3D printed tricalcium phosphate bone tissue engineering scaffolds: effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model
Solaiman Tarafdera,
Neal M. Daviesb,
Amit Bandyopadhyaya and
Susmita Bose*a
aW. M. Keck Biomedical Materials Research Laboratory, School of Mechanical and Materials Engineering, Washington State University, Pullman, WA 99164, USA. E-mail: sbose@wsu.edu; Tel: +1 509-335-7461
bDepartment of Pharmaceutical Sciences, College of Pharmacy, Washington State University, Pullman, WA 99164, USA
First published on 7th August 2013
Abstract
The presence of interconnected macro pores is important in tissue engineering scaffolds for guided tissue regeneration. This study reports in vivo biological performance of interconnected macro porous tricalcium phosphate (TCP) scaffolds due to the addition of SrO and MgO as dopants in TCP. We have used direct three dimensional printing (3DP) technology for scaffold fabrication followed by microwave sintering. Mechanical strength was evaluated for scaffolds with 500 μm, 750 μm, and 1000 μm interconnected designed pore sizes. Maximum compressive strength of 12.01 ± 1.56 MPa was achieved for Sr–Mg doped scaffold with 500 μm interconnected designed pore size. In vivo biological performance of the microwave sintered pure TCP and Sr–Mg doped TCP scaffolds was assessed by implanting 350 μm designed interconnected macro porous scaffolds in rat distal femoral defect. Sintered pore size of these 3D printed scaffolds were 311 ± 5.9 μm and 245 ± 7.5 μm for pure and SrO–MgO doped TCP scaffolds, respectively. These 3D printed scaffolds possessed multiscale porosity, i.e., 3D interconnected designed macro pores along with intrinsic micro pores. Histomorphology and histomorphometric analysis revealed a significant increase in osteoid like new bone formation, and accelerated mineralization inside SrO and MgO doped 3D printed TCP scaffolds as compared to pure TCP scaffolds. An increase in osteocalcin and type I collagen level was also observed in rat blood serum with SrO and MgO doped TCP scaffolds compared to pure TCP scaffolds. Our results show that these 3D printed SrO and MgO doped TCP scaffolds with multiscale porosity contributed to early healing through accelerated osteogenesis.
1. Introduction
The current gold standard for hard tissue reconstruction is autologous bone graft, but donor site morbidity and the need for a second surgery restrict their use for many patients.1–3 However, the use of allograft, an alternative to autograft, is severely restricted due to immunogenic response.4–6 Compositional similarities to bone mineral, excellent biocompatibility, bioactivity and non-immunogenicity of calcium phosphate (CaP) bioceramics are the primary reasons for their broad range of applications in hard tissue repair, regeneration and augmentation.7–11 CaP bioceramics also eliminates the necessity for second surgery required for autograft harvesting. Among other CaPs, the resorbable property of β-tricalcium phosphate (β-TCP) makes it an excellent candidate to be applicable as a biodegradable bone substitute for different orthopedic and dental applications.8,12,13 Biodegradable bone substitutes are eventually replaced by newly formed bone.Many trace elements such as Na+, Mg2+, Zn2+, Si4+, and Sr2+ are also present in the mineral phase of natural bone.12–14 In many cases, cation substitution in CaPs has been proven to improve mechanical properties and both in vitro and in vivo biological responses substantially. These changes in mechanical properties and biological responses are due to the changes in the physicochemical properties of CaPs such as crystallinity, microstructure and solubility caused by cation substitution. Our earlier work demonstrated that a β-TCP with desired mechanical properties can be obtained with appropriate dopants such as CaO, NaF, Ag2O, TiO2, MgO, SrO, SiO2, ZnO, and WO3 at the optimum concentration without affecting the inherent biocompatibility of β-TCP.13,15–18 Strontium (Sr2+), a trace element, which is about 0.035% of its calcium content in our skeleton system, has been shown to enhance bone regeneration when incorporated into synthetic bone graft.19 Bone regeneration by strontium is caused by its simultaneous stimulatory effect on osteoblast mediated bone formation, and inhibitory effect on osteoclast mediated bone resorption. Due to this tremendous impact of strontium on bone modeling and remodeling and its bone seeking property, strontium has also been used in osteoporotic drug as strontium ranelate. In recent years, magnesium based CaPs and alloys have brought noticeable attention from the scientific community in bone tissue engineering due to its enormous role in human physiology such as structure stabilization of many proteins, nucleic acids, acting as a cofactor in hundreds of enzymatic reactions, signal transduction modulation and cell proliferation.20 The effect of Mg2+ on bone and mineral metabolism was also demonstrated by low dietary Mg2+ intake.21
Natural bone is highly porous with interconnected pores. Mimicking the porous nature and pore interconnectivity of natural bone into synthetic bone graft materials is an endeavor put forward by the tissue engineering community. New tissue ingrowth through three dimensionally (3D) interconnected pores provides enhanced mechanical interlocking between surrounding host tissue and scaffold. Pore interconnectivity is also beneficial for nutrient and metabolic waste transport to and from the core of the scaffold, which is very crucial for proper vascularization.22–24 Tissue engineering scaffolds with 3D interconnected porosity can induce early stage osteogenesis from surrounding cells and tissues through cell attachment, migration, and hence tissue ingrowth and nutrient transport into interconnected macro pores.25–29 Apart from their biological role, architectural features of porous scaffolds, for instance, pore size, shape, interconnectivity and percent porosity play a significant role on mechanical properties of CaP ceramic scaffolds. However, a downside of porous scaffolds against their dense counterparts is their poor mechanical properties due to porosity.
