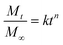Competing properties of mucoadhesive films designed for localized delivery of imiquimod
Sandeep K.
Ramineni
a,
Larry L.
Cunningham
Jr.
b,
Thomas D.
Dziubla
c and
David A.
Puleo
*a
aCenter for Biomedical Engineering, University of Kentucky, Lexington, KY, USA. E-mail: puleo@uky.edu; Fax: +1-859-257-1856; Tel: +1-859-257-2405
bCollege of Dentistry, University of Kentucky, Lexington, KY, USA
cDepartment of Chemical and Materials Engineering, University of Kentucky, Lexington, KY, USA
First published on 30th April 2013
Abstract
Oral mucosal delivery has gained prominence in the last two decades because the rich vasculature of the tissue enables rapid delivery and avoidance of first pass metabolism. Although commercial mucoadhesives are used for systemic delivery, systems are not currently available for treatment of local conditions. In the present work, mucoadhesive films are being developed for locally controlled release of an immune response modifier for preventing precancerous lesions from progressing to oral squamous cell carcinoma. Previous research showed that films composed of polyvinylpyrrolidone (PVP) and carboxymethylcellulose (CMC) released imiquimod in a sustained manner for 3 h. In continuing development of the system, additional key properties were investigated with changes in composition. While adhesive properties in pull-off (0.42 ± 0.03 to 1.1 ± 0.1 N cm−2) and shear adhesion (1.7 ± 0.25 to 5.6 ± 1.4 N cm−2) increased with increasing PVP content of films, tensile properties, such as modulus (6.9 ± 1.5 to 1.8 ± 0.2 MPa) and ultimate strength (4.2 ± 0.7 to 2.1 ± 0.02 MPa), decreased as PVP content increased. Release profiles of the films showed that an increased PVP content resulted in burst release and faster erosion compared to sustained release and slower erosion with more CMC. Studies of transport kinetics showed that the films doubled the amount of imiquimod localized within epithelium compared to drug in solution, increasing their potential for local treatment of oral dysplasia. The mucoadhesive drug delivery system based on CMC and PVP offers a wide range of these properties without addition of new constituents.
Introduction
Mucosal surfaces composed of epithelial cells and connective tissues are found in many regions, such as the oral cavity, nose, eyes, and gastrointestinal, respiratory, and reproductive tracts. In addition to its natural role in protecting underlying tissues, the potential for absorption of molecules via the rich vasculature of the oral cavity makes this an attractive route for drug delivery. Delivery through oral mucosa is rapid and avoids first pass metabolism of drugs.1 Furthermore, oral surfaces offer easy access, stable pH of 6.75 compared to the stomach and intestines whose pH ranges from 2 to 7, and rapid cell recovery.2Several mucoadhesive delivery systems have been developed for targeting mucous membranes encompassing buccal, gastrointestinal, vaginal, ocular, nasal and sublingual surfaces.1 Although some mucoadhesive formulations originated in 1947,3 this field grew significantly starting in the 1980s.2,3 The ability of these systems to adhere to mucosal surfaces increases residence time, bioavailability, provides high flux of drug, improves permeability, and retains structure of peptides and proteins.1 Commercialized systems, such as BEMA® technology (BioDelivery Sciences International) and trans-mucosal films (Watson Pharmaceuticals), exist for systemic drug delivery, but their fast erosion times (15–60 min) are not appropriate for localized treatment of diseases.
Oral squamous cell carcinoma (OSCC) is malignant form of cancer affecting squamous epithelial cells, which are present on all mucosal surfaces of the oral cavity, pharynx, and trachea. Current treatments, such as surgical resection, result in loss of tissue, which compromises normal function of the oral cavity.4 Radiation therapy of oral cancers has 100% incidence of painful post-treatment oral mucositis.5 Chemotherapy is associated with significant side effects, such as myelosuppression, mucositis, and hair loss, due to delivery of drug to healthy, as well as cancerous, tissues.6 A mucoadhesive system loaded with an immune response modifier, imiquimod, for potential local treatment of precancerous oral lesions was previously developed.7 Use of this delivery system would offer advantages of a non-invasive approach and reduced systemic effects of drugs.
