Solid-supported DNA for asymmetric synthesis: a stepping-stone toward practical applications†
Soyoung
Park
*a,
Keiichi
Ikehata
a and
Hiroshi
Sugiyama
*abc
aDepartment of Chemistry, Graduate School of Science, Kyoto University, Kitashirakawa-oiwakecho, Sakyo-ku, Kyoto 606-8502, Japan
bInstitute for Integrated Cell-Material Sciences (iCeMS), Kyoto University, Yoshida-ushinomiyacho, Sakyo-ku, Kyoto 606-8501, Japan
cCREST, Japan Science and Technology Corporation (JST), Sanbancho, Chiyoda-ku, Tokyo 102-0075, Japan. E-mail: hs@kuchem.kyoto-u.ac.jp; Fax: (+)81-75-753-3670; Tel: (+)81-75-753-4002
First published on 10th July 2013
Abstract
We developed an affordable solid-supported DNA and demonstrated that it could be used as a reusable chiral source for the copper(II)-catalyzed Diels–Alder reaction in water. This study will be a valuable stepping-stone for the industrial application of DNA-based asymmetric synthesis.
Stereoselective hybrid catalysts combining the catalytic power of a metal complex with the exquisite chirality of a biomolecule have received attention as alternative tools for the synthesis of enantiomerically pure compounds.1 Among various biomolecules, DNA has been in the spotlight recently as a promising chiral source for asymmetric catalysis because of its unique helical chirality and chemical stability compared with RNA and proteins. DNA is a cheap and readily available biopolymer. For example, 1 kg of bulk DNA can be purchased for $100–200, and 1 g of purified salmon testes DNA (st-DNA) for about $77.2 DNA-based hybrid catalysts have been applied successfully to various asymmetric carbon–carbon or carbon–heteroatom bond-forming reactions, such as Diels–Alder, Michael addition, and Friedel–Crafts alkylations.3–7
We are very interested in DNA-based asymmetric synthesis and have recently developed asymmetric intramolecular Friedel–Crafts alkylations with a DNA-based hybrid catalyst.8 To expand the utility of DNA in asymmetric catalysis, we decided to develop a new strategy by focusing on heterogeneous catalysis, which has received attention as an attractive strategy for green and sustainable chemistry. The heterogeneous catalytic systems are based on the immobilization of catalytically active metal complexes or chiral ligands to solid supports.9 The solid-supported catalysts or ligands can be recovered easily from the reaction mixtures by filtration and then recycled for the next reactions. The recovery and recyclability of solid-supported catalysts are important in providing an economic and efficient chemical process. In DNA-based asymmetric catalysis, the aqueous phase including DNA-Cu–ligand complexes can be reused after extraction of the products.4h However, to recover DNA itself, another process such as ethanol precipitation is needed.
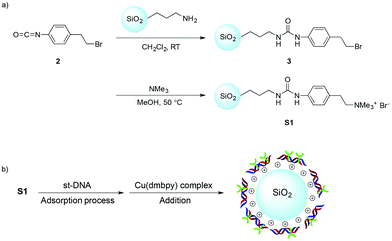
In this context, we devised a heterogeneous catalytic system based on the immobilization of DNA on a solid support.10,11 Surprisingly, asymmetric catalysis using solid-supported DNA has not been explored. Herein, we report that a solid-supported DNA prepared from st-DNA and ammonium-functionalized silica could be used as a reusable chiral source in the copper(II)-catalyzed Diels–Alder reaction in water.
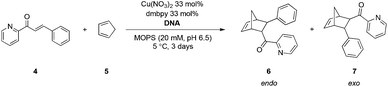
To immobilize DNA, we devised a noncovalent interaction strategy based on the anionic characteristic of the phosphate groups of DNA. In this study, DNA could be immobilized by the electrostatic interaction between anionic phosphate backbones and cationic quaternary ammonium groups. As a support we chose silica, which is used widely for the immobilization of various biomolecules and has been proven to provide excellent support for organic synthesis. Subsequently, ammonium-functionalized silica was prepared from a commercially available 3-aminopropyl-functionalized silica gel, as shown in Scheme 1a.