CaP scaffolds fabrication with complex architectural features is very challenging by conventional techniques since pore size, interconnectivity, distribution and volume fraction porosity cannot be precisely controlled.8,30,31 We have recently reported a successful direct 3D printing (3DP) fabrication of 3D interconnected porous TCP scaffolds with different pore size and volume fraction porosity.8,14 Both pure8 and doped14 3DP TCP scaffolds showed significantly higher compressive strength compared to scaffolds produced via other solid freeform fabrication (SFF) techniques. A significant increase in the mechanical strength of these 3DP interconnected macro porous TCP scaffolds was further achieved by microwave sintering compared to conventional sintering.8
Previously, we have reported that coexistence of strontium and magnesium in dense β-TCP scaffolds enhances compressive strength, in vitro degradation kinetics, osteoblast interactions, and in vivo bone remodeling.13,17 Here, we examined the effect of the presence of strontium and magnesium in 3DP macro porous β-TCP scaffolds on in vivo osteogenesis. How 3D interconnected porosity, and the presence of Sr2+ and Mg2+ influence in vivo osteogenesis was investigated in rat distal femoral defect model for 4, 8, 12 and 16 weeks. In vivo biodegradation of the SrO–MgO doped β-TCP scaffolds was measured by ion concentration assessment from rat urine using atomic absorption spectrophotometer (AAS), and compared to that of pure β-TCP control.
2. Materials and methods
2.1. Scaffold fabrication
Commercially available β-TCP powder with an average particle size of 550 nm was used (Berkeley Advanced Biomaterials Inc., Berkeley, CA) to make the scaffolds. 1 wt% SrO and 1 wt% MgO was mixed with β-TCP powder for SrO and MgO doped TCP scaffold fabrication. Powder mixing and drying was done according to our previously published procedure.17 Scaffolds with 350 μm designed pore size having a dimension of 3.4 mm in diameter and 5.2 mm in height were fabricated. The designed interconnected macro pores were square shaped and distributed orthogonally through the cylindrical shape in X, Y, and Z directions. A 3D printer (ProMetal®, ExOne LLC, Irwin, PA, USA) was used for these scaffold fabrication. Fig. 1 shows a schematic representation of the 3DP process. The process begins with laying a thin layer (4 to 6 mm) of powder bed in the build box platform. Depending on the predetermined layer thickness, a roller spreads the powder from feed bed onto the build bed. The print head moves across the loose powder bed selectively printing the liquid binder (solvent based binder, purchased from ProMetal®, ExOne LLC, Irwin, PA, USA) based on CAD model cross-sectional area of the designed part. The print head sets its position by moving back and forth in X direction along with the platform (feed bed and build bed) movement in Y direction. After each layer is printed, the powder bed with printed binder moves under a heater (fixed at a pre-set temperature) to expel the moisture and to limit binder spreading between the layers. The loose powder outside the part geometry acts as support for subsequent layers. The build platform then moves down by one layer thickness, while the feed platform moves up one layer (typically 20 μm). The roller then spreads a new layer of powder on top of the previous layer. This process continues by repeating these steps until the printing job is done. Cylindrical scaffolds of 7 mm in diameter and 10.5 mm in height with three different 3D interconnected square-shaped macropore sizes were designed for mechanical strength analysis. Square shaped designed macropores were 500 μm, 750 μm and 1000 μm in size, penetrating orthogonally through the cylindrical shape in X, Y and Z directions. Implants of 3.4 mm in diameter and 5.2 mm in height having 350 μm interconnected designed macropores were designed and made for the in vivo study. After 3D printing, the binder was allowed to harden at 175 °C for 90 min to form a green ceramic body. Dry ultrasonication and/or air blowing were used to remove the loosely adhering powder in the pores of the scaffolds. Scaffolds were then sintered in a 2.45 GHz 3 KW microwave furnace (MW-L0316 V, LongTech Co., Ltd, ChangSha, HuNan, P. R. China) at 1250 °C for 1 h.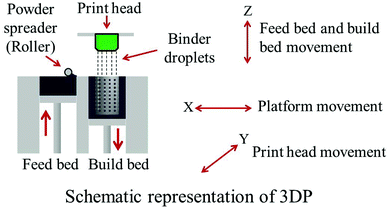 | ||
| Fig. 1 Schematic representation of three dimensional printing (3DP). | ||
2.2. Density, pore size, microstructure, phase, and mechanical strength analysis