Earlier work demonstrated sustained release of imiquimod for 3 h in vitro from mucoadhesive films containing polyvinylpyrrolidone (PVP) as film forming polymer and carboxymethylcellulose (CMC) as adhesive polymer.7 Other properties relevant for developing a mucoadhesive system, however, were not investigated. The aim of the present studies was to characterize adhesion strength, swelling, tensile properties, and transport kinetics as a function of film composition and to subsequently investigate changes in drug release profiles. A more complete understanding of PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC mucoadhesives allows tuning of the system for desired drug delivery, adhesive, and mechanical properties.
CMC mucoadhesives allows tuning of the system for desired drug delivery, adhesive, and mechanical properties.
Materials and methods
Chemicals and materials
Imiquimod (CalBiochem; White House Station, NJ) was incorporated into films that consisted of two polymers, PVP K-90 (Spectrum Chemicals; New Brunswick, NJ) and CMC (sodium salt, medium viscosity; Sigma, St. Louis, MO). Other chemicals and materials used were propyleneglycol, ethanol, acetonitrile (ACN), trifluoroacetic acid (TFA), 2-hydroxypropyl-β-cyclodextrin (cell culture tested; HPβCD), poly(ethylene-co-vinyl acetate) (18 wt% vinyl acetate; EVA), mucin from bovine submaxillary glands (Sigma-Aldrich; St. Louis, MO), and 15 mm Franz diffusion cells (PermeGear, Hellertown, PA).Fabrication of films
Mucoadhesive films were prepared as described previously.7 Briefly, the following three solutions were prepared concurrently, thoroughly mixed, and left overnight at 43 °C to remove bubbles: (1) 40% w/v aqueous solution of PVP mixed with ethanol at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v and followed by addition of 50% v/v propylene glycol; (2) 2% w/v aqueous solution of CMC; and (3) imiquimod solution (18 mg) using 2-hydroxy propyl-β-cyclodextrin and imiquimod complexes. The polymer solutions were cast in Teflon dishes and dried at 60 °C for a time specific to the particular type of film (described further in the Drying time section). The obtained films were peeled from the dishes and stored in a desiccator at 20% relative humidity for 24 h before use. Film formulations with varying contents of PVP and CMC were prepared as shown in Table 1. Blank films were used for the mechanical, adhesion, and swelling studies, while for release and transport studies, films were loaded with imiquimod. Samples (diameter, 1 cm unless otherwise noted) were punched from random points in the cast films for the following experiments.
1 v/v and followed by addition of 50% v/v propylene glycol; (2) 2% w/v aqueous solution of CMC; and (3) imiquimod solution (18 mg) using 2-hydroxy propyl-β-cyclodextrin and imiquimod complexes. The polymer solutions were cast in Teflon dishes and dried at 60 °C for a time specific to the particular type of film (described further in the Drying time section). The obtained films were peeled from the dishes and stored in a desiccator at 20% relative humidity for 24 h before use. Film formulations with varying contents of PVP and CMC were prepared as shown in Table 1. Blank films were used for the mechanical, adhesion, and swelling studies, while for release and transport studies, films were loaded with imiquimod. Samples (diameter, 1 cm unless otherwise noted) were punched from random points in the cast films for the following experiments.
Ratio of PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC CMC |
|||||
|---|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
| PVP (mL) | 2 | 2.5 | 3 | 3.5 | 4 |
| CMC (mL) | 12 | 11.25 | 9 | 7.5 | 6 |
| Drying time (h) | 7 | 8 | 9 | 10 | 13.5 |
Drying time
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC were cast and dried at 60 °C until negligible change in weight of film samples (±3%) was observed. The time required to reach this stage was recorded as steady state time (tst). Because the steady state water content can vary for different ratios of PVP
CMC were cast and dried at 60 °C until negligible change in weight of film samples (±3%) was observed. The time required to reach this stage was recorded as steady state time (tst). Because the steady state water content can vary for different ratios of PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC, the drying time to achieve equivalent contents was next identified.
CMC, the drying time to achieve equivalent contents was next identified.