 | ||
| Scheme 1 (a) Synthetic scheme of ammonium-functionalized silica S1. (b) Preparation of solid-supported DNA for asymmetric synthesis. | ||
Alkyl bromides were introduced to the silica using urea linkages formed by the reaction of amines with isocyanates. The quaternization of trimethylamines was performed using alkyl bromides on silica to give ammonium-functionalized silica, S1. To investigate the immobilization of st-DNA, the synthesized S1 was added to the buffer solution containing st-DNA, and the reaction mixture was allowed to be adsorbed at 5 °C. Commercially available 3-(trimethylammonium)propyl-functionalized silica gel, carbonate (S2), and silica gel 60 N (S3) were also examined as silica supports to immobilize st-DNA. Because DNA has maximal UV absorption at 260 nm, the immobilization of st-DNA on S1 was monitored by a decrease in the intensity of absorbance of the supernatant solution at a wavelength of 260 nm (Fig. 1). The S1 resulted in a decrease in the characteristic UV absorbance of the st-DNA solution albeit in a slow reaction. Thus, we concluded that silica-supported DNA, st-DNA/S1, was obtained by the electrostatic interaction between the anionic phosphate backbones of DNA and the cationic surfaces of silica. The silica-supported DNA was washed successively with water without significant leaching of DNA. Here, the DNA loading onto S1 was determined to be about 40 μg mg−1 silica. In contrast to S1, S2 and S3 showed no significant ability to immobilize st-DNA in this study.
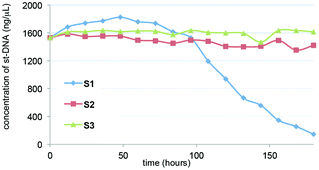 | ||
| Fig. 1 Progress of immobilization of st-DNA on ammonium-functionalized silica (S1). To st-DNA solution (2 mg of st-DNA in 1 mL of water), 50 mg of SN (N = 1–3) was added and mixed for 1 week. The DNA concentration was determined by the UV absorbance at 260 nm. | ||
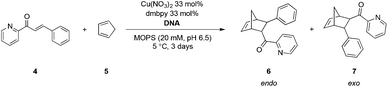
The utility of solid-supported DNA as a chiral source for asymmetric synthesis was investigated in the copper(II)-catalyzed Diels–Alder reaction of 2-azachalcone (4) and cyclopentadiene (5) in water. A catalytic suspension for the reaction was prepared by mixing the Cu(dmbpy) complex (33 mol%) with 50 mg of st-DNA/S1 in MOPS buffer solution (360 μL). To this, 2-azachalcone (4, 1 μmol, 2.78 mM) and cyclopentadiene (5, 66 mM) were added, and the reaction mixture was mixed by continuous rotation at 5 °C.
After 3 days, the product was obtained as a mixture of the endo (major) and exo (minor) isomers in 99% conversion. An endo/exo ratio of 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 94% ee for the endo isomer was found (Table 1, entry 2). Although slight diminution of ee was obtained compared with that from unsupported st-DNA (Table 1, entry 1), the present result demonstrates the potential of solid-supported DNA as a chiral source in asymmetric synthesis. By contrast, a controlled experiment using S1 without st-DNA yielded the product in low conversion with a very low ee.
1 and 94% ee for the endo isomer was found (Table 1, entry 2). Although slight diminution of ee was obtained compared with that from unsupported st-DNA (Table 1, entry 1), the present result demonstrates the potential of solid-supported DNA as a chiral source in asymmetric synthesis. By contrast, a controlled experiment using S1 without st-DNA yielded the product in low conversion with a very low ee.