Bulk densities of the sintered scaffolds were determined using mass and physical dimensions of the scaffolds. The bulk density takes into account both the closed and the open porosities in the scaffolds. Volume fraction porosity of the scaffolds were calculated from the apparent and the bulk densities, where apparent density was determined by Archimedes’ principle. Images for pore size measurement and surface morphologies of sintered 3D printed pure and SrO–MgO doped TCP scaffolds were taken using a field-emission scanning electron microscope (FESEM) (FEI Inc., Hillsboro, OR, USA) following gold sputter-coating (Technics Hummer V, CA, USA). Sintered pore size was calculated by averaging measurement from 3 samples for each pore size taking 3 different pores from each sample for both pure and Sr–Mg doped β-TCP scaffolds, respectively. Phase analysis of sintered pure and SrO–MgO doped β-TCP scaffolds was carried out by X-ray diffraction (XRD) using a PW 3040/00 Xpert MPD system (Philips, Eindhoven, The Netherlands) with Cu Kα radiation and a Ni filter. Samples were scanned over a 2θ range of 20° to 60° at a step size of 0.05° and a count time of 0.5 s per step. Compressive strength of microwave sintered SrO–MgO doped β-TCP scaffolds was determined using a screw-driven universal testing machine (AG-IS, Shimadzu, Tokyo, Japan) with a constant crosshead speed of 0.33 mm min−1. Compressive strength was calculated based on the maximum load at failure and initial sample dimension. For compressive strength analysis, ten samples (n = 10) were used from each composition.2.3. In vivo study
A total number of 24 male Sprague-Dawley rats (Simonsen Laboratories, Gilroy, CA, USA) with an average body weight of 300 g were used in the present study. Table 1 presents experimental detail for animal study.| Animal model | Time points (weeks) | Number of animals | Comment |
|---|---|---|---|
| Rat distal femoral defect model | 4, 8, 12 and 16 | 4 rats at each time points (4 × 4 = 16 rats) | Histology and histomorphometry |
| Each rat underwent a bilateral surgery: both pure and Sr–Mg doped 3DP β-TCP scaffolds were implanted in contralateral femurs | |||
| 16 | 4 + 4 = 8 rats | Type I collagen and osteocalcin were measured from blood serum collected at the time of sacrifice after 16 week. Ca2+, Sr2+ and Mg2+ concentration were measured from urine collected at different time points | |
| Each rat underwent a unilateral surgery: 3DP pure β-TCP scaffolds were implanted in 4 rats and marked as control group, while 3DP Sr–Mg doped β-TCP scaffolds were implanted in other 4 rats and marked as treatment group |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–acetone mixture, and 100% acetone) series. After embedding samples in Spurr's resin, each undecalcified implant block was sectioned perpendicular to the implant surface using a low speed diamond blade. After polishing, the sections were stained by modified Masson Goldner's trichrome stain and observed under a light microscope [Olympus BH-2, Olympus America Inc., USA]. Image J software (National Institute of Health) was used for osteoid area fraction (osteoid area/total area, %) and bone area fraction (bone area/total area, %) analysis from 800 μm width and 800 μm height tissue sections, n = 8 (2 × 4, two stained tissue sections from each rat). Masson Goldner's trichrome stained tissue sections were used for osteoid and bone area analysis.
1 ethanol–acetone mixture, and 100% acetone) series. After embedding samples in Spurr's resin, each undecalcified implant block was sectioned perpendicular to the implant surface using a low speed diamond blade. After polishing, the sections were stained by modified Masson Goldner's trichrome stain and observed under a light microscope [Olympus BH-2, Olympus America Inc., USA]. Image J software (National Institute of Health) was used for osteoid area fraction (osteoid area/total area, %) and bone area fraction (bone area/total area, %) analysis from 800 μm width and 800 μm height tissue sections, n = 8 (2 × 4, two stained tissue sections from each rat). Masson Goldner's trichrome stained tissue sections were used for osteoid and bone area analysis.2.4. Statistical analysis
Data for density, porosity, sintered pore size, compressive strength, osteoid area, bone area, type I collagen, osteocalcin concentration, and ions concentration are presented as mean ± standard deviation. Statistical analysis was performed on compressive strength, osteoid area, bone area, type I collagen, and osteocalcin concentration level using student's t-test, and p value <0.05 was considered significant.3. Results
3.1. Density, pore size, microstructure, phase and mechanical strength