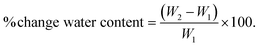 | (1) |
Ratio of PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC CMC |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|---|---|---|---|---|---|
| Drying times (h) | 5 | 6 | 7 | 9 | 13 |
| 6 | 7 | 8 | 10 | 14 | |
| 7 | 8 | 9 | 13 | 15 |
For each film composition, one time point was chosen such that all film types had equivalent water content.
Tensile properties
Following removal from Teflon dishes, dumbbell-shaped (gauge width = 5 mm, gauge length = 10 mm) samples were cut from each film and fixed between the grips of a Bose ELF 3300 mechanical testing system. After preloading to 0.1 N, test specimens were deformed at a displacement rate of 3 mm s−1.8–10 The recorded load and displacement values were used to calculate the Young's modulus, ultimate tensile strength (UTS), and percentage elongation.Adhesion studies
Drug release and erosion studies
Based on their tensile and adhesive properties, only the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films were further investigated. Samples were attached to the wall of 6 mL polyethylene vials to limit release of drug to only one side. These samples were immersed in SS and incubated at 37 °C with shaking at 150 rpm. Supernatants were collected and stored at predetermined intervals followed by replacement with fresh SS. Concentrations of imiquimod released into the supernatants were measured using fluorescence spectroscopy at excitation and emission wavelengths of 250 and 340 nm, respectively.
CMC films were further investigated. Samples were attached to the wall of 6 mL polyethylene vials to limit release of drug to only one side. These samples were immersed in SS and incubated at 37 °C with shaking at 150 rpm. Supernatants were collected and stored at predetermined intervals followed by replacement with fresh SS. Concentrations of imiquimod released into the supernatants were measured using fluorescence spectroscopy at excitation and emission wavelengths of 250 and 340 nm, respectively.
Cumulative release profiles of films were analyzed using the Korsmeyer–Peppas mathematical model:16
Erosion (mass loss) studies were performed in a similar way. The initial sample weight (W1) was recorded before the study, and final weight (W2) was measured after drying the degraded samples at 43 °C overnight. Mass loss was calculated and plotted against degradation time.
Swelling studies
Two types of swelling studies were performed on 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films and pure PVP and CMC films to further understand properties of the films.
CMC films and pure PVP and CMC films to further understand properties of the films.
Transport kinetics and permeability characteristics
Transport kinetics of imiquimod released from films were analyzed on porcine buccal tissues that were frozen until use. Upon thawing, sections of 500 μm thick were prepared using a sledge microtome to separate the underlying connective tissue from epithelium and used immediately. The tissue sections were mounted in a Franz cell such that epithelial side faced the donor compartment. Mucoadhesive samples were applied to the mucosal surface of tissue contained in the Franz cell. After filling the receptor compartment with SS, care was taken to ensure the tissue surface was always in contact with the solution.Supernatant was collected from the receptor compartment at predetermined intervals and replaced with fresh SS. Acetate buffer (100 mM, pH 4.0) was freshly prepared and added at a ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) to all collected samples to solubilize drug before measurement. Experiments were run for 24 h, after which residual film was solubilized completely in 50
50 (v/v) to all collected samples to solubilize drug before measurement. Experiments were run for 24 h, after which residual film was solubilized completely in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 acetate buffer
50 acetate buffer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SS to quantify the remaining drug. Tissue sections were also immersed overnight in 50
SS to quantify the remaining drug. Tissue sections were also immersed overnight in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 acetate buffer
50 acetate buffer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SS to extract retained imiquimod. The amount of drug in all samples was determined by reverse phase high performance liquid chromatography (HPLC) using a Shimadzu Prominence system equipped with a Phenomenex C18 column. The mobile phase used was 40
SS to extract retained imiquimod. The amount of drug in all samples was determined by reverse phase high performance liquid chromatography (HPLC) using a Shimadzu Prominence system equipped with a Phenomenex C18 column. The mobile phase used was 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 ACN to water containing 1% TFA at a flow rate of 1 mL min−1. Imiquimod was measured using a UV detector at a wavelength of 242 nm.
60 ACN to water containing 1% TFA at a flow rate of 1 mL min−1. Imiquimod was measured using a UV detector at a wavelength of 242 nm.
