a tissue culture plate. However, as the scaffold itself does not include any signal for differentiation, differentiation is induced solely by the soluble inducers added to the growth media. Soluble inducers commonly used for neural differentiation include neurotrophic factors (nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) etc.) and retinoic acid (RA).Another approach is by embedding the soluble inducers mentioned above into the scaffold in order to provide a gradual release or by tethering these inducers to the surface of the scaffold through functional groups, in order to provide spatial organization to cells. In the case of gradual release, the scaffold is not bioactive but has a role in differentiation by releasing inducers at desired concentrations over a longer time period. When tethering the inducers to the surface, the scaffold is bioactive, however its bioactivity is not directly related to the differentiation process. Bioactive groups on the material’s surface are presented so that they bind to the soluble inducers and present them similar to the extracellular matrix components (such as heparan sulfate proteoglycans) presenting growth factors to the cells. Soluble factors can also be immobilized on material surfaces directly by covalent attachment without the use of the growth factor affine proteoglycans.13
Functionalization of the surface with bioactive signals is a method used for differentiation induction directed by scaffold. Bioactive groups can be either chemical groups inspired by the natural environment of cells in a specific tissue, like the brain, or functional groups of natural inducers such as peptides found in neural differentiation-inducing proteins. Besides the addition of bioactive signals, scaffolds can also be functionalized by tuning their mechanical properties and physical morphology in order to support neural cell survival and differentiation. Since they present bioactive groups, these scaffolds can be used alone for the induction of differentiation, depending on the complexity of the final cell type and potency of the starting cell for differentiation into desired cell phenotypes (Fig. 2). Such a scaffold can induce differentiation into different neuronal subtypes. However, it might not be sufficient for maturation of the induced cells into functional cells. Additional inducers can be added to the culture medium to promote maturation or the scaffold can be functionalized with multiple bioactive groups to overcome the maturation problem.
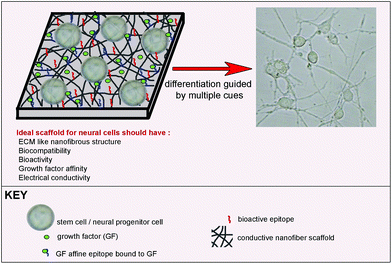 | ||
| Fig. 2 Bioactive scaffold design for neural differentiation of stem cells. | ||
2.1. Use of synthetic scaffolds in combination with soluble inducers
Nonbioactive synthetic scaffolds can be used in combination with soluble inducers. These scaffolds combined with soluble inducers are not actively involved in the differentiation process; however, they support differentiation by providing a 3D environment for cells that is more similar to their natural environment when compared to two-dimensional (2D) tissue culture surfaces. Neurons can have outgrowths in all directions within 3D scaffolds, thus 3D scaffolds are more accurate representations of in vivo tissue architecture, compared to 2D scaffolds. They provide in vivo-like cell–cell interactions which increases cellular survival and leads to more realistic gene expression and cellular behavior. 2D cultures have less compatibility with in vivo systems. For example, it has been shown that dopaminergic neurons isolated from an embryonic brain display longer viability in 3D systems when compared to monolayer 2D cultures.14 On the other hand, nutrient deprivation in 3D scaffolds causes more severe alterations in the expression of specific genes, cell proliferation and viability as well as productivity. Also, it was shown that mechanical injuries cause a more severe response in cells grown in 3D neural cultures than 2D scaffolds even when they are subjected to the same strain and strain rate.15These scaffolds do not have bioactive groups and they usually do not induce differentiation by themselves, except for when stiffness is used to control differentiation pathway.8–12 Hence, defining an optimal cocktail of soluble inducers such as growth factors plays an essential role in the success of differentiation; however, it is costly and complex. Inducers are often chosen by considering the extracellular signals that play a role in neural cell survival and differentiation in the central nervous system. This approach is beneficial in terms of the ease of material synthesis as the material is not further modified and it is sufficient that the material does not interfere with cell survival. However, the lack of bioactivity makes the use of additional inducers, such as growth factors, inevitable. The selection of soluble inducers and their concentrations should be optimized to achieve the differentiation of cells cultured on nonbioactive scaffolds. Several different materials, mostly polymers, are used for the production of such scaffolds, examples of which are given below.
Nanofibrous poly-L-lactic acid (PLLA) scaffolds with high porosity were used for differentiation of neural stem cells (NSCs) and found to support neurite outgrowth.16 Poly(ethylene-co-vinyl alcohol) (EVAL) membranes are another type of polymeric scaffold used for neural cell culture. Rat NSCs cultured on these scaffolds differentiated into neurons and astrocytes in the presence of basic fibroblast growth factor (bFGF). However, polyvinyl alcohol (PVA) scaffolds used in the same study did not support cell viability.17 Although they do not contain bioactive signals, such scaffolds provide an initial environment for cells to produce their own microenvironment. NSCs encapsulated in a biodegradable polyethylene glycol (PEG) hydrogel in the presence of bFGF were observed to produce fibronectin, an indication of the production of a suitable microenvironment. These cells later differentiated into neurons and astrocytes, as demonstrated by immunohistochemistry and western blot analyses. Differentiated neurons were found to express synaptic protein synaptophysin and they were responsive to the neurotransmitter GABA (Fig. 3) indicating the functionality of de novo differentiated neurons.18 Human bone marrow mesenchymal stem cells (MSCs) cultured on polyesters of 3-hydroxyalkanoate scaffolds in the presence of neural induction media (serum-free DMEM containing bFGF, IBMX, INDO and insulin) differentiated into neural cells with a better efficiency when compared to those grown on polylactic acid (PLA) scaffolds. In comparison, 3D scaffolds supported differentiation better than 2D polymer films and smaller pore sizes resulted in more effective differentiation, while scaffolds with larger pore sizes lead to a promotion of proliferation.19 In addition to MSCs, NSCs were also found to effectively differentiate into neural cells on polyhydroxyalkanoates (PHA) with better efficiency when cultured on 3D scaffolds rather than 2D films of the same polymers. Among the three different PHA scaffolds used, poly(3-hydroxybutyrate) (PHB), copolymer of 3-hydroxybutyrate and 4-hydroxybutyrate (P3HB4HB) and copolymer of 3-hydroxybutyrate and 3-hydroxyhexanoate (PHBHHx), PHBHHx was found to support neural differentiation of NSCs with better efficiency as demonstrated by higher levels of β-III tubulin expression by western blot and immunostaining.20 Microspheres composed of copolymers of PHA were also used for neural cell culture. PC-12 cells, primary cortical neurons (CN), and neuronal progenitor cells (NPC) were cultured on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) microspheres along with the soluble growth factor, BDNF. PHBV microspheres supported the growth and proliferation of PC-12 cells and the differentiation of NPCs into neurons. However, the level of maturation of differentiated NPCs was lower when compared to the full maturation of CNs.21
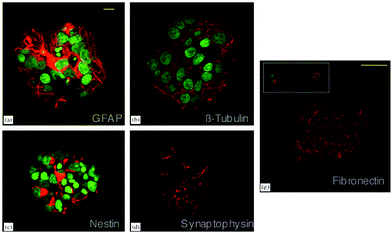 | ||
| Fig. 3 Differentiation of NSCs into astrocytes (a) and neurons (b) in PEG hydrogels. Expression of nestin by undifferentiated NSCs (c), synaptophysin by differentiated neurons (d) and fibronectin by neural cells (e) are also shown. Scale bar represents 10 μm (a–d) and 50 μm (e). Reprinted with permission from ref. 18. | ||
Another nonbioactive polymeric scaffold for neural regeneration was fabricated by ink-jet microdispensing PLA/polycaprolactone (PCL) in 80/20 proportions. In this study, genetically modified human embryonic kidney cells (EcR-293 cells), which can mimic Schwann cells by secreting NGF upon induction, were utilized. A PLA/PCL scaffold was found to support cell adhesion and cell growth.22 Another genetically modified cell line used for neural differentiation was NSCs which were altered to express TrkC and neurotrophin-3 (NT-3). These cells differentiated on macroporous poly(lactic-co-glycolic acid) (PLGA) scaffolds without the use of additional soluble inducers. TrkC and NT-3 expression by NSCs favored neuronal differentiation over astrocyte and oligodendrocyte differentiation.23
Polymeric scaffolds fabricated by electrospinning have also been used for neural differentiation of stem cells. Polyurethane (PU) scaffolds with high porosity produced by electrospinning technique were used for the differentiation of human embryonic stem cells (hESCs) into neurons. The hESCs cultured on PU scaffolds were induced with a neural induction medium composed of Neuronal A basal medium supplemented with N2 and B27 supplements along with epidermal growth factor (EGF) and bFGF. Differentiated cells were positive for β-III tubulin, an early neural marker, MAP2ab, a mature neuron marker, and tyrosine hydroxylase (TH), a dopaminergic cell marker. Differentiated cells did not express glial fibrillary acidic protein (GFAP), indicating neural differentiation in the absence of astrocytic differentiation. The morphology of the differentiated cells, as demonstrated by scanning electron microscopy (SEM) images, also resembled neuronal morphology24 (Fig. 4).