Sintered pure TCP and SrO–MgO doped TCP scaffolds are shown in Fig. 2. Interconnected designed macropores are clearly visible in the sintered scaffolds. Density, designed and sintered pore size of the scaffolds are presented in Table 2. Pore size has a great influence on new bone formation through tissue in-growth and vascularization. In general, minimum effective recommended pore diameter is 100 μm for successful bone tissue regeneration through transport of essential nutrients and oxygen for cell survivability.32,33 However, interconnected macro pore sizes in the range of 200–350 μm are recommended for osteogenesis and vascularization.34 Keeping this in mind, we have designed our scaffolds for in vivo implantation with designed 350 μm pore size, which resulted in 245 ± 7.5 μm and 311 ± 5.98 μm pore size for SrO–MgO doped and pure TCP scaffolds, respectively. The XRD spectra of the pure and Sr–Mg doped TCP scaffolds are shown in Fig. 3. XRD patterns of the sintered pure and SrO–MgO doped TCP scaffolds were compared with the as received pure β-TCP (JCPDS # 09-0169) powder. Sintered pure TCP showed the presence of some α-TCP (JCPDS # 09-0348) peaks indicating high temperature β to α phase transformation. However, SrO–MgO doped TCP did not show β to α phase transformation. Surface morphology of the pure and doped TCP scaffolds is presented in Fig. 4. Higher densification and smaller grains are observed in the SrO–MgO doped TCP scaffolds. Fig. 4 also shows the presence of large number of intrinsic and residual micro pores (<20 μm in size) uniformly distributed on the scaffold struts/walls. Fig. 5 presents the compressive strength of the microwave sintered Sr–Mg doped TCP scaffolds. Fig. 5 also shows the comparison of the compressive strength obtained in this study with the previously reported values8 for conventionally and microwave sintered pure TCP. A maximum compressive strength value of 12.01 ± 1.56 MPa was achieved for 500 μm interconnected designed pore size Sr–Mg doped scaffold as compared to 10.95 ± 1.28 MPa8 for pure TCP.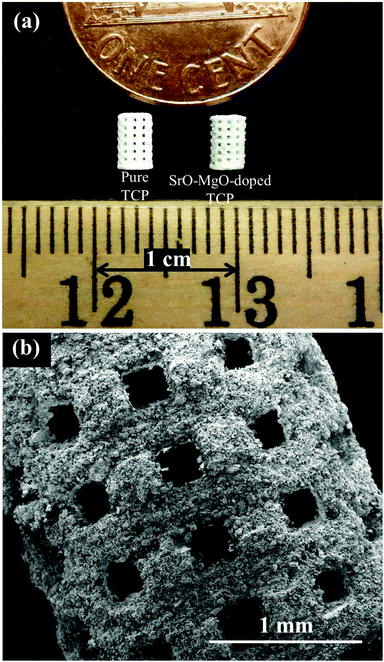 | ||
| Fig. 2 (a) Photograph of the microwave sintered TCP and Sr–Mg doped TCP scaffolds, and (b) a high magnification SEM image of the pure TCP scaffold. | ||
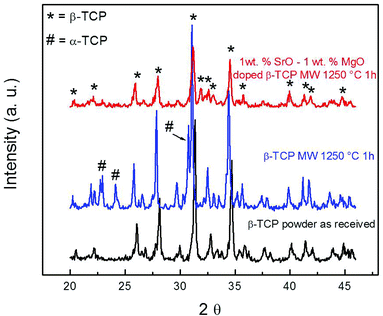 | ||
| Fig. 3 XRD patterns of 3DP pure β-TCP scaffolds and Sr/Mg doped β-TCP scaffolds sintered at 1250 °C for 1 h in a microwave furnace. | ||
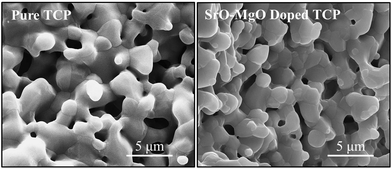 | ||
| Fig. 4 Surface morphology of microwave sintered 3DP pure TCP and SrO and MgO doped TCP scaffolds. | ||
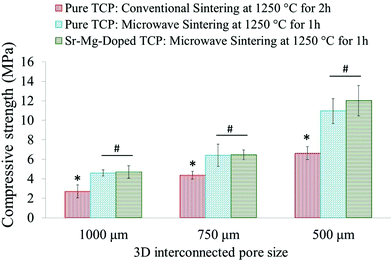 | ||
| Fig. 5 Compressive strength comparison of 3DP microwave sintered Sr–Mg doped TCP scaffolds with the previously reported8 microwave sintered and conventionally sintered pure TCP (*p < 0.05, conventionally sintered pure TCP vs. microwave sintered pure and Sr–Mg doped TCP; #p > 0.05, microwave sintered pure TCP vs. Sr–Mg doped TCP; n = 10). | ||
| Designed pore size (μm) | Sintered pore size (μm) | Bulk density (%) | Total open porosity after sintering (%) | Designed porosity (%) | |
|---|---|---|---|---|---|
| Sr–Mg doped TCP | 500 | 361 ± 9.1 | 45.06 ± 3.05 | 41.63 ± 2.09 | 27 |
| 750 | 545 ± 8.3 | 37.12 ± 1.55 | 47.05 ± 1.52 | 35 | |
| 1000 | 724 ± 5.7 | 31.44 ± 2.71 | 54.74 ± 3.28 | 41 | |
| 350 | 245 ± 7.5 | 41.41 ± 2.72 | 46.82 ± 3.19 | 35 | |
| Pure TCP | 350 | 311 ± 5.9 μm8 | 38.78 ± 3.64 | 49.44 ± 4.64 |
3.2. In vivo study
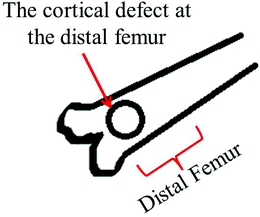 | ||
| Fig. 6 Schematic of the rat distal femoral cortical defect model (anterior view). | ||
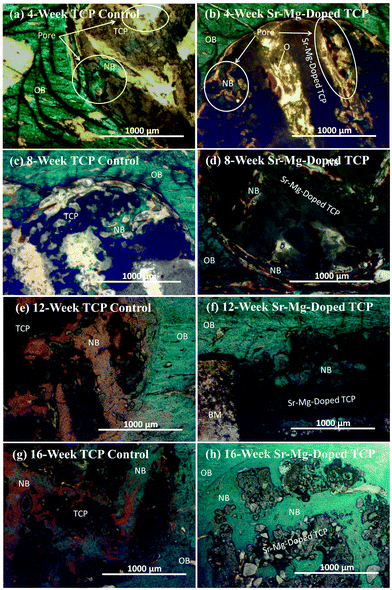 | ||
| Fig. 7 Photomicrograph of 3DP pure TCP implants (a, c, e and g), and Sr/Mg doped TCP implants (b, d, f and h) showing the development of new bone formation and bone remodeling inside the interconnected macro and intrinsic micro pores of the 3DP scaffolds after 4, 8, 12 and 16 weeks in rat distal femur model. Modified Masson Goldner's trichrome staining of transverse section. OB: old bone, NB: new bone, O: osteoid, and BM: bone marrow. Color description: dark grey/black = scaffold; orange/red = osteoid; green/bluish = new mineralized bone (NMB)/old bone. | ||
Complete bone formation was observed inside the interconnected macro and intrinsic micro pores after 12 and 16 weeks in both pure and doped TCP as shown in Fig. 7(e–h). However, there was no difference between host bone (old bone) and newly formed bone in SrO–MgO doped TCP scaffold after 12 week. On the other hand, there was still a difference between newly formed bone and old bone in pure TCP implant even after 16 week. This is the manifestation of complete mineralization process of the newly formed bone due to the presence of SrO and MgO in TCP scaffolds. This indicates the presence of SrO and MgO in 3DP TCP scaffolds favored early stage wound healing compared to pure TCP when implanted in rat distal femur. Fig. 8 and 9 show histomorphometric analysis of osteoid like new bone area and total bone area, respectively. Fig. 8 shows that the presence of SrO and MgO in TCP induced increased osteoid like new bone formation at early time points. Increased early stage bone formation was induced by SrO–MgO doped TCP compared to pure TCP scaffolds as shown in Fig. 9. No significant difference in bone formation was observed between pure and SrO–MgO doped TCP after 16 weeks. Histological evaluation and histomorphometric analysis revealed that the treatment group (doped TCP scaffolds) facilitated higher osteoid like bone at early stage, and completely mineralized bone later, which may indicate quicker bone formation and mineralization process.