|
|
||||
|---|---|---|---|---|
| Entry | DNA | Conversion (%) | endo/exo | ee (%) |
| a Entry 1 was performed with 1.3 M st-DNA, 1.5 mM Cu(dmbpy), 1 mM 2-azachalcone, and 15 mM cyclopentadiene in 15 mL MOPS buffer (20 mM, pH 6.5) for 3 days at 5 °C. (Also see ref. 4a.) b Entries 2 and 3 were performed with 50 mg of st-DNA/S1 or 50 mg of S1, 0.9 mM Cu(dmbpy), 2.78 mM 2-azachalcone, and 66 mM cyclopentadiene in 360 μL MOPS buffer (20 mM, pH 6.5) for 3 days at 5 °C. | ||||
| 1a | st-DNA | 93 | 99/1 | 99 |
| 2b | st-DNA/S1 | 99 | 99/1 | 94 |
| 3b | S1 | 14 | 91/9 | <3 |
Next, we investigated the reusability of st-DNA/S1 in the Diels–Alder reaction (Table 2). After the reaction was complete, the st-DNA/S1 was recovered and washed thoroughly with water, methanol, and ethyl acetate, and then dried under reduced pressure. The recovered st-DNA/S1 was used for the next reaction cycle by adding fresh Cu(dmbpy) solution. Subsequently, the st-DNA/S1 could be reused for 10 cycles to afford greater than 93% conversion for every cycle, although the enantioselectivity decreased slightly. These results indicate that st-DNA/S1 can be used as a reusable chiral source for asymmetric catalytic reactions.
|
|
|||
|---|---|---|---|
| Cyclea | Conversion (%) | endo/exo | ee (%) |
| a All cycles were carried out with 50 mg of st-DNA/S1, 0.9 mM Cu(dmbpy), 2.78 mM 2-azachalcone (1 μmol) and 66 mM cyclopentadiene in 360 μL MOPS buffer (20 mM, pH 6.5) for 3 days at 5 °C. | |||
| 1 | 99 | 99/1 | 94 |
| 2 | 99 | 98/2 | 94 |
| 3 | 93 | 98/2 | 93 |
| 4 | 96 | 98/2 | 93 |
| 5 | 99 | 98/2 | 92 |
| 6 | 99 | 98/2 | 90 |
| 7 | 98 | 98/2 | 89 |
| 8 | 99 | 98/2 | 88 |
| 9 | 94 | 97/3 | 85 |
| 10 | 99 | 98/2 | 89 |
In conclusion, we have developed an affordable and easy-to-prepare solid-supported DNA, st-DNA/S1, and we have demonstrated that this st-DNA/S1 could be applied successfully to the Diels–Alder reaction and reused readily for 10 cycles. To our knowledge, this is the first example showing that solid-supported DNA can be applied to asymmetric synthesis. Although the present results are a preliminary investigation for practical applications and more improvements are needed, we believe that the development of solid-supported DNA will be a valuable stepping-stone for the industrial application of DNA-based asymmetric synthesis such as a continuous flow system. Further studies will explore more details of the present reaction system to improve the productivity and efficiency.
Acknowledgements
We express our sincere thanks for the CREST grant from the Japan Science and Technology Corporation (JST), grants from the WPI program (iCeMS, Kyoto University), and for the global COE program from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. A.R. expresses sincere thanks to the Japan Society for the Promotion of Science (JSPS) for a postdoctoral fellowship.Notes and references
- G. Roelfes and B. L. Feringa, Angew. Chem., Int. Ed., 2005, 44, 3230–3232 CrossRef CAS.
- http://sigmaaldrich.com/catalog .
- For reviews, see: (a) S. Park and H. Sugiyama, Angew. Chem., Int. Ed., 2010, 49, 3870–3878 CrossRef CAS; (b) E. W. Dijk, A. J. Boersma and B. L. Feringa G. Roelfes, Org. Biomol. Chem., 2010, 8, 3868–3873 RSC; (c) F. Rosati and G. Roelfes, ChemCatChem, 2010, 2, 916–927 CrossRef CAS; (d) A. J. Boersma, R. P. Megens, B. L. Feringa and G. Roelfes, Chem. Soc. Rev., 2010, 39, 2083–2092 RSC; (e) S. K. Silverman, Angew. Chem., Int. Ed., 2010, 49, 7180–7201 CrossRef CAS.