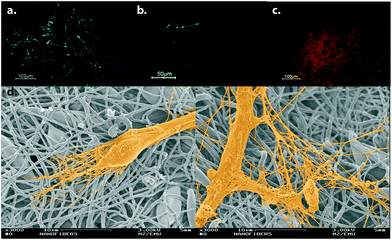 | ||
| Fig. 4 Neural differentiation of hESCs on electrospun PU scaffolds. Differentiated cells express β-III tubulin (a), MAP2ab (b) and TH (c). (d) SEM micrographs of differentiated cells are demonstrated. Reprinted with permission from ref. 24. | ||
Soluble factors can be used in combination with extracellular matrix proteins for a more efficient induction of neural differentiation. Poly(lactic-co-glycolic acid) (PLGA)/poly(L-lactic acid) (PLLA) polymer scaffolds with pore sizes between 250–500 μm coated with fibronectin or Matrigel™ were used for neural differentiation of hESC by adding RA into the growth media. The PLGA/PLLA scaffold provided a biodegradable 3D matrix and fibronectin/Matrigel™ coating which, along with RA, served as neural differentiation inducers. The hESCs grown on these scaffolds formed rosette-like structures with a similar morphology to an embryonic neural tube and expressed nestin and β-III tubulin. These structures were also found to express neurofilament (NF) by RNA analysis.25
Encapsulating differentiation inducer proteins in scaffolds provides sustained release over a long period depending on the scaffold material. Synthetic materials are commonly used to entrap neural inducer proteins to provide a gradual release for supporting neural differentiation. In one study, PLGA conduits and NT-3 were combined to generate NT-3-loaded PLGA carriers and this scaffold was used to co-culture NSCs and Schwann cells. Sustained release of NT-3 from the scaffold lasted up to 4 weeks. Immunoreactivity against MAP2 showed the differentiation of NSCs into neurons. In addition, synaptic structures and myelin sheaths were detected in the co-culture by double-immunostaining and electron microscopy analyses. Synapses between cells were excitable and capable of releasing synaptic vesicles under depolarization conditions. These results indicated a positive effect of NT-3 release from PLGA on the differentiation of NSCs into neurons, the development of synaptic connections and the myelination of neurites.26
NGF-encapsulating scaffolds are also commonly used in neural cell culture. A biodegradable electrospun copolymer of ε-caprolactone and ethyl ethylene phosphate (PCLEEP) with encapsulated NGF was used for the culture of PC-12 cells. Sustained release of NGF from the scaffold was observed for 3 months, resulting in neurite outgrowth of PC-12 cells.27 Sustained release of NGF from PCL was obtained for 28 days by using PCL-bovine serum albumin (BSA)-NGF nanofibers that were also fabricated by electrospinning. The incorporation of BSA into fibers enhanced the sustained release and homogeneous distribution of NGF when compared to PCL fibers without BSA incorporation.28 Hydrogels of polymers are also used for sustained release of neural inducers. NGF-loaded lysine-incorporated poly(2-hydroxyethylmethacrylate) [p(HEMA)] hydrogels resulted in the slow release of NGF due to the positive charge of the hydrogel provided by the lysine moieties. Dorsal root ganglion (DRG) neurons cultured in these hydrogels extended much longer neurites when compared to the soluble NGF-treated cells.29 The p(HEMA) microporous gels with a gradient of immobilized NGF were used for PC-12 neurite outgrowth assay. In this study, neurites grew in the direction of higher concentration of NGF in the gradient.30
The sustained release of another neuronal induction factor, RA, was also obtained by encapsulating RA within aligned PCL nanofibers. MSC neuronal fate was affected by both nanofiber topography and controlled RA release. Without RA release, nanofiber topography was not sufficient to induce synaptophysin expression from MSCs, emphasizing the importance of the combined effect of topography and sustained release.31
Chemical conjugation of growth factors to electrospun nanofibers is also effective in inducing neural differentiation, even more effective than physical adsorption, which leads to burst release, as shown by Cho et al. In this study, amine-terminated PEG-poly(ε-caprolactone) conjugates were electrospun to obtain random and aligned nanofibers. NGF was chemically conjugated to free amine ends of PEG on the surface of the fibers. MSCs seeded on these NGF-conjugated scaffolds trans-differentiated into neural cells after 7 days, as evidenced by the expression of both immature (nestin and β-III tubulin) and mature (MAP-2) neural markers by RT-PCR and immunostaining analysis. The expression of neural markers was at the highest level on NGF-conjugated aligned scaffolds when compared to random fibers and NGF-adsorbed fibers.32
2.2. Physical, chemical or biological functionalization of scaffolds for promotion of neural differentiation
Chemically, physically and biologically functionalized scaffolds can hold several characteristics of the natural environment of cells at the same time. Bioactivation can be achieved through modulating the scaffold by addition of small chemical groups inspired by specific chemicals found in different tissues (e.g. phosphate in bone) as well as by presenting short peptide sequences on the surface of the scaffold that are functional domains of inducer proteins. Physical properties of tissues including elasticity, stiffness, roughness and electrical conductivity are other important parameters that should be considered for scaffold functionalization. In this section, different approaches of scaffold functionalization for neural cell culture are explained in detail.In a pioneering study, polyacrylamide gels with elasticities between 0.1–1 kPa were coated with collagen I and used for the direct induction of neural differentiation of hMSCs without a requirement for any soluble factor. The hMSCs obtained a neuronal morphology (Fig. 5) with the expression of a wide array of neural markers, including commitment (nestin), early differentiation (β-III tubulin), and mature neural cell markers (NF and MAP2) as demonstrated by immunofluorescence, western blot and microarray analyses. After three weeks of culture on these soft substrates, hMSCs committed to neural cell fate irreversibly even under the influence of myogenic and osteogenic inducers.9
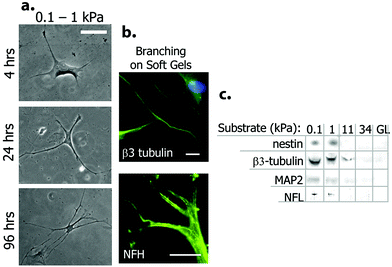 | ||
| Fig. 5 Neural differentiation of hMSCs on soft polyacrylamide gel scaffolds. (a) Change in cell morphology when cultured on the substrate with low stiffness. Note the neural projection-like branches. (b) Expression of neural markers β-III tubulin and NF by differentiated cells. (c) Western blot analysis of neural markers for cells grown on PA gels with different elasticities. Glass (GL) surface was used as control. Reprinted with permission from ref. 9. | ||
Laminin-coated methacrylamide chitosan (MAC) hydrogels with different stiffness values were also used for analysis of stem cell behavior with respect to changing stiffness. The proliferation and differentiation of NSCs were found to be promoted on the softest scaffolds with elasticities less than 1 kPa (Fig. 6).10 MAC hydrogels with a similar stiffness to brain tissue were also functionalized by IFN-γ and resulted in neural differentiation of NSCs more effectively than brain-derived neurotrophic factor (BDNF)-treated NSCs.35 Polydimethylsiloxane (PDMS) substrates with differing stiffness produced by using varying proportions of cross-linking agents were also used for NSC culture. In this study, astrocyte differentiation occurred at the highest level on soft substrates while oligodendrocyte differentiation rate (induced by addition of thyroid hormone) was found to be independent of substrate stiffness. The number of differentiated neurons was also observed to be independent of stiffness, while maturation of these differentiated neurons was highly dependent on the degree of stiffness. Neurite length and expression of synaptic proteins were promoted on scaffolds with stiffness values near to that of brain tissue (Fig. 7).12
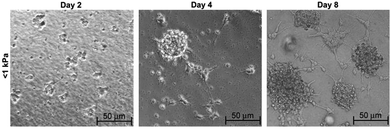 | ||
| Fig. 6 Differentiation of NSCs in neurobasal media on soft MAC hydrogel of <1 kPa substrate stiffness over 8 days of culture. Reprinted with permission from ref. 10. | ||
 | ||
| Fig. 7 Differentiation of NSCs into neurons on tissue culture plate (a) and PDMS substrates with changing stiffness; 750 kPa (b) and 12 kPa (c). Reprinted with permission from ref. 12. | ||
In another study, glass surfaces coated with the electroactive silsesquioxane precursor N-(4-aminophenyl)-N′-(4′-(3-triethoxysilyl-propyl-ureido) phenyl-1,4-quinonenediimine) (ATQD) were covalently modified with cyclic RGD peptide. PC-12 cells were reported to extend neurites on these surfaces even in the absence of NGF. Addition of NGF further enhanced the level of neurite extension (Fig. 8).39
 | ||
| Fig. 8 Neurite extension of PC-12 cells on electroactive surfaces. Tissue culture plate without (a), and with NGF (b), electroactive ATQD-RGD without (c) and with NGF (d) (50 ng mL−1). Reprinted with permission from ref. 39. | ||
Aligned PLLA nanofibers produced by electrospinning technique modified by bFGF and laminin immobilization via heparin interaction could efficiently induce neurite extension from DRG. Neurites of DRG cells were extended in the direction of alignment, which has considerable importance for nerve bridging in clinical applications (Fig. 9).40 Aligned PLCL nanofibers also induced DRG neurite alignment through the direction of fibers and coating PLCL fibers with multi-walled carbon nanotubes was found to lead to extension of much longer neurites. CNTs used in this study were ionically modified. This coating increased the hydrophilicity of the PLCL fibers, which was attributed to longer neurite production. In addition, the CNT coating provided electrical conductivity to the non-conducting PLCL fibers, making the environment more suitable for neural cells.41 Much longer neurites were produced by PC-12 cells cultured on CNT-coated PLCL nanofibers compared to the uncoated PLCL fibers, similar to the response of DRG cells.42
 | ||
| Fig. 9 Neurite outgrowth from DRG cells on PLLA nanofibers demonstrated by neurofilament staining. DRG cells on (A) random nanofibers, (B) aligned nanofibers, and (C) aligned nanofibers immobilized with bFGF. Reprinted with permission from ref. 40. | ||
Embryonic stem cells (ESCs) seeded on aligned electrospun PCL fibers exhibited neurite growth in the same direction as the alignment, similar to DRG cells on PLLA and PLCL fibers, after retinoic acid treatment (Fig. 10). In addition, the number of differentiated astrocytes was less on aligned PCL fibers compared to cells cultured on random PCL fibers.43 Nanoscale ridge-groove patterns of polyurethane acrylate (PUA) fabricated by UV-assisted capillary force lithography were also used to direct selective neural differentiation of ESCs without the use of any soluble inducers. ESCs seeded on aligned PUA substrates differentiated into neural cells that express neural markers Tuj1, HuC/D and MAP2, but not GFAP, indicating the absence of astrocytes. Cells cultured on flat PUA surfaces didn't express any of these neural markers. Some of the cells on the ridge-groove patterns even differentiated into serotonergic and GABAergic neurons as demonstrated by the expression of serotonin and GABA.44
 | ||
| Fig. 10 Neural differentiation of ESCs on random (a,b) and aligned (c,d) PCL fibers. Immunohistochemistry is performed for a neural cell marker, Tuj1. Reprinted with permission from ref. 43. | ||