Permeability and transport kinetics of control solutions (imiquimod solubilized in acetate buffer) and imiquimod-loaded 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 films were compared. Care was taken to ensure that mucoadhesive films and control solutions had equal amounts of drug (0.26 mg). The cumulative amount of drug permeated through tissue per unit area was calculated and plotted as a function of time. Flux (Q) of drug was then calculated from the slope of the linear portion of the curve.
1 films were compared. Care was taken to ensure that mucoadhesive films and control solutions had equal amounts of drug (0.26 mg). The cumulative amount of drug permeated through tissue per unit area was calculated and plotted as a function of time. Flux (Q) of drug was then calculated from the slope of the linear portion of the curve.
Statistical analysis
All experiments were conducted in triplicate and repeated at least once to demonstrate reproducibility of results. The results are expressed as mean ± standard deviation. While unpaired two-tail student t-tests were used to compare instantaneous release of drug, degradation and swelling, ANOVA with the Tukey post-hoc test was used for tensile, adhesive studies, and transport kinetics and permeability studies. Results were considered statistically significant if p < 0.05.Results
Drying time
Film samples (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC) were observed to lose water when dried for extended periods of time. Although significant changes were observed through the first 5 h, mass decreases slowed thereafter, and negligible change (±3%) was observed between 11 and 24 h for all types of films. Hence, 24 h was chosen as the steady state drying time point (tst) at which minimal water content of films was achieved.
CMC) were observed to lose water when dried for extended periods of time. Although significant changes were observed through the first 5 h, mass decreases slowed thereafter, and negligible change (±3%) was observed between 11 and 24 h for all types of films. Hence, 24 h was chosen as the steady state drying time point (tst) at which minimal water content of films was achieved.
After determining that maximal water loss occurred by 24 h, fresh mucoadhesive films were prepared by drying for different times (tI,n). Samples were then punched and dried again for 24 h (tst) to find the water content. Irrespective of film type, all samples lost water, and as expected, the percentage change in water content decreased with increased initial drying time (tI,n) (Fig. 1). Based on these observations, drying times for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2
2, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 2
2, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 films were chosen to be 7, 8, 9, 10, and 13.5 h, respectively, to achieve a uniform water content of 39 ± 2.5%. Smooth, bubble-free, and flexible films of each type were obtained after drying for their respective times. Tackiness of films increased with increasing PVP content of films, which made handling slightly difficult.
1 films were chosen to be 7, 8, 9, 10, and 13.5 h, respectively, to achieve a uniform water content of 39 ± 2.5%. Smooth, bubble-free, and flexible films of each type were obtained after drying for their respective times. Tackiness of films increased with increasing PVP content of films, which made handling slightly difficult.
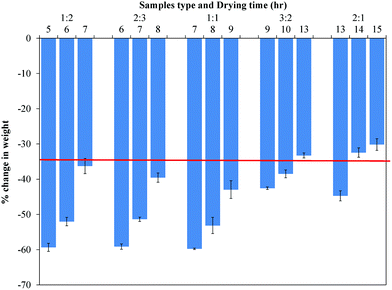 | ||
| Fig. 1 Percentage change in weight of mucoadhesive films at different initial drying times. Data are mean ± standard deviation (n ≥ 3). Initial drying times were chosen such that all films had comparable water content of 39% (red line). | ||
Tensile properties
The stress–strain curves of all film types, except those with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC, showed two slopes before failure (Fig. 2a). Elastic modulus was calculated from the initial slope, with the second slope representing plastic deformation. As shown in Fig. 2b, both elastic modulus and UTS decreased significantly (p < 0.0001) with increasing PVP content. Elastic modulus ranged from 6.9 ± 1.5 to 1.8 ± 0.2 MPa and UTS ranged from 4.2 ± 0.7 to 2.1 ± 0.02 MPa for 1
CMC, showed two slopes before failure (Fig. 2a). Elastic modulus was calculated from the initial slope, with the second slope representing plastic deformation. As shown in Fig. 2b, both elastic modulus and UTS decreased significantly (p < 0.0001) with increasing PVP content. Elastic modulus ranged from 6.9 ± 1.5 to 1.8 ± 0.2 MPa and UTS ranged from 4.2 ± 0.7 to 2.1 ± 0.02 MPa for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 films, respectively. Percentage elongation, however, significantly (p < 0.0001) increased with PVP content, ranging from 129.2 ± 13.5 to 394 ± 47.3% for 1
1 films, respectively. Percentage elongation, however, significantly (p < 0.0001) increased with PVP content, ranging from 129.2 ± 13.5 to 394 ± 47.3% for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 films, respectively (Fig. 2b). A detailed presentation of significant differences between each type of film is shown in Table 3.