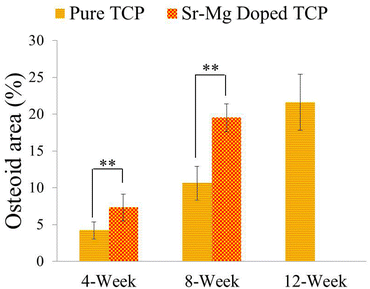 | ||
| Fig. 8 Histomorphometric analysis of osteoid area fraction (osteoid area/total area, %) from 800 μm width and 800 μm height tissue sections (**p < 0.05, *p > 0.05, n = 8). Completely mineralized bone formation was observed in presence of SrO and MgO in TCP after 12 weeks, hence no osteoid area was observed. | ||
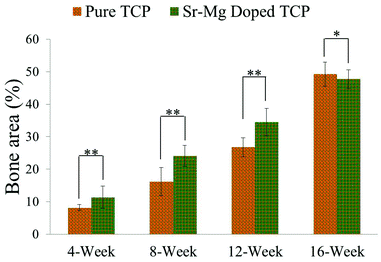 | ||
| Fig. 9 Histomorphometric analysis of bone area fraction (total newly formed bone area/total area, %) from 800 μm width and 800 μm height tissue sections (**p < 0.05, *p > 0.05, n = 8). Bone area is the summation of osteoid like new bone plus newly formed mineralized bone. | ||
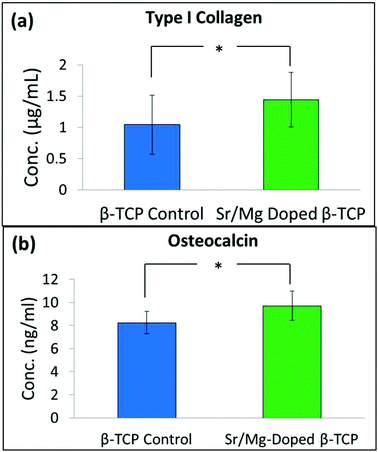 | ||
| Fig. 10 Concentration of type I collagen (a), and osteocalcin (b) in serum of the control group (received only pure 3DP TCP scaffolds), and the treatment group (received Sr/Mg doped 3DP TCP scaffolds) rats after 16 weeks (*P > 0.05, n = 4). | ||
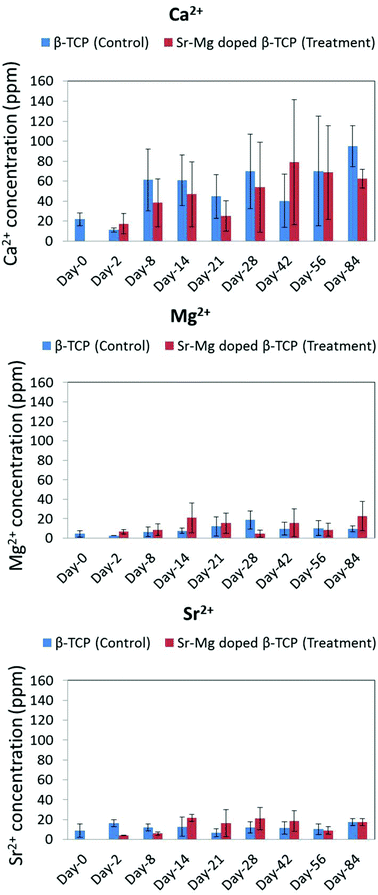 | ||
| Fig. 11 Ca2+, Mg2+ and Sr2+ concentration in control group (received only pure TCP) and treatment group (received only Sr/Mg doped TCP) rat urine at different time points. | ||
4. Discussion
Three dimensional printing (3DP), a solid freeform fabrication (SFF) technique, offers the advantage of direct scaffold fabrication from calcium phosphate (CaP) powder. Another great advantage of 3DP is the ability to make patient-specific bone graft substitute. The major drawbacks of scaffold fabrication techniques such as precise control of the pore size, distribution and interconnectivity can be overcome using 3DP. This study along with our previous study8 demonstrates the successful use of 3DP for CaP scaffold fabrication with complex architectural features. Microstructural features revealed the presence of multiscale porosity, i.e., designed macro and intrinsic micro pores, in the sintered scaffolds. The difference between designed porosity and sintered porosity is due to the presence of intrinsic and residual micro pores in the scaffold struts as shown in Fig. 4. Presence of micro pores in the scaffold strut/wall is an integral part of the 3DP because of the absence of dense sintering (i.e., no pressure, uniaxial or isostatic, was applied to compact the samples at any stage of the process).8 The presence of multiscale porosity and 3D microenvironment in tissue engineering scaffolds increases the overall performance by increased osteoconduction and osseointegration.36–38Addition of dopants in β-TCP can influence its phase stability, microstructure, grain size, mechanical strength and strength degradation kinetics. Pure β-TCP is usually thermally stable up to 1125 °C, and α-TCP becomes the stable phase between 1125 and 1430 °C. Unlike pure TCP, the absence of α-TCP formation at 1250 °C sintering temperature in SrO–MgO doped TCP indicates the phase stability. This high-temperature phase stability is probably due to the presence of Mg2+. Substitution of Mg2+ into β-TCP stabilizes the β phase at a higher temperature by delaying the β to α phase transformation.13,17 The ionic radius of Ca2+, Sr2+ and Mg2+ are 0.99 Å, 1.13 Å and 0.69 Å, respectively. Sr2+ substitution for Ca2+ leads to an increase in the size of the unit cell of the β-TCP lattice, while substitution of Mg2+ for Ca2+ makes the unit cell smaller. However, the combined substitution of Sr2+ and Mg2+ [(1.13 Å + 0.69 Å)/2 = 0.91 Å] in β-TCP causes a reduction in the unit cell parameters. The lattice contraction caused by Sr2+ and Mg2+ co-substitution into Ca2+ sites increases the stability of the β-TCP phase and increases the β to α transformation temperature as well.39 A similar or higher compressive strength of the microwave sintered Sr–Mg doped 3DP TCP scaffolds are obtained in this study (Fig. 5) as compared to our recently reported values for microwave sintered pure TCP scaffolds.