Aligned electrospun nanofibers were also used for NSC differentiation. The direction of NSC elongation and neurite outgrowth was found to be parallel to the direction of PLLA fibers for aligned scaffolds (Fig. 11). Also, the rate of NSC differentiation was higher for PLLA nanofibers than that for microfibers, indicating that the aligned nanofibrous PLLA scaffold could have more potential use in neural tissue engineering than microscale aligned fibers.45 Effects of PLLA nanofibers might vary with respect to different properties of these fibers such as fiber diameter or density. For example, when DRG cells were cultured on highly aligned PLLA electrospun fibers, the direction and extent of neurite extension and Schwann cell migration from DRG explants was found to be influenced significantly by fiber diameter. The length of neurites was shorter on small diameter fibers (293 nm) when compared to the large diameter ones (1325 nm).46 In another study, increasing the PLLA fiber density was found to be correlated to an increase in neurite density, without affecting the length of the extending neurites (Fig. 12).47 The effect of topography on NSC differentiation has also been demonstrated with several other polymeric fibers as scaffolds. NSCs cultured on aligned poly(ε-caprolactone)/gelatin scaffolds exhibited neurite outgrowth parallel to the fiber direction. In this study, gelatin was found to further promote differentiation in addition to scaffold alignment.48
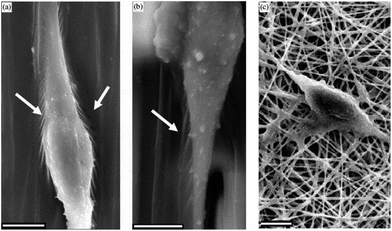 | ||
| Fig. 11 SEM images of NSCs differentiated on aligned nanofibers (a), microfibers (b) and random nanofibers (c) of PCL. Arrows indicate sites of interaction between cells and the scaffold. Reprinted with permission from ref. 45. | ||
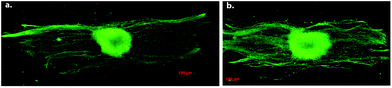 | ||
| Fig. 12 Effect of fiber density on the number of neurites produced by DRG cells cultured on a. low density, and b. high density aligned electrospun PLLA scaffolds for five days. Immunohistochemistry is performed for neurofilament. Reproduced with permission from ref. 47. | ||
Neuritogenesis and major neurite (axon) formation was demonstrated to be accelerated in primary motor neurons cultured on electrospun PLLA nanofibers (0.6–0.8 μm diameter) when compared to PLLA films and glass substrates. However, there was no difference between random and aligned fibers in the acceleration of neurite formation, and the minor neurite density and neurites were shorter in cells grown on fibers.49
Besides the aligned polymeric fibers, nanogratings were also used to produce aligned surfaces for stem cell differentiation. Aligned PDMS nanogratings lead to neural differentiation of hMSCs, even in the absence of any soluble factor, as evidenced by upregulation of the neural markers nestin, β-III tubulin, MAP2 and synaptophysin. Cells seeded on flat PDMS showed significantly lower expression of these neural markers, while the addition of retinoic acid increased their expression levels. Also, nanogratings were found to induce neural differentiation better than micropatterned PDMS substrates.50 Micropatterned PDMS surfaces coated with poly-L-lysine (PLL) and laminin can also serve as effective neurite guidance surfaces for NSCs but the channel width should be properly defined for proper alignment and adequate neuron density. Smaller micropatterns were found to provide a perfect alignment, but hindered neurite development. On the other hand, larger micropatterns provided higher neuron density but neurites escaped out of the microchannel.51 Precisely-sized micropatterned poly(methyl methacrylate) (PMMA) grooved scaffolds were used for mature astrocyte differentiation from radial glia-like cells (RGLC) in vitro without adding any soluble or substrate-adsorbed biochemical factors. RGLC were highly organized and the cells aligned along a 2 μm-patterned PMMA line. They expressed both nestin and Pax6, and generated different intermediate progenitors. These micropatterned surfaces also supported and directed axonal growth and neuronal migration.52
Surface topography also affects the resting membrane potential and voltage-gated calcium channel responsiveness, which are important parameters in the determination of the functionality of a differentiated neural cell. Neural progenitor cells grown on polystyrene microbead arrayed substrates exerted more negative resting membrane potentials and higher voltage-gated calcium channel responsiveness when compared to cells grown on flat polystyrene surfaces, indicating that surface roughness can direct the differentiation of stem cells into more functional neural cells.53
Besides topography, surface geometry was also found to have profound effects on cellular behaviors including adhesion, proliferation and differentiation. Poly(ε-caprolactone) nanowires fabricated by a solvent free nanoscale template technique were found to support PC-12 cell adhesion, proliferation and viability better when compared to smooth PCL surfaces. Cells were found to interact with PCL nanowires via their lamellopodia and filopodia, as evidenced by SEM imaging. Neural differentiation was evidenced by the presence of neurite extensions as well as NF and TH expression evidenced by immunofluorescence analysis, while neurite extension was not observed on a smooth PCL surface.54
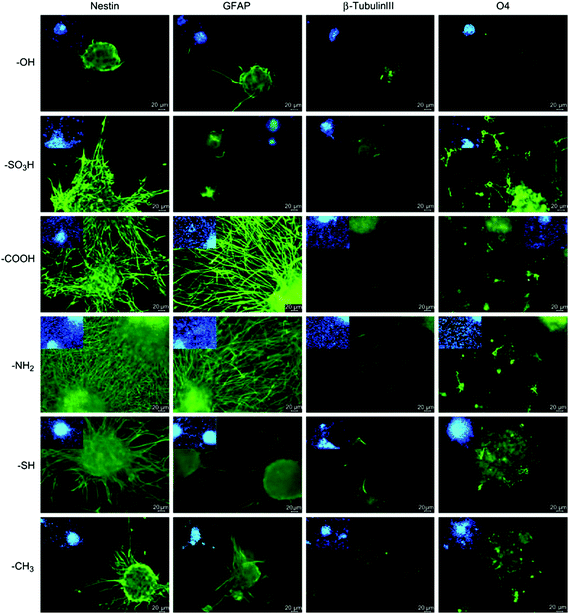 | ||
| Fig. 13 Differentiation of NSCs on chemically functionalized glass substrates as demonstrated by expression of markers for astrocytes (GFAP), neurons (β-III tubulin) and oligodendrocytes (O4). Undifferentiated NSCs are shown by nestin expression. Reprinted with permission from ref. 55. | ||
Another method of functionalizing polymeric scaffolds is through mimicking the chemical structure of key proteins in neural differentiation. Acetylcholine-like polymers were synthesized by click chemistry and free radical polymerization from a bioactive unit mimicking acetylcholine, dimethylaminomethyl methacrylate (DMAEMA) and a bioinert unit, poly(ethylene glycol) monomethyl ether-glycidyl methacrylate (MePEG-GMA). Polymeric surfaces consisting of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (mol/mol) of MePEG-GMA to DMAEMA were found to support adhesion, normal morphology and neuronal outgrowth of hippocampal neurons effectively.56
60 (mol/mol) of MePEG-GMA to DMAEMA were found to support adhesion, normal morphology and neuronal outgrowth of hippocampal neurons effectively.56
The introduction of specific chemical groups on polymeric scaffolds can also be used for modification of surface tension, which in turn was found to affect neurite outgrowth. Embryonic cortical neurons that were cultured on electrospun PLLA and PLGA fibers treated with KOH to change surface tension grew longer neurites on the surfaces with the lowest tension, which were also the most hydrophobic scaffolds used in the study.57 Chemical heterogeneity of the surface, which leads to surface disorder and the formation of local gradients in the surface free-energy, is another parameter that was found to have a role in neuritogenesis. PC-12 cells seeded on a self-assembled monolayer of alkylsiloxanes, which were highly disordered with high levels of chemical heterogeneity, could extend neurites within 48 h even without NGF treatment.58
In order to find an optimal combination of inducer molecules and biomaterials for NSC differentiation, a combinatorial protein display was carried out to screen mixtures of a variety of biomaterials with different soluble signals. Natural and synthetic matrices (fibronectin (FN), laminin (LN), PLL, RGD, IKVAV, PEI; poly(ethyleneimine)) containing different growth factors (EGF, NGF, CNTF, NT-3) were immobilized onto gold surfaces by photo-assisted patterning of an alkanethiol self-assembled monolayer as spots. NSCs were grown on these spots, each with different combinations of matrices and growth factors, and their differentiation was analyzed by immunohistochemistry. This study revealed FN/CNTF and RGD/CNTF to be the most efficient inducers of astroglial differentiation and LN/NGF, FN/NGF, RGD/NGF, FN/NT-3 and RGD/NT-3 as the most effective neuronal differentiation inducers among the studied combinations.59
In another study, methylcellulose (MC) scaffolds functionalized with laminin-1 (MC-x-LN1) were used for culturing primary murine neurospheres. MC-x-LN1 was found to enhance both NSC survival and maturation. In addition, lower levels of apoptotic activity were observed on MC-x-LN1 when compared to unmodified MC controls. The expression of neuronal and oligodendrocyte precursor markers showed a higher differentiation level on laminin-functionalized scaffolds when compared to cells on unmodified MC surfaces.60
Aerogels are also an important class of materials with tunable chemical, physical, and surface properties. Polyurea crosslinked silica aerogels (PCSA) were coated with PLL, basement membrane extract (BME), or laminin-1 and used for a culture of DRG neurons. Interactions of DRG neurons were tracked on PCSA and PLL, BME, and laminin-coated aerogels. In this study, laminin was found to be the most effective surface treatment for the attachment and growth of DRG neurons on the PCSA surface.61
Polymeric scaffolds can also be functionalized by producing fibers of a mixture of a polymer and an ECM protein by electrospinning technique. Electrospun blended PLLA/laminin fibers promoted neural differentiation of PC-12 cells in the presence of NGF significantly more efficiently than the unblended PLLA nanofibers.62 PLCL/collagen fibrous scaffolds fabricated by electrospinning were used for neural differentiation of MSCs. Neural induction was performed gradually, first culturing MSCs in pretreatment media composed of β-mercaptoethanol, EGF, and bFGF; and then in the neural induction media consisting of N2 supplement, β-mercaptoethanol, insulin, EGF, NGF, and BDNF. MSCs on PCL/collagen fibers gained a neuronal phenotype with multipolar elongations along with the expression of neural markers NF and nestin (Fig. 14).63
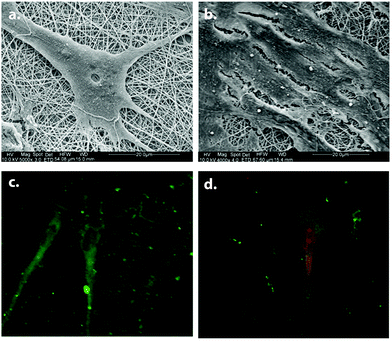 | ||
| Fig. 14 Neural differentiation of MSCs on PLCL/collagen scaffolds. SEM images of differentiated (a) and undifferentiated (b) MSCs. Neurofilament (green) and nestin (red) expressions of differentiated MSCs are shown in (c) and (d) respectively. Reprinted with permission from ref. 63. | ||
In such a study, functional domains of ECM proteins were produced as a fusion protein to the Escherichia coli outer membrane protein, OmpA, and attached to gold-coated surfaces. These bioactive surfaces effectively induced neurite extension and branching of neurites in PC-12 cells, as well as differentiation of NSCs.64 Differentiation of fetal NSCs was promoted on superporous p(HEMA-AEMA) hydrogels modified with laminin-derived IKVAV, when compared to unmodified p(HEMA-AEMA). NSCs cultured on IKVAV-p(HEMA-AEMA) scaffolds expressed higher levels of β-III tubulin, NF and synaptophysin.65 Polyamide electrospun nanofibers covalently attached to neuroactive tenascin C-derived peptides were found to promote neuritogenesis and neurite extension in primary neurons isolated from several different regions of brain, when compared to those cultured on unmodified polyamide scaffolds and PLL-coated glass cover slips.66
In another study, poly(HEMA-co-AEMA) nerve guidance channels were produced by a fiber templating technique and modified with laminin-derived peptides (YIGSR and IKVAV). Primary chick DRG cells cultured in these channels could effectively extend neurites. There was no statistical difference in terms of neurite length between the cells in these channels and those on a PLL/laminin positive control surface, indicating that laminin-derived epitopes are as efficient as laminin itself in the induction of neurite outgrowth.67
Self-assembled peptide scaffold systems contain functional domains found in ECM proteins or other neural differentiation inducer proteins, which bind to cell surface receptors. Peptide amphiphile (PA) nanofibers with cell specific signals can be produced by synthesis of the epitope of a signal protein and linking this peptide to an alkyl tail along with hydrophobic amino acids. PA molecules form nanofibers in aqueous solutions by self-assembly through charge neutralization and hydrogen bonding. These nanofibers present epitopes on their surface, directly available to cell surface receptors. PA nanofibers with laminin epitope (IKVAV) were found to induce selective differentiation of neural progenitor cells into neurons while suppressing astrocyte differentiation.68 PA nanofibers can present multiple epitopes at the same time. PA nanofibers, formed from laminin-derived IKVAV-PAs along with growth factor affine heparan sulfate-mimicking PAs, stimulated PC-12 differentiation cooperatively. Neurite outgrowth was promoted when cells were cultured on scaffolds presenting both functional groups. In addition, cells were found to extend neurites on these scaffolds even in the presence of inhibitory chondroitin sulfate proteoglycans.69 Heparan sulfate-mimicking PAs were found to have affinity towards NGF, providing an increase in the local concentration of NGF in close vicinity of cells. More importantly, NGF does not lose its bioactivity after interaction with these PA nanofibers, which was evident from its inductive effect on neurite outgrowth (Fig. 15).70