1 films, respectively (Fig. 2b). A detailed presentation of significant differences between each type of film is shown in Table 3.
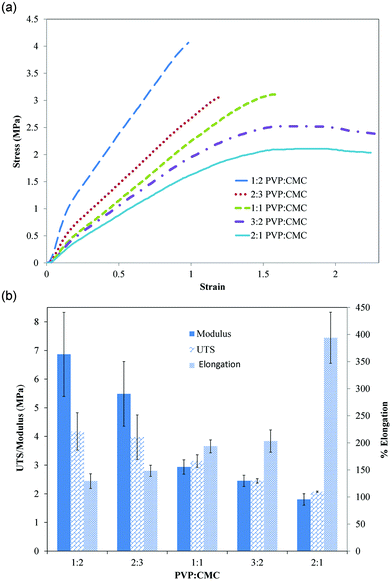 | ||
| Fig. 2 (A) Representative stress–strain curves for different types of mucoadhesive films. (B) Modulus, UTS, and percentage of elongation of all mucoadhesive film compositions tested. Results of statistical analysis are shown in Table 3. Data are mean ± standard deviation (n ≥ 3). | ||
| Sample | Modulus | UTS | % Elongation | Max pull-off adhesive strength | Max shear adhesive strength | Shear work of adhesion |
|---|---|---|---|---|---|---|
| *ns = not significant (p > 0.05); * = (p < 0.05); ** = (p < 0.01); *** = (p < 0.001). | ||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 vs. 2 2 vs. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
ns | ns | ns | * | ns | ns |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 vs. 1 2 vs. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
*** | * | * | * | ns | ns |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 vs. 3 2 vs. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
*** | *** | ** | ** | ns | * |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 vs. 2 2 vs. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
*** | *** | *** | *** | *** | *** |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 vs. 1 3 vs. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
** | ns | ns | ns | ns | ns |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 vs. 3 3 vs. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
** | ** | * | ns | ns | ns |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 vs. 2 3 vs. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
*** | *** | *** | * | *** | ** |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vs. 3 1 vs. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
ns | ns | ns | ns | ns | ** |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vs. 2 1 vs. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
ns | * | *** | * | *** | *** |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 vs. 2 2 vs. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
ns | ns | *** | ns | ** | ns |
Films became soft, tacky, and viscoelastic as PVP content increased. This viscoelastic behavior was more evident in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 film types in the form of strain recovery. When films were elongated until just before breakage (x) and returned to half of that elongated length (x/2), the films were observed to recover from this strain in less than 30 seconds (final length of films = initial length + x/2) as shown in Fig. 3.
1 film types in the form of strain recovery. When films were elongated until just before breakage (x) and returned to half of that elongated length (x/2), the films were observed to recover from this strain in less than 30 seconds (final length of films = initial length + x/2) as shown in Fig. 3.