Initiation of distinct new bone formation was observed inside the macro pores, and fibrous interzone (FIZ) of both pure and SrO–MgO doped TCP after 4 week of healing. However, no apparent distinction was observed between pure and doped TCP after 4 weeks (Fig. 7). Increased osteoid like-new bone formation was observed after 8 weeks healing in the SrO–MgO doped TCP. Osteoid, non-mineralized bone formation initiates during ECM protein secretion by osteoblasts.40 A distinct difference in the healing pattern was observed between pure and Sr–Mg doped TCP after 12 weeks (Fig. 7). All micro and interconnected macro pores were completely filled with new mineralized bone in SrO–MgO doped TCP. Matured osteoid filled with deposited mineral is known as mineralized bone.41 Although all micro and interconnected macro pores in pure TCP were also found completely filled with new bone (reddish color in Fig. 7), part of it remains yet to be turned into mineralized bone. Mineralization of the newly formed bone in pure TCP scaffolds was not complete even after 16 weeks. Mineralization starts within a few days of osteoid formation and could take several months. ECM proteins play a key role as a point of mineral nucleation.42 Osteoblast cells are the source of ECM proteins, and both Sr2+ and Mg2+ ions have stimulatory effects on these bone forming cells. A significant increase in osteoid-like new bone formation due to the presence of SrO and MgO in TCP was observed by histomorphometric analysis as shown in Fig. 8.
Both micro and interconnected macro pores facilitated the infiltration of osteoprogenitor cells, which emphasizes the presence of multiscale porosity in tissue engineering scaffolds. Osteogenic scaffolds help the recruitment of osteoprogenitor cells to the site of injury. Sr2+ can induce osteogenesis through increased β-catenin formation that enhances osteogenic differentiation of mesenchymal stem cells.43 Although the role of Mg2+ in osteogenesis is not well understood, current literature results are pointing that Mg2+ can induce osteogenesis. Our earlier studies showed increased cellular attachment, proliferation and ALP production by osteoblasts in presence of magnesium in calcium phosphate bioceramics.12,44 Recent reported results demonstrate that magnesium doped calcium phosphates increases regenerated bone formation resulting in enhanced osteogenesis in both animal (rabbit) and human, respectively.45,46 It has also been shown that magnesium can play a role in angiogenesis through nitric oxide production in endothelial cells.47 Thus, the presence of Sr2+ in the doped TCP was beneficial for osteogenic differentiation of mesenchymal stem cells present in the bone marrow, which infiltrated inside the interconnected macro porous 3DP scaffolds. Our results show that SrO–MgO doped interconnected macro porous 3DP TCP scaffolds facilitate the healing process with early new bone formation and accelerated mineralization as compared to pure TCP, when tested in the rat model. Histomorphometric analysis of total newly formed bone area (both osteoid and mineralized bone) also showed a significant increase in bone formation in the SrO–MgO doped TCP scaffolds compared to pure TCP (Fig. 9). Thus, the presence of Sr2+ and Mg2+ in TCP is beneficial for enhanced osteogenesis.
Collagen, particularly type I collagen, is the most abundant protein in most connective tissues. Approximately, 80% of bone protein is type I collagen.48 In addition to secreting non-collagenous proteins such as, osteocalcin and osteopontin, type I collagen is also secreted by osteoblast cells during the mineralization process.49 Osteocalcin is a bone specific extra cellular matrix (ECM) protein and circulates in blood.50 Osteocalcin concentration in serum is a sensitive marker of bone turnover, which can directly be related to bone formation.50,51 Rats implanted with SrO–MgO doped TCP showed an increased level of type I collagen and osteocalcin concentration in the serum even after 16 weeks, although the difference between undoped and doped TCP was not significant (Fig. 10). We could probably see a significant difference in type I collagen and osteocalcin concentration at early time points if we could have collected blood samples. Collecting blood samples from small animals like the rat at different time intervals over 16 weeks is extremely challenging and can put extra stress on the animals, which may cause death of the test animal. Type I collagen and osteocalcin concentration in the rats with these interconnected macro porous pure and SrO–MgO doped TCP scaffolds are higher than the reported values from their dense counterparts.13 This difference indicates that the presence of both micro and interconnected macro pores facilitated increased ECM protein production to support increased cellular activity.