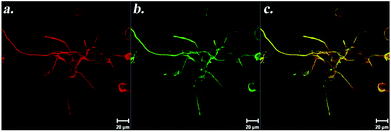 | ||
| Fig. 15 Immunostaining of PC-12 cells against β-III tubulin (a) and Syn1 (b) on NGF-treated heparan sulfate-mimicking nanofibers. Panel (c) shows merged images of β-III tubulin and Syn1 on the same cells. Reprinted with permission from ref. 70. | ||
The PA nanofibers were also used in a spinal cord injury (SCI) model in mice. IKVAV-PA injection led to a reduction in astrogliosis and cell death while increasing the number of oligodendroglia at the site of injury. IKVAV peptide alone was not able to promote recovery, which showed that both the nanofiber structure of the PA and the IKVAV sequence were required.71 The anatomical basis of the behavioural recovery coming from the injection of IKVAV-PA was analyzed in a separate study and the major factor for this improvement was found to be the increased density of serotonergic fibers close to the lesion.72
The mechanical properties of PA nanofiber scaffolds can also be modified by using different proportions of signal incorporated PAs and non-bioactive but mechanically more stable molecules. Such an approach leads to the formation of more stable and stiffer gels.73 In addition, their gelation kinetics can be modified by changing the amino acid sequences that are important for structural properties of the fibers. By including more hydrophilic and bulky amino acids, gelation can be slowed down without disrupting bioactivity, which can be important for in vivo applications such as injections.74
The PA gels can also be used for efficient delivery of biological molecules to the treatment site. One such example is the delivery of Sonic hedgehog (SHH) via monodomain gels containing aligned PA nanofibers. SHH protein has important roles in peripheral nerve regeneration. SHH signalling was inhibited in rats and their cavernous nerve was crushed leading peripheral nerve damage to form the animal model in this study. SHH was then delivered to the crushed cavernous nerve by the PA gel and promoted regeneration, suppressed penile apoptosis and improved erectile function. Such a treatment might be crucial in regeneration of the cavernous nerve in prostatectomy and diabetic patients where SHH levels are decreased.75 A hybrid matrix approach with the combination of type I collagen and peptide amphiphile nanofibers was also shown to support neuronal survival, morphogenesis, and fine control over dendrite and axon growth of Purkinje cells (PC). While collagen provided a favorable mechanical support, the laminin epitope concentration was adjusted to control the matrix bioactivity by using PAs. Therefore, this system enabled the adjustment of laminin epitope density to control bioactivity without affecting its structural integrity.76
Self-assembled peptide nanofibers produced from alternating basic, hydrophobic and acidic amino acids (e.g., RADA16) are also used for neural cell culture. They were initially reported to support the adhesion, neurite extension and synapse formation of PC-12 cells as well as primary neural cells.77 These scaffolds better enhanced neural differentiation when they were functionalized with epitopes of ECM proteins (laminin, collagen, and fibronectin) and bone marrow homing peptides (BMHP1, and BMHP2).78 RADA scaffolds were also synthesized by incorporating an FGL motif, a synthetic FGF receptor derived from a neural cell adhesion molecule (NCAM). DRG neurons cultured on these scaffolds extended much longer neurites when compared to bare (RADA)16 scaffolds, and the number of cells extending the neurites was higher in FGL-incorporated scaffolds.79 When (RADA)16 scaffolds were mixed with laminin for biofunctionalization, NSC differentiation was enhanced in 3D culture.80 (RADA)16-I scaffolds were also successful in axonal regeneration of an in vivo acute optic tract injury, and provided visual recovery.81
Peptide nanofibers can also be used as delivery vehicles for the sustained delivery of cytokines. (RADA)16 scaffolds modified by the addition of negatively or positively charged amino acids were used for sustained delivery of vascular endothelial growth factor, bFGF, and BDNF. Positively charged growth factors could be released more readily from positively charged scaffolds, while they were released slowly from negatively charged scaffolds.82 Glycine spacers were shown to increase both the stability and functional motif exposure of the nanofibers. Nanofibers with more glycine residues incorporated between the self-assembling sequence (RADA)16-I and the bioactive sequence (PFSSTKT, derived from BMHP1) supported NSCs adhesion, proliferation and differentiation more effectively than those with shorter spacers.83
Short peptide epitopes derived from ECM components were also used to produce peptide-modified silica thin gels for neural differentiation induction. Embryonic carcinoma stem cells were cultured on laminin-derived YIGSR, fibronectin-derived RGD and tenascin-derived NID peptide-modified surfaces in the presence of retinoic acid. RGD/YIGSR-modified substrates induced longer neurite formation, while the RGD/YIGSR/NID substrate resulted in an increased number of neurites per field.84
Mussel adhesive protein-inspired immobilization strategies are also useful in the attachment of bioactive signals to organic and inorganic materials for the modification of scaffolds. Growth factors and adhesion peptides containing amine and thiol groups were immobilized onto the polydopamine (PD)-coated polymer substrates for NSCs differentiation. ECM protein-derived adhesion peptides (fibronectin [RGD] and laminin [YIGSR]) and neurotrophic factors (NGF and glial cell line-derived neurotrophic (GDNF)) were immobilized by using this strategy. These scaffolds promoted differentiation and proliferation of human fetal brain-derived NSCs and human induced pluripotent stem cell-derived NSCs. Enhancement of neuronal differentiation of human fetal NSCs in PS-PD substrates was revealed by immunostaining and qRT-PCR analyses of Tuj1. Immunostaining and qRT-PCR of astrocyte marker GFAP demonstrated that immobilization of GDNF, C(K)GGYIGSR, and KGGRGD enhanced glial differentiation (astrocyte lineage) of human NSCs on the PS-PD substrates.85
Nanofiber-like viruses can also be used for the presentation of cell surface receptor binding epitopes on the scaffold surface. M13 phages were genetically engineered to express IKVAV and RGD peptides as their coat proteins at high density and they were drop-cast in order to produce aligned nanofibrous scaffolds. NSCs could easily differentiate into neurons on these viral scaffolds and produced neurites in the same direction as the alignment of the viral nanofibers.86
3. Identification methods for de novo differentiated neurons
The methods used in the evaluation of the effect of culture conditions (i.e. scaffold or medium components) are critical for the assessment of the effectiveness of the biomaterials. Analyses of already differentiated neurons or differentiation of neural progenitor cells are simpler due to the limited cell fates and end with more reliable results, however, analysis of the differentiation of stem cells from other tissues requires more effort. Use of more than one method should be preferred in order to avoid unreliable results. For instance, although the distinct morphology of neural cells is useful to get an idea about the identity of the differentiated cell, it might be misleading if used alone. Cellular toxicity is known to cause cell body shrinkage along with some extensions that might be confusing due to its resemblance to neural cell morphology.87 Hence, morphological observations should be supported with the expression of lineage-specific genes and functional tests to verify that the differentiated cell is able to conduct action potentials. Several methods are used for the analysis of different aspects of neural differentiation. Morphological observations are mainly based on optical microscopy images that are useful in terms of neurite outgrowth analysis. A variety of softwares are available for neurite outgrowth-based measurements from optical microscopy images. Among these softwares, Image J is the most commonly used one. To evaluate the effect of scaffolds on neurite outgrowth, the total length of neurites per image,12,28,29,39–43,45–47,49,51,56,57,62,64,66,67,69,70,76,77,79,83,86 mean number of neurites extended by a single cell,38,49,58,64 number of branches in a specified area,64 and percentage of cells extending neurites27,28,30,38,49,60,66,69,79 are commonly measured by using Image J. The imaging of cells with electron microscopy is also useful in morphological analysis of differentiated cells as it provides more detailed images at higher magnifications. Growth cone morphology and the morphology of the extended neurites can be easily observed in SEM images.16,19,20,24,39,45,48,53,54,61–63,69,78,86 Morphology of synapses can be analyzed in fine detail by transmission electron microscopy imaging.26Since morphological observations alone are not sufficient to define the differentiation status, they can be supported with molecular information such as the expression of marker genes. There are various lineage-specific marker genes defined for neural progenitor cells, neurons, astrocytes and oligodendrocytes, and some of these even specify the subtypes of neurons.88Table 3 gives a list of the most commonly used marker genes and analysis methods. Expression of these genes can be analyzed both at the RNA level (by microarray and RT-PCR methods) and protein level (by western blot, immunostaining and ELISA methods). It is important to check protein levels, even if mRNA levels rise indicating differentiation. mRNA levels, although informative, might be misleading in some cases where gene expression levels at the transcriptional and translational levels differ.89,90
By using morphological observations along with expression analysis of various marker genes, a differentiated cell's identity can be defined. However, in order to verify whether a neuron is capable of conducting action potentials and making synapses like its natural counterparts, additional tests are required to verify its functionality. Electrophysiological recordings of single cells by patch clamp is quite useful for such an analysis.76 Commercially available calcium-binding dyes can also be used to analyze stimulation based on calcium ion flux in differentiated neurons. Stimulation by neurotransmitters or high extracellular K+ ion concentrations leading to depolarization results in an increase in the intracellular calcium ion levels. Fluorescence upon binding of intracellular calcium to the dye can be imaged with confocal microscopy in a time series to obtain a plot of calcium spikes over time.18,53 Another fluorescent imaging method developed as a functionality test for neurons is based on the release of synaptic vesicles upon depolarization of the cell membrane by high extracellular K+ ion concentrations. A fluorescent dye, (N-3-triethylammoniumpropyl)-4-(4-(dibutylamino)styryl), is used to stain endocytic synaptic vesicles and track their release upon depolarization.23,26,77 Resting membrane potentials can also be measured by using a cell permeant potentiometric fluorescent dye, tetramethylrhodamine methyl ester. Intracellular and extracellular fluorescence intensities obtained by confocal imaging can be used to calculate the cells’ resting membrane potentials.53
In summary, a combination of morphological observations, expression level analysis of lineage specific markers and neuronal activity analysis methods are suggested to be used together in order to correctly identify the de novo differentiated neurons.