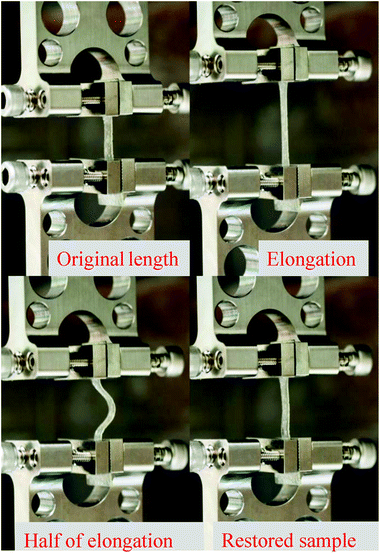 | ||
Fig. 3 Time-lapse images showing strain recovery behavior of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP 1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films. When the film was deformed and then returned to its original length, the polymer chains rearranged to recover the initial deformation. CMC films. When the film was deformed and then returned to its original length, the polymer chains rearranged to recover the initial deformation. | ||
Adhesive properties
Detachment of samples in both pull-off and shear adhesion studies occurred only at the interface between polymer and tissue/mucin. The average maximum adhesive strength (force per unit area) required to detach mucoadhesive films from porcine buccal tissue increased significantly with PVP content (p < 0.0003) from 0.42 ± 0.03 to 1.1 ± 0.1 N cm−2 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mucoadhesive films, respectively (Fig. 4a). Increasing PVP content, however, did not significantly affect work of adhesion (Fig. 4a). For shear adhesion, both the maximum shear strength and work of adhesion required to peel mucoadhesive films from mucin-coated membranes significantly increased with PVP content (p < 0.0001) (Fig. 4b). The maximum shear adhesive strength increased (p < 0.0001) from 1.7 ± 0.25 to 5.6 ± 1.4 N cm−2 and work of adhesion increased (p < 0.0001) from 4.3 ± 1.1 to 12.9 ± 0.84 N cm−2 for 1
1 mucoadhesive films, respectively (Fig. 4a). Increasing PVP content, however, did not significantly affect work of adhesion (Fig. 4a). For shear adhesion, both the maximum shear strength and work of adhesion required to peel mucoadhesive films from mucin-coated membranes significantly increased with PVP content (p < 0.0001) (Fig. 4b). The maximum shear adhesive strength increased (p < 0.0001) from 1.7 ± 0.25 to 5.6 ± 1.4 N cm−2 and work of adhesion increased (p < 0.0001) from 4.3 ± 1.1 to 12.9 ± 0.84 N cm−2 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mucoadhesive films, respectively. A detailed presentation of statistically significant differences between each type of film is shown in Table 3.
1 mucoadhesive films, respectively. A detailed presentation of statistically significant differences between each type of film is shown in Table 3.
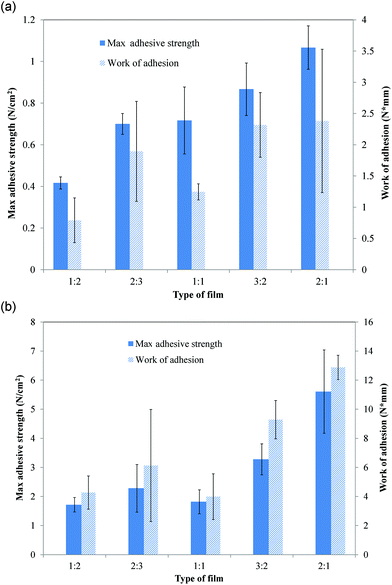 | ||
| Fig. 4 (A) Maximum pull-off adhesive strength (force/unit area) and work of adhesion for films on porcine buccal tissue. (B) Maximum shear adhesive strength (force/unit area) and shear work of adhesion for films on mucin-coated membranes. Results of statistical analysis are shown in Table 3. Data are mean ± standard deviation (n ≥ 3). | ||
Drug release, erosion, and mathematical modeling
The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PVP
2 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films were able to achieve sustained release of imiquimod for up to 3 h with a burst at 160 min and continuously decreasing release thereafter (Fig. 5). For 2
CMC films were able to achieve sustained release of imiquimod for up to 3 h with a burst at 160 min and continuously decreasing release thereafter (Fig. 5). For 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 films, however, most of the imiquimod was released over the first hour, after which release continually decreased over time (Fig. 5). Cumulative release profiles for both types of films coupled with their respective mass loss profiles are shown in Fig. 6. For 1
1 films, however, most of the imiquimod was released over the first hour, after which release continually decreased over time (Fig. 5). Cumulative release profiles for both types of films coupled with their respective mass loss profiles are shown in Fig. 6. For 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PVP
2 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC, the profile of drug release closely followed erosion of the films (Fig. 6a). The 2
CMC, the profile of drug release closely followed erosion of the films (Fig. 6a). The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 films, however, began eroding early and eroded faster, reflected by the initial mass loss being greater than the percentage of drug released up to 40 min (Fig. 6b). In addition, imiquimod release from 2
1 films, however, began eroding early and eroded faster, reflected by the initial mass loss being greater than the percentage of drug released up to 40 min (Fig. 6b). In addition, imiquimod release from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films occurred faster than from 1
CMC films occurred faster than from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 films. Inconsistencies in the last few time points of the mass loss measurements were attributed to difficulties in handling of the viscous and mostly eroded films. Mathematical modeling of release profiles based on the Korsmeyer–Peppas equation showed ‘n’ values of 1.03 for 1
2 films. Inconsistencies in the last few time points of the mass loss measurements were attributed to difficulties in handling of the viscous and mostly eroded films. Mathematical modeling of release profiles based on the Korsmeyer–Peppas equation showed ‘n’ values of 1.03 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PVP
2 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films and 0.89 for 2
CMC films and 0.89 for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films.