- (a) G. Roelfes and B. L. Feringa, Angew. Chem., Int. Ed., 2005, 44, 3230–3232 CrossRef CAS; (b) G. Roelfes, A. J. Boersma and B. L. Feringa, Chem. Commun., 2006, 635–637 RSC; (c) A. J. Boersma, B. L. Feringa and G. Roelfes, Org. Lett., 2007, 9, 3647–3650 CrossRef CAS; (d) D. Coquière, B. L. Feringa and G. Roelfes, Angew. Chem., Int. Ed., 2007, 46, 9308–9311 CrossRef; (e) N. Sancho Oltra and G. Roelfes, Chem. Commun., 2008, 6039–6041 RSC; (f) E. W. Dijk, B. L. Feringa and G. Roelfes, Tetrahedron: Asymmetry, 2008, 19, 2374–2377 CrossRef CAS; (g) A. J. Boersma, J. E. Klijn, B. L. Feringa and G. Roelfes, J. Am. Chem. Soc., 2008, 130, 11783–11790 CrossRef CAS; (h) A. J. Boersma, B. L. Feringa and G. Roelfes, Angew. Chem., Int. Ed., 2009, 48, 3346–3348 CrossRef CAS; (i) F. Rosati, A. J. Boersma, J. E. Klijn, A. Meetsma, B. L. Feringa and G. Roelfes, Chem.–Eur. J., 2009, 15, 9596–9605 CrossRef CAS; (j) A. J. Boersma, D. Coquière, D. Geerdink, F. Rosati, B. L. Feringa and G. Roelfes, Nat. Chem., 2010, 2, 991–995 CrossRef CAS; (k) R. P. Megens and G. Roelfes, Org. Biomol. Chem., 2010, 8, 1387–1393 RSC; (l) A. J. Boersma, B. Bruin, B. L. Feringa and G. Roelfes, Chem. Commun., 2012, 48, 2394–2396 RSC.
- N. Shibata, H. Yasui, S. Nakamura and T. Toru, Synlett, 2007, 1153–1157 CrossRef CAS.
- P. Fournier, R. Fiammengo and A. Jäschke, Angew. Chem., Int. Ed., 2009, 48, 4426–4429 CrossRef CAS.
- (a) C. Wang, G. Jia, J. Zhou, Y. Li, Y. Liu, S. Lu and C. Li, Angew. Chem., Int. Ed., 2012, 51, 9352–9355 CrossRef CAS; (b) S. Roe, D. J. Ritson, T. Garner, M. Searle and J. E. Moses, Chem. Commun., 2010, 46, 4309–4311 RSC; (c) C. Wang, Y. Li, G. Jia, Y. Liu, S. Lu and C. Li, Chem. Commun., 2012, 48, 6232–6234 RSC.
- S. Park, K. Ikehata, R. Watabe, Y. Hidaka, A. Rajendran and H. Sugiyama, Chem. Commun., 2012, 48, 10398–10400 RSC.
- For reviews, see: (a) Q.-H. Fan, Y.-M. Li and A. S. C. Chan, Chem. Rev., 2002, 102, 3385–3466 CrossRef CAS; (b) N. Madhavan, C. W. Jones and M. Weck, Acc. Chem. Res., 2008, 41, 1153–1165 CrossRef CAS; (c) D. Zaramella, P. Scrimin and L. J. Prins, J. Am. Chem. Soc., 2012, 134, 8396–8399 CrossRef CAS.
- For immobilization of oligonucleotides onto silica, see: (a) S. R. Rasmussen, M. R. Larsen and S. E. Rasmussen, Anal. Biochem., 1991, 198, 138–142 CrossRef CAS; (b) L. R. Hilliard, X. Zhap and W. Tan, Anal. Chim. Acta, 2002, 470, 51–56 CrossRef CAS; (c) D. Zhang, Y. Chen, H.-Y. Chen and X. H. Xia, Anal. Bioanal. Chem., 2004, 379, 1025–1030 CrossRef CAS; (d) X. Su, L. Hu, L. Kong and X. Lei, J. Chromatogr., A, 2007, 1154, 132–137 CrossRef CAS.
- During our preparation of the manuscript for submission, Grass and co-workers have reported the encapsulation of DNA by using submicron-sized silica particles with ammonium surface functionality. See: D. Paunescu, R. Fuhrer and R. N. Grass, Angew. Chem., Int. Ed., 2013, 52, 4269–4272 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3bm60134j |
| This journal is © The Royal Society of Chemistry 2013 |