4. Conclusion
Neural differentiation is a complex task and selective differentiation into a specific subgroup of neural cells is even much harder. Despite the complexity of the process, directing neural cell differentiation is crucial to overcome functional loss of neurons caused by traumatic injuries or neurodegenerative disorders. Since conventional therapies have low rates of success in functional recovery after neural loss, the utilization of bioactive materials is considered as a promising approach for neural regeneration. Biomaterials can be applied in clinics either through the transplantation of in vitro differentiated cells into the lesion site; or transplantation of drug/growth factor-loaded biomaterials to direct in vivo differentiation of endogenous stem cells. Although biomaterials containing natural materials have the advantage of being biocompatible, they have disadvantages due to immunological reactions and the risk of cross-contamination. Synthetic materials can alternatively be used as the building blocks of scaffolds for tissue engineering as they have the advantage of low immunogenicity. In order to make synthetic materials more biocompatible and bioactive, the mechanical and chemical properties of scaffold materials can be altered, or scaffolds can be modified with biological signals for directing the cell fate determination. Many different approaches and methods are applied for the development of artificial scaffolds to be used in neural regeneration. These include the use of a variety of polymers, alone or in combination either with a different type of polymer or with natural materials, and the use of self-assembling synthetic materials, inspired by nature, such as peptide nanofibers (summarized in Tables 1 and 2). Despite promising results obtained from the application of different scaffolds, comparative studies are required to ensure their efficiency and safety, and to determine optimal materials and methods for neural differentiation, so that they can be used for the treatment of neural injuries and neurodegeneration in clinics.| Substrate | Cells used | Ref. |
|---|---|---|
| Acetylcholine-like polymers | Hippocampal neurons | 56 |
| Glass | PC-12, NSC | 39, 55 |
| Gold | PC-12, NSC | 64 |
| M13 phages (nanofiber-like viruses) | NSC | 86 |
| Methacrylamide chitosan hydrogels | NSC | 10, 35 |
| Methylcellulose | Primary murine neurospheres | 60 |
| Peptide amphiphile nanofibers | Neural progenitor cells, PC-12, Purkinje cells | 68–76 |
| Peptide nanofibers (RADA16) | PC-12, NSC, DRG | 77–83 |
| Polyacrylamide gels | MSC | 9 |
| Polyamide nanofibers | Primary neurons from brain | 66 |
| Polycaprolactone (PCL) fibers | ESC, MSC, EcR-293 cells | 22, 31, 43 |
| Polydimethylsiloxane (PDMS) | NSC, MSC | 12, 50, 51 |
| Poly(ethylene-co-vinyl alcohol) | NSC | 17 |
| Polyethylene glycol (PEG) | NSC | 18, 56 |
| Polyhydroxyalkanoates (PHA) | MSC, NSC | 19, 21 |
| Poly(lactic-co-glycolic acid) (PLGA) fibers | NSC, ESC, cortical neurons | 23, 25, 26, 57 |
| Poly(lactide-co-ε-caprolactone) (PLCL) | PC-12, DRG, MSC | 38, 41, 42, 63 |
| Poly-L-lactic acid (PLLA) | ESC, MSC, NSC, DRG, cortical neurons | 16, 25, 37, 38, 40–42, 45, 46, 57, 63 |
| Polydopamine substrate | NSC | 85 |
| Poly(methyl methacrylate) (PMMA) | Radial glia-like cells | 52 |
| Poly(2-hydroxyethylmethacrylate) (p(HEMA)) | DRG cells, PC-12, NSC | 37, 65, 67 |
| Polyurea cross-linked silica aerogels | DRG | 61 |
| Polyurethane scaffolds | ESC | 24, 29, 44, 61 |
| Poly(ε-caprolactone) | PC-12, MSC, NSC | 27, 28, 32, 48 |
| Silica thin gels | Embryonic carcinoma cells | 84 |
| Substrate | Functionalization | Cells used | Result | Ref. |
|---|---|---|---|---|
| Modification for providing control over substrate stiffness | ||||
| Polyacrylamide gels | Stiffness in 0.1–1 kPa range, coating with collagen I | MSC | Neuronal morphology along with neural marker expressions | 9 |
| Methacrylamide chitosan hydrogels | Stiffness modification and coating with laminin | NSC | Proliferation and differentiation is promoted on softest scaffolds | 10 |
| Methacrylamide chitosan hydrogels | Stiffness modification and functionalization with IFN-γ | NSC | Neural differentiation | 35 |
| PDMS | Stiffness modification | NSC | Differentiation into astrocytes and maturation of differentiated neurons highest on softest substrates | 12 |
| Providing electrical conductivity by modifications | ||||
| PLLA | Electrospinning blending with carbon nanotubes | ESC | Neural differentiation is promoted | 37 |
| PLCL | Electrospinning blending with PAni | PC-12 | Neurite outgrowth is enhanced | 38 |
| Glass | Silsesquioxane precursor ATQD covalently attached to RGD peptide | PC-12 | Neurite outgrowth even in the absence of NGF | 39 |
| Providing alignment in substrate | ||||
| PLLA fibers | Aligned fibers | NSC, DRG | NSC differentiation. Neurite extension in the direction of alignment, both for NSCs and DRGs | 45, 46 |
| Aligned fibers modified with bFGF and laminin immobilization by heparin interaction | DRG | Neurite extension in the direction of alignment | 40 | |
| PLCL nanofibers | Aligned fibers coated with carbon nanotubes | DRG | Longer neurites growing in the direction of alignment | 41 |
| PCL fibers | Aligned fibers | ESC | After RA treatment, lower number of astrocytes on aligned fibers along with neurite extension in the direction of alignment | 43 |
| Polyurethane acrylate | Nanoscale ridge-groove patterns | ESC | Selective neural differentiation even in the absence of any soluble factor | 44 |
| Poly(ε-caprolactone) | Aligned fibers produced by electrospinning blending with gelatin | NSC | Differentiation is promoted, neurite extension in the direction of alignment | 48 |
| PDMS | Aligned nanogratings | MSC | Upregulation of neural markers | 50 |
| Aligned channels by micropatterning and coating with poly-L-lysine and laminin | Alignment of neurites differs with channel width | 51 | ||
| PMMA | Microgrooved patterns | Radial glia-like cells | Cell alignment along with directed axonal growth | 52 |
| PA nanofibers | Aligned PA nanofibers releasing SHH | In vivo study for cavernous nerve regeneration | Cavernous nerve regeneration, suppression in penile apoptosis and improvement in erectile function | 75 |
| Chemical modifications | ||||
| Glass | Introduction of chemical groups; –OH, –SO3H, –NH2, –SH, –COOH, –CH3 | NSCs | Differentiation to oligodendrocytes on –SO3H-modified surface, astrocytes on –COOH-modified surface; neurons only on –NH2-modified surface | 55 |
| Acetylcholine like polymers | Synthesized by using acetylcholine mimicking dimethylaminomethyl methacrylate | Hippocampal neurons | Cells adhere, preserve normal morphology and extend neurites | 56 |
| PLLA or PLGA fibers | Modification of surface tension by KOH treatment | Cortical neurons | Longer neurites on surfaces with lowest tension | 57 |
| Use of ECM proteins for biofunctionalization | ||||
| Methylcellulose | Laminin-1 functionalization | Primary murine neurospheres | NSC survival and maturation | 60 |
| PLLA fibers | PLLA/laminin fibers produced by electrospinning blending | PC-12 | Neural differentiation is enhanced | 62 |
| PLCL | PLCL/collagen fibers produced by electrospinning blending | MSC | Neuronal phenotype and expression of NF and nestin | 63 |
| Polyurea cross-linked silica aerogels | Poly-L-lysine/basement membrane extract/laminin-1 coating | DRG | Better attachment and growth of DRG on laminin-coated surface | 61 |
| Peptide nanofibers (RADA16) | Mixing with laminin | NSC | Differentiation is improved in 3D culture | 80 |
| Use of ECM-derived short peptides for biofunctionalization | ||||
| Gold | ECM proteins fused to E. coli outer membrane protein | PC-12, NSC | Neurite outgrowth and branching | 64 |
| Peptide amphiphile (PA) nanofibers | Laminin-derived epitope (IKVAV) incorporation | Neural progenitor cells | Selective differentiation into neurons, suppression of astrocyte differentiation | 68 |
| PA nanofibers | IKVAV incorporation | In vivo study for SCI treatment | Reduction in astrogliosis and cell death, increase in oligodendroglia cell number at injury site | 71, 72 |
| PA nanofibers | Heparan sulfate-mimicking epitope incorporation | PC-12 | Neurite outgrowth provided by NGF affinity of nanofibers | 70 |