In most time points, a non-significant higher Ca2+ concentration was observed in the urine of control group rats with undoped TCP implants as compared to rats with SrO–MgO doped TCP scaffolds (Fig. 11). The high error bar in Fig. 11 is due to small sample size (n = 4). The ion concentration data gives us some idea about in vivo degradation of the pure TCP and Sr–Mg doped TCP scaffolds in spite of the non-significant difference between the control and treatment group. An overall higher Ca2+ concentration in the urine of control group rats with undoped TCP scaffolds compared to rats with doped TCP scaffolds indicates a relatively faster in vivo degradation of pure TCP compared to doped TCP. Sr2+ and Mg2+ ions are also part of the body's physiologic system, but are present at a significantly lower concentration than Ca2+. Moreover, due to the low concentration of Sr2+ and Mg2+ in the scaffolds, no apparent difference in these ion concentrations was observed in the urine between rats with undoped and doped scaffolds. The effect of Mg2+ ion on bone formation was also reported in postmenopausal women subjects, where oral Mg2+ supplementation was administered as magnesium citrate (1830 mg day−1) for 30 days.52 Similar to our observation in the rat model in this study, an increased serum osteocalcin level was also observed, where no difference in serum Mg2+ concentration was observed between Mg2+ supplemented and unsupplemented postmenopausal women group.52
In our previous studies with dense TCP, we have shown that the SrO and MgO co-substitution in β-TCP influences the microstructure, density, mechanical strength and both in vitro and in vivo bioactivity.13,17 This study demonstrates the beneficial effects of multiscale porosity, and SrO and MgO doping in 3D printed interconnected macro porous TCP scaffolds for bone tissue engineering applications. A successful bone defect repair was accelerated by the presence of interconnected macro pores and intrinsic micro pores. 3D interconnected porosity facilitated pathways for nutrient transport, adequate cell penetration, and vascularization for ingrowth tissue. All these factors contributed to accelerated healing by enhanced osteogenesis.
5. Conclusion
This study presents 3D printed interconnected macro porous TCP scaffolds with high mechanical strength and improved in vivo biological performance achieved by the addition of SrO and MgO as dopants in TCP. Increased bone formation was induced by the addition of SrO and MgO doping in TCP as compared to undoped TCP. These dopants also caused increased ECM formation and accelerated mineralization, when tested in rat femoral defects as compared to pure TCP. The effect of interconnected macro and intrinsic micro pores on enhanced osteogenesis, and bone tissue ingrowth was observed. The presence of SrO and MgO in TCP on early healing was exhibited by increased bone formation and accelerated mineralization of the newly formed bone as compared to undoped TCP. Thus, SrO–MgO doped 3D printed interconnected macro porous TCP scaffolds show very promising applications in bone tissue engineering for early healing.Funding source
National Institutes of Health, NIBIB (Grant # NIH-R01-EB-007351), and M. J. Murdock charitable trust to acquire the 3D printing system.Acknowledgements
The authors would like to thank Valerie Lynch-Holm and Christine Davitt from Franceschi Microscopy and Imaging Center at Washington State University for their technical assistance with histology and immunohistochemistry. The authors acknowledge the assistance provided by Dr. Jody Takemoto and Dr. Connie Remsberg.References
- U. Kneser, D. J. Schaefer, E. Polykandriotis and R. E. Horch, J. Cell. Mol. Med., 2006, 10, 7–19 CrossRef CAS.
- S. T. Becker, P. H. Warnke, E. Behrens and J. Wiltfang, J. Oral Maxillofac. Surg., 2011, 69, 48–53 CrossRef.
- J. S. Silber, D. G. Anderson, S. D. Daffner, B. T. Brislin, J. M. Leland, A. S. Hilibrand, A. R. Vaccaro and T. J. Albert, Spine, 2003, 28, 134–139 CrossRef.
- G. Zimmermann and A. Moghaddam, Injury, 2011, 42(Supplement 2), S16–S21 CrossRef.
- R. H. Gross, J. Pediatr. Orthop., 2012, 32, 100–105 CrossRef.
- A. Kolk, J. Handschel, W. Drescher, D. Rothamel, F. Kloss, M. Blessmann, M. Heiland, K.-D. Wolff and R. Smeets, J. Craniomaxillofac. Surg., 2012, 28, 706–718 Search PubMed.
- K. de Groot, Bioceramics of calcium phosphate, CRC Press, 1983 Search PubMed.
- S. Tarafder, V. K. Balla, N. M. Davies, A. Bandyopadhyay and S. Bose, J. Tissue Eng. Regen. Med., 2013, 7, 631–641 CrossRef CAS.
- R. Z. LeGeros, Chem. Rev., 2008, 108, 4742–4753 CrossRef.
- S. Bose and S. Tarafder, Acta Biomater., 2012, 8, 1401–1421 CrossRef CAS.
- C. Rey, Biomaterials, 1990, 11, 13–15 CrossRef CAS.
- A. Bandyopadhyay, S. Bernard, W. Xue and S. Bose, J. Am. Ceram. Soc., 2006, 89, 2675–2688 CrossRef CAS.
- S. S. Banerjee, S. Tarafder, N. M. Davies, A. Bandyopadhyay and S. Bose, Acta Biomater., 2010, 6, 4167–4174 CrossRef CAS.
- G. A. Fielding, A. Bandyopadhyay and S. Bose, Dent. Mater., 2012, 28, 113–122 CrossRef CAS.
- Z. Seeley, A. Bandyopadhyay and S. Bose, Mater. Sci. Eng., C, 2008, 28, 11–17 CrossRef CAS.
- Z. Seeley, A. Bandyopadhyay and S. Bose, J. Biomed. Mater. Res. A, 2007, 82, 113–121 CrossRef.
- S. Bose, S. Tarafder, S. S. Banerjee, N. M. Davies and A. Bandyopadhyay, Bone, 2011, 48, 1282–1290 CrossRef CAS.
- J. Dhal, G. Fielding, S. Bose and A. Bandyopadhyay, J. Biomed. Mater. Res. B, Appl. Biomater., 2012, 100B, 1836–1845 CrossRef CAS.
- S. Pors Nielsen, Bone, 2004, 35, 583–588 CrossRef CAS.
- S. Bose, G. Fielding, S. Tarafder and A. Bandyopadhyay, Trends Biotechnol., 2013 DOI:10.1016/j.tibtech.2013.06.005.
- R. K. Rude, H. E. Gruber, H. J. Norton, L. Y. Wei, A. Frausto and J. Kilburn, Bone, 2005, 37, 211–219 CrossRef CAS.