CMC films.
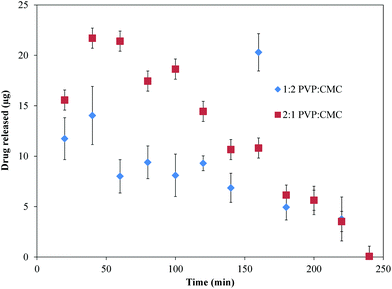 | ||
Fig. 5 Instantaneous release of imiquimod from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2 2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP 1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films. Data are mean ± standard deviation (n ≥ 3). CMC films. Data are mean ± standard deviation (n ≥ 3). | ||
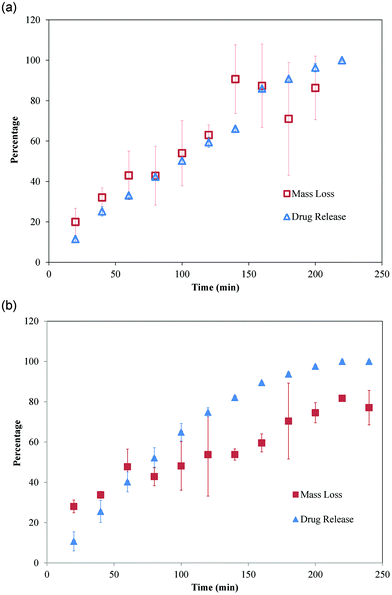 | ||
Fig. 6 Cumulative imiquimod release profile coupled with erosion profile for (A) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and (B) 2 2 and (B) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP 1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films. Data are mean ± standard deviation (n ≥ 3). CMC films. Data are mean ± standard deviation (n ≥ 3). | ||
Swelling
Conventional swelling studies indicated rapid mass changes, which resulted in indices reaching up to 500 and 200 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films, respectively (Fig. 7a). The 1
CMC films, respectively (Fig. 7a). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PVP
2 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC film swelling indices reached 200 in the first five min and 400 at 60 min. The rate of swelling subsequently decreased, and a further increase of only 100 was observed over the next 90 min. In contrast, 2
CMC film swelling indices reached 200 in the first five min and 400 at 60 min. The rate of swelling subsequently decreased, and a further increase of only 100 was observed over the next 90 min. In contrast, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films reached swelling index of 200 by 40 min and started decreasing from 60 min as samples began losing mass. Although both film types became viscous and difficult to handle after 130 min, 1
CMC films reached swelling index of 200 by 40 min and started decreasing from 60 min as samples began losing mass. Although both film types became viscous and difficult to handle after 130 min, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 films maintained their integrity, unlike 2
2 films maintained their integrity, unlike 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 films, which started eroding as observed visually. Swelling indices of the films were significantly different (p < 0.05 to p < 0.001), except at the first time point of 10 s.
1 films, which started eroding as observed visually. Swelling indices of the films were significantly different (p < 0.05 to p < 0.001), except at the first time point of 10 s.