| PA nanofibers | IKVAV incorporation along with heparan sulfate-mimicking epitopes | PC-12 | Neurite outgrowth is promoted by cooperative effect of the two epitopes | 69 |
| PA nanofibers | Mixing collagen I with PAs carrying laminin-derived epitopes to produce a hybrid matrix | Purkinje cells | Improved mechanical support and adjustment of laminin epitope density is provided for control over dendrite and axon growth | 76 |
| Peptide nanofibers (RADA16) | Epitopes derived from laminin, collagen, fibronectin and bone marrow homing peptides are incorporated | NSC | Neural differentiation is enhanced | 78 |
| Peptide nanofibers (RADA16) | FGL motif (a synthetic FGF receptor derived from NCAM) is incorporated | DRG | Higher percentage of cells extend neurites and neurites are much longer | 79 |
| Silica thin gels | Modified with laminin (YIGSR)/fibronectin (RGD)/tenascin (NID)-derived epitopes | Embryonic carcinoma cells | Longer neurite outgrowth on RGD/YIGSR, increased number of neurites on RGD/YIGSR/NID | 84 |
| PS | Immobilization of fibronectin (RGD), laminin (YIGSR)-derived peptides and NGF, GDNF on polydopamine-coated PS | NSC | Enhancement in differentiation | 85 |
| M13 phages (nanofiber-like viruses) | Genetic engineering for expression of IKVAV and RGD epitopes on phage surface | NSC | Neural differentiation and outgrowth of neurites in the same direction of phage alignment | 86 |
| Polyamide nanofibers | Covalently attached Tenascin–C-derived peptides | Primary neurons from brain | Neurite outgrowth | 66 |
| p(HEMA-co-AEMA) nerve guidance channels | Modified with laminin-derived peptides (YIGSR and IKVAV) | DRG cells, NSC | Neurite outgrowth of DRG, differentiation of NSC | 65, 67 |
| Marker | Function | Ref. | Methods used for analysis of expression |
|---|---|---|---|
| NSC markers | |||
| Nestin | Intermediate filament | 91 | Immunostaining12,17,18,20,25,32,43,44,50,52,53,55,59,60,63,65,78,83,85 |
| Western blot9,52 | |||
| RT-PCR10,19,32,37,50 | |||
| PAX6 | Transcription factor controlling progenitor cell identity and neural fate | 92, 93 | Western blot52 |
| RT-PCR10 | |||
| Neuron markers | |||
| β-III tubulin | Microtubule protein specifically expressed in early stages of neural commitment. Recognized by Tuj1 antibody | 94 | Immunostaining9,10,12,16,18,20,23–25,31,32,35,38,43,49–53,55,59–61,64,65,68,70,76,78,80,83–86 |
| Western blot9,18,20,23,26,38 | |||
| RT-PCR10,12,19,32,37 | |||
| Neurofilament (NF) | Intermediate filament found in axons | 95 | Immunostaining9,40,45–47,49,54,57,62,63,65 |
| Western blot9 | |||
| RT-PCR25,50 | |||
| Microtubule-associated protein-2 (MAP2) | Provide stability to microtubules in dendrites | 96, 97 | Immunostaining21,23,24,26,31,32,37,44,49,50,56,76 |
| MAP2ab: specific for mature neurons | Western blot9 | ||
| MAP2c: expressed both in neurons and neural progenitors | RT-PCR31,32,37,50 | ||
| Tau | Provides stability to microtubules in axons | 97 | Immunostaining56 |
| Neuron-specific enolase (NSE) | Glycolytic enzyme | 98, 99 | Immunostaining17 |
| RT-PCR10,37 | |||
| Neuronal nuclear protein (NeuN) | DNA binding nuclear protein found in mature neurons. Recently identified as Fox3 gene product acting as tissue specific splicing factor | 100–102 | Immunostaining76 |
| Doublecortin (DCX) | Microtubule binding protein. Expressed in progenitor cells committed to neuronal; sharply reduced expression after expression of mature neural markers (i.e. NeuN) | 103 | RT-PCR10 |
| Synaptophysin | Presynaptic vesicle membrane glycoprotein | 104 | Immunostaining18,31,50,65,69,70,76 |
| Synapsin1 | Presynaptic vesicle-associated phosphoprotein | 105 | Immunostaining23,26 |
| Growth-associated protein 43 (GAP43) | Localized in growth cone, GAP43 guides axonal growth and modulates formation of new connections | 106 | Western blot38 |
| Tyrosine hydroxylase (TH) | Rate-limiting enzyme of catecholamine biosynthesis. Essential for dopamine synthesis | 107 | Immunostaining24,54,80 |
| RT-PCR50 | |||
| Astrocyte markers | |||
| Glial fibrillary acidic protein (GFAP) | Intermediate filament | 108 | Immunostaining10,12,17,18,20,21,23,24,26,31,35,43,44,50–52,55,59,60,68,78,83,85 |
| Western blot18,26,52 | |||
| RT-PCR10,12,19,31,50 | |||
| S100B | Calcium binding protein | 109 | RT-PCR10 |
| Oligodendrocyte markers | |||
| Galactosylcerebroside (GalC) | A glycolipid in myelin | 110 | Immunostaining10,83 |
| OLIG1 | Basic-helix-loop-helix (bHLH) transcription factor required in oligodendrocyte fate specification | 111 | RT-PCR10 |
| Myelin oligodendrocyte glycoprotein (MOG) | A surface marker of oligodendrocytes involved in myelin structure. Expressed late in oligodendrocyte maturation | 112,113 | RT-PCR10 |
| 2′,3′-Cyclic-nucleotide 3′-phosphodiesterase (CNPase, identified by RIP antibody) | An enzyme found in myelin | 114–116 | RT-PCR10 |
| Immunostaining10,35,43,55,60,83 | |||
| O4 | A sulfolipid (sulfatide) found in myelin. Used as a surface marker for oligodendrocytes | 117 | Immunostaining43,55,60,83 |
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ATQD | N-(4-Aminophenyl)-N′-(4′-(3-triethoxysilyl-propyl-ureido) phenyl-1,4-quinonenediimine) |
| BDNF | Brain-derived neurotrophic factor |
| bFGF | Basic fibroblast growth factor |
| BME | Basement membrane extract |
| BMHP | Bone marrow homing peptides |
| BSA | Bovine serum albumin |
| CN | Cortical neurons |
| CNT | Carbon nanotube |
| DMAEMA | Dimethylaminomethyl methacrylate |
| DMEM | Dulbecco's Modified Eagle Medium |
| DRG | Dorsal root ganglion |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| ESCs | Embryonic stem cells |
| EVAL | Ethylene-co-vinyl alcohol |
| FN | Fibronectin |
| GABA | γ-Aminobutyric acid |
| GAP-43 | Growth-associated protein 43 |
| GDNF | Glial cell line-derived neurotrophic factor |
| GF | Growth factor |
| GFAP | Glial fibrillary acidic protein |
| hESCs | Human embryonic stem cells |
| IBMX | 3-Isobutyl-1-1-methylxanthine |
| INDO | Indomethacin |
| LN | Laminin |
| MAC | Methacrylamide chitosan |
| MC | Methylcellulose (MC) |
| MePEG-GMA | Monomethyl ether-glycidyl methacrylate |
| MSCs | Mesenchymal stem cells |
| NCAM | Neural cell adhesion molecule |
| NF | Neurofilament |
| NGF | Nerve growth factor |
| NPC | Neuronal progenitor cells |
| NSCs | Neural stem cells |
| NSE | Neuron specific enolase |
| NT-3 | Neurotrophin-3 |
| PA | Peptide amphiphile |
| P3HB4HB | Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) |
| PAni | Polyaniline |
| PC | Purkinje cells |
| PCL | Polycaprolactone |
| PCLEEP | Poly(ε-caprolactone-co-ethylethylene phosphate) |
| PCSA | Polyurea crosslinked silica aerogels |
| PD | Polydopamine |
| PDMS | Polydimethylsiloxane |
| PEG | Polyethylene glycol |
| PEI | Poly(ethyleneimine) |
| PHA | polyhydroxyalkanoates |
| PHB | Poly(3-hydroxybutyrate) |
| PHBHHx | Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| p(HEMA) | Poly(2-hydroxyethylmethacrylate) |
| PLA | Polylactic acid |
| PLCL | Poly(lactide-co-ε-caprolactone) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PMMA | Poly(methyl methacrylate) |
| PLL | Poly-L-lysine |
| PLLA | Poly-L-lactic acid |
| PU | Polyurethane |
| PUA | Polyurethane acrylate |
| PVA | Polyvinyl alcohol |
| RA | Retinoic acid |
| RGLC | Radial glia-like cells |
| SCI | Spinal cord injury |
| SEM | Scanning electron microscopy |
| TH | Tyrosine hydroxylase |
Acknowledgements
This work was partially supported by the Scientific and Technological Research Council of Turkey (TUBITAK) grant number 111M410. M.S. is supported by TUBITAK BIDEB fellowship. A.B.T. and M.O.G. acknowledge support from the Turkish Academy of Sciences Distinguished Young Scientist Award (TUBA GEBIP).References
- J. Avila, Nat. Med., 2010, 16, 1372 CrossRef CAS PubMed
.
- E. H. Lo, Nat. Med., 2010, 16, 1205–1209 CrossRef CAS PubMed
.
- H. Cao, T. Liu and S. Y. Chew, Adv. Drug Delivery Rev., 2009, 61, 1055–1064 CrossRef CAS PubMed
.
- W. Potter, R. E. Kalil and W. J. Kao, Front. Biosci., 2008, 13, 806–821 CrossRef CAS PubMed
.
- H. Shin, S. Jo and A. G. Mikos, Biomaterials, 2003, 24, 4353–4364 CrossRef CAS
.
- V. Mukhatyar, L. Karumbaiah, J. Yeh and R. Bellamkonda, Adv. Mater., 2009, 21, 4670–4679 CAS
.
- S. M. Dellatore, A. S. Garcia and W. M. Miller, Curr. Opin. Biotechnol., 2008, 19, 534–540 CrossRef CAS PubMed
.