- H. Seyednejad, D. Gawlitta, R. V. Kuiper, A. de Bruin, C. F. van Nostrum, T. Vermonden, W. J. A. Dhert and W. E. Hennink, Biomaterials, 2012, 33, 4309–4318 CrossRef CAS.
- A. C. Jones, C. H. Arns, D. W. Hutmacher, B. K. Milthorpe, A. P. Sheppard and M. A. Knackstedt, Biomaterials, 2009, 30, 1440–1451 CrossRef CAS.
- G. Fielding and S. Bose, Acta Biomater., 2013 DOI:10.1016/j.actbio.2013.07.009.
- H. R. R. Ramay and M. Zhang, Biomaterials, 2004, 25, 5171–5180 CrossRef CAS.
- L. G. Sicchieri, G. E. Crippa, P. T. de Oliveira, M. M. Beloti and A. L. Rosa, J. Tissue Eng. Regen. Med., 2012, 6, 155–162 CrossRef CAS.
- S. Yang, K.-F. Leong, Z. Du and C.-K. Chua, Tissue Eng., 2002, 8, 1–11 CrossRef CAS.
- Q. Q. Qi, J. D. Chen, S. Z. Gao, J. Bu and Z. P. Qiu, Adv. Mater. Res., 2011, 236–238, 1897–1901 CrossRef CAS.
- S. Bose, M. Roy and A. Bandyopadhyay, Trends Biotechnol., 2012, 30, 546–554 CrossRef CAS.
- B. Derby, Science, 2012, 338, 921–926 CrossRef CAS.
- A. Atala, F. K. Kasper and A. G. Mikos, Sci. Transl. Med., 2012, 4, 160rv12–160rv12 CrossRef CAS.
- J. Rouwkema, N. C. Rivron and C. A. van Blitterswijk, Trends Biotechnol., 2008, 26, 434–441 CrossRef CAS.
- S. F. Hulbert, F. A. Young, R. S. Mathews, J. J. Klawitter, C. D. Talbert and F. H. Stelling, J. Biomed. Mater. Res., 1970, 4, 433–456 CrossRef CAS.
- C. M. Murphy, M. G. Haugh and F. J. O'Brien, Biomaterials, 2010, 31, 461–466 CrossRef CAS.
- A. G. Robling, A. B. Castillo and C. H. Turner, Annu. Rev. Biomed. Eng., 2006, 8, 455–498 CrossRef CAS.
- S. K. Lan Levengood, S. J. Polak, M. B. Wheeler, A. J. Maki, S. G. Clark, R. D. Jamison and A. J. Wagoner Johnson, Biomaterials, 2010, 31, 3552–3563 CrossRef CAS.
- P. Habibovic, H. Yuan, C. M. van der Valk, G. Meijer, C. A. van Blitterswijk and K. de Groot, Biomaterials, 2005, 26, 3565–3575 CrossRef CAS.
- K. A. Hing, B. Annaz, S. Saeed, P. A. Revell and T. Buckland, J. Mater. Sci. Mater. Med., 2005, 16, 467–475 CrossRef CAS.
- S. Kannan, F. Goetz-Neunhoeffer, J. Neubauer, S. Pina, P. M. C. Torres and J. M. F. Ferreira, Acta Biomater., 2010, 6, 571–576 CrossRef CAS.
- N. A. Sims and J. H. Gooi, Semin. Cell Dev. Biol., 2008, 19, 444–451 CrossRef CAS.
- H. F. Rios and W. V. Giannobile, Principles of bone biology and regeneration in Implant Site Development, ed. M. Sonick and D. Hwang, John Wiley & Sons, 2011 Search PubMed.
- Y. Wang, T. Azaïs, M. Robin, A. Vallée, C. Catania, P. Legriel, G. Pehau-Arnaudet, F. Babonneau, M.-M. Giraud-Guille and N. Nassif, Nat. Mater., 2012, 11, 724–733 CrossRef CAS.
- F. Yang, D. Yang, J. Tu, Q. Zheng, L. Cai and L. Wang, Stem Cells, 2011, 29, 981–991 CrossRef CAS.
- W. Xue, K. Dahlquist, A. Banerjee, A. Bandyopadhyay and S. Bose, J. Mater. Sci.: Mater. Med., 2008, 19, 2669–2677 CrossRef CAS.
- E. Landi, G. Logroscino, L. Proietti, A. Tampieri, M. Sandri and S. Sprio, J. Mater. Sci.: Mater. Med., 2008, 19, 239–247 CrossRef CAS.
- L. Canullo, F. Heinemann, T. Gedrange, R. Biffar and C. Kunert-Keil, Clin. Oral Implants Res., 2013, 24, 398–406 CrossRef.
- J. A. Maier, D. Bernardini, Y. Rayssiguier and A. Mazur, Biochim. Biophys. Acta, Mol. Basis Dis., 2004, 1689, 6–12 CrossRef CAS.
- S. Viguet-Carrin, P. Garnero and P. Delmas, Osteoporos. Int., 2006, 17, 319–336 CrossRef CAS.
- A. M. Ferreira, P. Gentile, V. Chiono and G. Ciardelli, Acta Biomater., 2012, 8, 3191–3200 CrossRef CAS.
- J. Iwamoto, Y. Sato, T. Takeda and H. Matsumoto, Nutr. Rev., 2011, 69, 162–167 CrossRef.
- P. Szulc, M. C. Chapuy, P. J. Meunier and P. D. Delmas, J. Clin. Invest., 1993, 91, 1769–1774 CrossRef CAS.
- H. Aydın, O. Deyneli, D. Yavuz, H. Gözü, N. Mutlu, I. Kaygusuz and S. Akalın, Biol. Trace Elem. Res., 2010, 133, 136–143 CrossRef.
| This journal is © The Royal Society of Chemistry 2013 |