- A. Banerjee, M. Arha, S. Choudhary, R. S. Ashton, S. R. Bhatia, D. V. Schaffer and R. S. Kane, Biomaterials, 2009, 30, 4695–4699 CrossRef CAS PubMed
.
- A. J. Engler, S. Sen, H. L. Sweeney and D. E. Discher, Cell, 2006, 126, 677–689 CrossRef CAS PubMed
.
- N. D. Leipzig and M. S. Shoichet, Biomaterials, 2009, 36, 6868–6878 Search PubMed
.
- K. Saha, A. J. Keung, E. F. Irwin, Y. Li, L. Little, D. V. Schaffer and K. E. Healy, Biophys. J., 2008, 95, 4426–4438 CrossRef CAS PubMed
.
- A. I. Teixeira, S. Ilkhanizadeh, J. A. Wigenius, J. K. Duckworth, O. Inganas and O. Hermanson, Biomaterials, 2009, 30, 4567–4572 CrossRef CAS PubMed
.
- J. Y. Lee, C. A. Bashur, C. A. Milroy, L. Forciniti, A. S. Goldstein and C. E. Schmidt, IEEE Trans. NanoBiosci., 2012, 11, 15–21 CrossRef PubMed
.
- J. W. Fawcett, R. A. Barker and S. B. Dunnett, Exp. Brain Res., 1995, 106, 275–282 CrossRef CAS
.
- D. K. Cullen and M. C. LaPlaca, J. Neurotrauma, 2006, 23, 1304–1319 CrossRef PubMed
.
- F. Yang, R. Murugan, S. Ramakrishna, X. Wang, Y. X. Ma and S. Wang, Biomaterials, 2004, 25, 1891–1900 CrossRef CAS PubMed
.
- T. H. Young and C. H. Hung, Biomaterials, 2005, 26, 4291–4299 CrossRef CAS PubMed
.
- M. J. Mahoney and K. S. Anseth, Biomaterials, 2006, 27, 2265–2274 CrossRef CAS PubMed
.
- L. Wang, Z. H. Wang, C. Y. Shen, M. L. You, J. F. Xiao and G. Q. Chen, Biomaterials, 2010, 31, 1691–1698 CrossRef CAS PubMed
.
- X. Y. Xu, X. T. Li, S. W. Peng, J. F. Xiao, C. Liu, G. Fang, K. C. Chen and G. Q. Chen, Biomaterials, 2010, 31, 3967–3975 CrossRef CAS PubMed
.
- W. Chen and Y. W. Tong, Acta Biomater., 2012, 8, 540–548 CrossRef CAS PubMed
.
- D. Radulescu, Mater. Sci. Eng., C, 2007, 27, 534–539 CrossRef CAS PubMed
.
- Y. Xiong, Y. S. Zeng, C. G. Zeng, B. L. Du, L. M. He, D. P. Quan, W. Zhang, J. M. Wang, J. L. Wu, Y. Li and J. Li, Biomaterials, 2009, 30, 3711–3722 CrossRef CAS PubMed
.
- B. Carlberg, M. Z. Axell, U. Nannmark, J. Liu and H. G. Kuhn, Biomed. Mater., 2009, 4, 45004 CrossRef PubMed
.
- S. Levenberg, N. F. Huang, E. Lavik, A. B. Rogers, J. Itskovitz-Eldor and R. Langer, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 12741–12746 CrossRef CAS PubMed
.
- Y. Xiong, J. X. Zhu, Z. Y. Fang, C. G. Zeng, C. Zhang, G. L. Qi, M. H. Li, W. Zhang, D. P. Quan and J. Wan, Int. J. Nanomed., 2012, 7, 1977–1989 CrossRef CAS PubMed
.
- S. Y. Chew, J. Wen, E. K. Yim and K. W. Leong, Biomacromolecules, 2005, 6, 2017–2024 CrossRef CAS PubMed
.
- C. M. Valmikinathan, S. Defroda and X. Yu, Biomacromolecules, 2009, 10, 1084–1089 CrossRef CAS PubMed
.
- S. J. Jhaveri, M. R. Hynd, N. Dowell-Mesfin, J. N. Turner, W. Shain and C. K. Ober, Biomacromolecules, 2009, 10, 174–183 CrossRef CAS PubMed
.
- T. Kapur and M. Shoichet, J. Biomed. Mater. Res., 2004, 68A, 235–243 CrossRef CAS PubMed
.
- X. Jiang, H. Q. Cao, L. Y. Shi, S. Y. Ng, L. W. Stanton and S. Y. Chew, Acta Biomater., 2012, 8, 1290–1302 CrossRef CAS PubMed
.
- Y. I. Cho, J. S. Choi, S. Y. Jeong and H. S. Yoo, Acta Biomater., 2010, 12, 4725–4733 CrossRef PubMed
.
- P. C. Georges, W. J. Miller, D. F. Meaney, E. S. Sawyer and P. A. Janmey, Biophys. J., 2006, 90, 3012–3018 CrossRef CAS PubMed
.
- A. L. Zajac and D. E. Discher, Curr. Opin. Cell Biol., 2008, 20, 609–615 CrossRef CAS PubMed
.
- N. D. Leipzig, C. Xu, T. Zahir and M. S. Shoichet, J. Biomed. Mater. Res., Part A, 2009, 93, 625–633 Search PubMed
.
- A. L. Hodgkin and A. F. Huxley, J. Physiol., 1952, 117, 500–544 CAS
.
- M. Kabiri, M. Soleimani, I. Shabani, K. Futrega, N. Ghaemi, H. H. Ahvaz, E. Elahi and M. R. Doran, Biotechnol. Lett., 2012, 34, 1357–1365 CrossRef CAS PubMed
.
- S. H. Bhang, S. I. Jeong, T. J. Lee, I. Jun, Y. B. Lee, B. S. Kim and H. Shin, Macromol. Biosci., 2012, 12, 402–411 CrossRef CAS PubMed
.
- Y. Guo, M. Li, A. Mylonakis, J. Han, A. G. MacDiarmid, X. Chen, P. I. Lelkes and Y. Wei, Biomacromolecules, 2007, 8, 3025–3034 CrossRef CAS PubMed
.
- S. Patel, K. Kurpinski, R. Quigley, H. Gao, B. S. Hsiao, M. M. Poo and S. Li, Nano Lett., 2007, 7, 2122–2128 CrossRef CAS PubMed
.
- G. Z. Jin, M. Kim, U. S. Shin and H. W. Kim, Neurosci. Lett., 2011, 501, 10–14 CrossRef CAS PubMed
.
- G. Z. Jin, M. Kim, U. S. Shin and H. W. Kim, Cell Biol. Int., 2011, 35, 741–745 CrossRef CAS PubMed
.
- J. Xie, S. M. Willerth, X. Li, M. R. Macewan, A. Rader, S. E. Sakiyama-Elbert and Y. Xia, Biomaterials, 2009, 30, 354–362 CrossRef CAS PubMed
.
- M. R. Lee, K.
W. Kwon, H. Jung, H. N. Kim, K. Y. Suh, K. Kim and K. S. Kim, Biomaterials, 2010, 31, 4360–4366 CrossRef CAS PubMed
.
- F. Yang, R. Murugan, S. Wang and S. Ramakrishna, Biomaterials, 2005, 26, 2603–2610 CrossRef CAS PubMed
.
- H. B. Wang, M. E. Mullins, J. M. Cregg, C. W. McCarthy and R. J. Gilbert, Acta Biomater., 2010, 6, 2970–2978 CrossRef CAS PubMed
.
- H. B. Wang, M. E. Mullins, J. M. Cregg, A. Hurtado, M. Oudega, M. T. Trombley and R. J. Gilbert, J. Neural Eng., 2009, 6, 016001 CrossRef PubMed
.
- L. Ghasemi-Mobarakeh, M. P. Prabhakaran, M. Morshed, M. H. Nasr-Esfahani and S. Ramakrishna, Biomaterials, 2008, 29, 4532–4539 CrossRef CAS PubMed
.
- C. C. Gertz, M. K. Leach, L. K. Birrell, D. C. Martin, E. L. Feldman and J. M. Corey, Dev. Neurobiol., 2010, 70, 589–603 CrossRef CAS PubMed
.
- E. K. Yim, S. W. Pang and K. W. Leong, Exp. Cell Res., 2007, 313, 1820–1829 CrossRef CAS PubMed
.
- A. Beduer, C. Vieu, F. Arnauduc, J. C. Sol, I. Loubinoux and L. Vaysse, Biomaterials, 2012, 33, 504–514 CrossRef CAS PubMed
.
- M. Mattotti, Z. Alvarez, J. A. Ortega, J. A. Planell, E. Engel and S. Alcantara, Biomaterials, 2012, 33, 1759–1770 CrossRef CAS PubMed
.
- Z. Z. Wu, W. S. Kisaalita, L. Wang, A. L. Zachman, Y. Zhao, K. Hasneen, D. Machacek and S. L. Stice, Biotechnol. Bioeng., 2010, 106, 649–659 CrossRef CAS PubMed
.
- S. L. Bechara, A. Judson and K. C. Popat, Biomaterials, 2010, 31, 3492–3501 CrossRef CAS PubMed
.
- Y. J. Ren, H. Zhang, H. Huang, X. M. Wang, Z. Y. Zhou, F. Z. Cui and Y. H. An, Biomaterials, 2009, 30, 1036–1044 CrossRef CAS PubMed
.
- Q. Tu, L. Li, Y. Zhang, J. Wang, R. Liu, M. Li, W. Liu, X. Wang and L. Ren, Biomaterials, 2011, 32, 3253–3264 CrossRef CAS PubMed
.
- D. R. Nisbet, S. Pattanawong, N. E. Ritchie, W. Shen, D. I. Finkelstein, M. K. Horne and J. S. Forsythe, J. Neural Eng., 2007, 4, 35–41 CrossRef CAS PubMed
.
- G. Lamour, A. Eftekhari-Bafrooei, E. Borguet, S. Soues and A. Hamraoui, Biomaterials, 2010, 31, 3762–3771 CrossRef CAS PubMed
.
- M. Nakajima, T. Ishimuro, K. Kato, I. K. Ko, I. Hirata, Y. Arima and H. Iwata, Biomaterials, 2007, 28, 1048–1060 CrossRef CAS PubMed
.
- S. E. Stabenfeldt, G. Munglani, A. J. Garcia and M. C. LaPlaca, Tissue Eng. A, 2010, 16, 3747–3758 CrossRef CAS PubMed
.
- F. Sabri, J. A. Cole, M. C. Scarbrough and N. Leventis, PLoS One, 2012, 7, e33242 CAS
.
- H. S. Koh, T. Yong, C. K. Chan and S. Ramakrishna, Biomaterials, 2008, 29, 3574–3582 CrossRef CAS PubMed
.
- M. P. Prabhakaran, J. R. Venugopal and S. Ramakrishna, Biomaterials, 2009, 30, 4996–5003 CrossRef CAS PubMed
.
- M. J. Cooke, T. Zahir, S. R. Phillips, D. S. Shah, D. Athey, J. H. Lakey, M. S. Shoichet and S. A. Przyborski, J. Biomed. Mater. Res., Part A, 2009, 93, 824–832 Search PubMed
.
- S. Kubinova, D. Horak, N. Kozubenko, V. Vanecek, V. Proks, J. Price, G. Cocks and E. Sykova, Biomaterials, 2010, 23, 5966–5975 CrossRef PubMed
.
- I. Ahmed, H. Y. Liu, P. C. Mamiya, A. S. Ponery, A. N. Babu, T. Weik, M. Schindler and S. Meiners, J. Biomed. Mater. Res., Part A, 2006, 76A, 851–860 CrossRef CAS PubMed
.
- T. T. Yu and M. S. Shoichet, Biomaterials, 2005, 26, 1507–1514 CrossRef CAS PubMed
.
- G. A. Silva, C. Czeisler, K. L. Niece, E. Beniash, D. A. Harrington, J. A. Kessler and S. I. Stupp, Science, 2004, 303, 1352–1355 CrossRef CAS PubMed
.
- B. Mammadov, R. Mammadov, M. O. Guler and A. B. Tekinay, Acta Biomater., 2012, 8, 2077–2086 CrossRef CAS PubMed
.
- R. Mammadov, B. Mammadov, M. O. Guler and A. B. Tekinay, Biomacromolecules, 2012, 13, 3311–3319 CrossRef CAS PubMed
.
- V. M. Tysseling-Mattiace, V. Sahni, K. L. Niece, D. Birch, C. Czeisler, M. G. Fehlings, S. I. Stupp and J. A. Kessler, J. Neurosci., 2008, 28, 3814–3823 CrossRef CAS PubMed
.
- V. M. Tysseling, V. Sahni, E. T. Pashuck, D. Birch, A. Hebert, C. Czeisler, S. I. Stupp and J. A. Kessler, J. Neurosci. Res., 2010, 88, 3161–3170 CrossRef CAS PubMed
.
- J. M. Anderson, A. Andukuri, D. J. Lim and H. W. Jun, ACS Nano, 2009, 3, 3447–3454 CrossRef CAS PubMed
.
- K. L. Niece, C. Czeisler, V. Sahni, V. Tysseling-Mattiace, E. T. Pashuck, J. A. Kessler and S. I. Stupp, Biomaterials, 2008, 29, 4501–4509 CrossRef CAS PubMed
.
- N. L. Angeloni, C. W. Bond, Y. Tang, D. A. Harrington, S. Zhang, S. I. Stupp, K. E. McKenna and C. A. Podlasek, Biomaterials, 2011, 32, 1091–1101 CrossRef CAS PubMed
.
- S. Sur, E. T. Pashuck, M. O. Guler, M. Ito, S. I. Stupp and T. Launey, Biomaterials, 2012, 33, 545–555 CrossRef CAS PubMed
.
- T. C. Holmes, S. de Lacalle, X. Su, G. Liu, A. Rich and S. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 6728–6733 CrossRef CAS
.
- F. Gelain, D. Bottai, A. Vescovi and S. Zhang, PLoS One, 2006, 1, e119 Search PubMed
.
- Z. Zou, Q. Zheng, Y. Wu, X. Guo, S. Yang, J. Li and H. Pan, J. Biomed. Mater. Res., Part A, 2010, 95A, 1125–1131 CrossRef CAS PubMed
.
- S. Ortinau, J. Schmich, S. Block, A. Liedmann, L. Jonas, D. G. Weiss, C. A. Helm, A. Rolfs and M. J. Frech, BioMed. Eng. Online, 2010, 9, 70 CrossRef PubMed
.
- R. G. Ellis-Behnke, Y. X. Liang, S. W. You, D. K. Tay, S. Zhang, K. F. So and G. E. Schneider, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 5054–5059 CrossRef CAS PubMed
.
- F. Gelain, L. D. Unsworth and S. Zhang, J. Controlled Release, 2010, 145, 231–239 CrossRef CAS PubMed
.
- F. Taraballi, A. Natalello, M. Campione, O. Villa, S. M. Doglia, A. Paleari and F. Gelain, Front. Neuroeng., 2010, 3, 1 CrossRef CAS PubMed
.
- S. S. Jedlicka, K. M. Little, D. E. Nivens, D. Zemlyanov and J. L. Rickus, J. Mater. Chem., 2007, 17, 5058–5067 RSC
.
- K. Yang, J. S. Lee, J. Kim, Y. B. Lee, H. Shin, S. H. Um, J. B. Kim, K. I. Park, H. Lee and S. W. Cho, Biomaterials, 2012, 33, 6952–6964 CrossRef CAS PubMed
.
- A. Merzlyak, S. Indrakanti and S. W. Lee, Nano Lett., 2009, 9, 846–852 CrossRef CAS PubMed
.
- P. Lu, A. Blesch and M. H. Tuszynski, J. Neurosci. Res., 2004, 77, 174–191 CrossRef CAS PubMed
.
- C. N. Svendsen, A. Bhattacharyya and Y. T. Tai, Nat. Rev. Neurosci., 2001, 2, 831–834 CrossRef CAS PubMed
.
- Y. Guo, P. Xiao, S. Lei, F. Deng, G. G. Xiao, Y. Liu, X. Chen, L. Li, S. Wu, Y. Chen, H. Jiang, L. Tan, J. Xie, X. Zhu, S. Liang and H. Deng, Acta Biochim. Biophys. Sin., 2008, 40, 426–436 CrossRef CAS
.
- T. Maier, M. Guell and L. Serrano, FEBS Lett., 2009, 583, 3966–3973 CrossRef CAS PubMed
.
- U. Lendahl, L. B. Zimmerman and R. D. McKay, Cell, 1990, 60, 585–595 CrossRef CAS
.
- G. Estivill-Torrus, H. Pearson, V. van Heyningen, D. J. Price and P. Rashbass, Development, 2002, 129, 455–466 CAS
.
- J. Ericson, P. Rashbass, A. Schedl, S. Brenner-Morton, A. Kawakami, V. van Heyningen, T. M. Jessell and J. Briscoe, Cell, 1997, 90, 169–180 CrossRef CAS
.
- D. Caccamo, C. D. Katsetos, M. M. Herman, A. Frankfurter, V. P. Collins and L. J. Rubinstein, Lab. Invest., 1989, 60, 390–398 CAS
.
- F. C. Huneeus and P. F. Davison, J. Mol. Biol., 1970, 52, 415–428 CrossRef CAS
.
- A. Matus, Annu. Rev. Neurosci., 1988, 11, 29–44 CrossRef CAS PubMed
.
- C. Viereck, R. P. Tucker, L. I. Binder and A. Matus, Neuroscience, 1988, 26, 893–904 CrossRef CAS
.
- D. Schmechel, P. J. Marangos and M. Brightman, Nature, 1978, 276, 834–836 CrossRef CAS
.
- T. Kirino, M. W. Brightman, W. H. Oertel, D. E. Schmechel and P. J. Marangos, J. Neurosci., 1983, 3, 915–923 CAS
.
- R. J. Mullen, C. R. Buck and A. M. Smith, Development, 1992, 116, 201–211 CAS
.
- B. K. Dredge and K. B. Jensen, PLoS One, 2011, 6, e21585 Search PubMed
.
- K. K. Kim, R. S. Adelstein and S. Kawamoto, J. Biol. Chem., 2009, 284, 31052–31061 CrossRef CAS PubMed
.
- J. P. Brown, S. Couillard-Despres, C. M. Cooper-Kuhn, J. Winkler, L. Aigner and H. G. Kuhn, J. Comp. Neurol., 2003, 467, 1–10 CrossRef CAS PubMed
.
- B. Wiedenmann and W. W. Franke, Cell, 1985, 41, 1017–1028 CrossRef CAS
.
- P. De Camilli, R. Cameron and P. Greengard, J. Cell Biol., 1983, 96, 1337–1354 CrossRef CAS
.
- L. I. Benowitz and A. Routtenberg, Trends Neurosci., 1997, 20, 84–91 CrossRef CAS
.
- S. C. Daubner, T. Le and S. Wang, Arch. Biochem. Biophys., 2011, 508, 1–12 CrossRef CAS PubMed
.
- S. K. Pixley, Y. Kobayashi and J. de Vellis, J. Neurosci. Res., 1984, 12, 525–541 CrossRef CAS PubMed
.
- D. B. Zimmer, E. H. Cornwall, A. Landar and W. Song, Brain Res. Bull., 1995, 37, 417–429 CrossRef CAS
.
- A. Rostami, P. A. Eccleston, D. H. Silberberg, M. Hirayama, R. P. Lisak, D. E. Pleasure and S. M. Phillips, Brain Res., 1984, 298, 203–208 CrossRef CAS
.
- Q. Zhou and D. J. Anderson, Cell, 2002, 109, 61–73 CrossRef CAS
.
- N. J. Scolding, S. Frith, C. Linington, B. P. Morgan, A. K. Campbell and D. A. Compston, J. Neuroimmunol., 1989, 22, 169–176 CrossRef CAS
.
- T. G. Johns and C. C. Bernard, J. Neurochem., 1999, 72, 1–9 CrossRef CAS
.
- P. Kursula, Amino Acids, 2006, 34, 175–185 CrossRef PubMed
.
- S. U. Kim, F. A. McMorris and T. J. Sprinkle, Brain Res., 1984, 300, 195–199 CrossRef CAS
.
- M. Watanabe, Y. Sakurai, T. Ichinose, Y. Aikawa, M. Kotani and K. Itoh, J. Neurosci. Res., 2006, 84, 525–533 CrossRef CAS PubMed
.
- R. Bansal, A. E. Warrington, A. L. Gard, B. Ranscht and S. E. Pfeiffer, J. Neurosci. Res., 1989, 24, 548–557 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2013 |