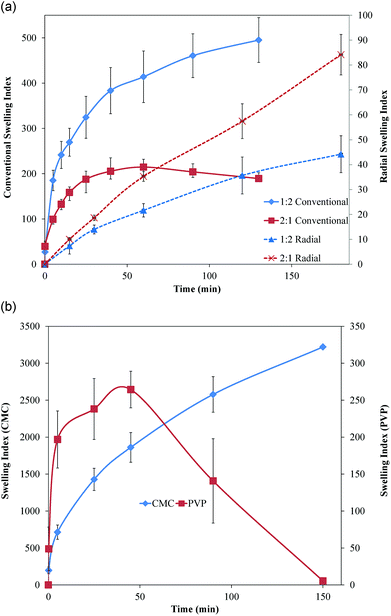 | ||
Fig. 7 (A) Conventional and radial swelling profiles for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2 2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP 1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films. (B) Conventional swelling profiles for pure PVP and CMC films. Data are mean ± standard deviation (n ≥ 3). CMC films. (B) Conventional swelling profiles for pure PVP and CMC films. Data are mean ± standard deviation (n ≥ 3). | ||
In contrast to the conventional swelling studies, measurement of radial swelling on agar showed that 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films swelled more than did the 1
CMC films swelled more than did the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PVP
2 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films (Fig. 7a). The swelling indices of both films were less than 100, but both films continued to swell radially after 180 min, unlike the conventional mass gain studies in which swelling plateaued and the samples began losing mass. At longer times, loss of the samples’ circular shape made further measurements difficult. Swelling indices of both film types were significantly different (p < 0.05 to <0.0025), except at the first time point of 15 min.
CMC films (Fig. 7a). The swelling indices of both films were less than 100, but both films continued to swell radially after 180 min, unlike the conventional mass gain studies in which swelling plateaued and the samples began losing mass. At longer times, loss of the samples’ circular shape made further measurements difficult. Swelling indices of both film types were significantly different (p < 0.05 to <0.0025), except at the first time point of 15 min.
Conventional swelling profiles for films containing only CMC showed a high index of 3000 in 150 min with monotonically increasing swelling (Fig. 7b). Unlike CMC films, PVP-only films reached their maximum swelling index of 264 in 45 min and then quickly started losing mass, being completely eroded by the end of 150 min (Fig. 7b).
Transport kinetics and permeability
Imiquimod in all samples was successfully separated from tissue particles and polymer components using HPLC. While imiquimod had a sharp peak at the retention time of 3.3 min, solubilized molecules of tissue had a broad peak 3.8 min; PVP and CMC were found with the injection peak. Imiquimod was detectable within a linear concentration range from 60 ng mL−1 to 7.8 μg mL−1.Transport of imiquimod through porcine buccal tissue into simulated saliva was controlled by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mucoadhesive films. The average flux rates of imiquimod through buccal mucosal tissue were 1.25 ± 0.39, 1.11 ± 0.12, and 4.98 ± 0.91 μg cm−2 h−1 for 1
1 mucoadhesive films. The average flux rates of imiquimod through buccal mucosal tissue were 1.25 ± 0.39, 1.11 ± 0.12, and 4.98 ± 0.91 μg cm−2 h−1 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mucoadhesive films and the control solution, respectively (Fig. 8a). The mucoadhesive films significantly (p < 0.01) decreased flux of imiquimod through tissue compared to control solutions, while no significant difference in flux was observed between 1
1 mucoadhesive films and the control solution, respectively (Fig. 8a). The mucoadhesive films significantly (p < 0.01) decreased flux of imiquimod through tissue compared to control solutions, while no significant difference in flux was observed between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 film types. Imiquimod retained in tissue after 24 h increased with the use of mucoadhesive films (30%) and was observed to be double the amount of imiquimod retained from control solutions (15%) (Fig. 8b). The amount of imiquimod transported through tissue into simulated saliva was 3.5 fold higher for control solutions compared to when films were used (p < 0.001).
1 film types. Imiquimod retained in tissue after 24 h increased with the use of mucoadhesive films (30%) and was observed to be double the amount of imiquimod retained from control solutions (15%) (Fig. 8b). The amount of imiquimod transported through tissue into simulated saliva was 3.5 fold higher for control solutions compared to when films were used (p < 0.001).
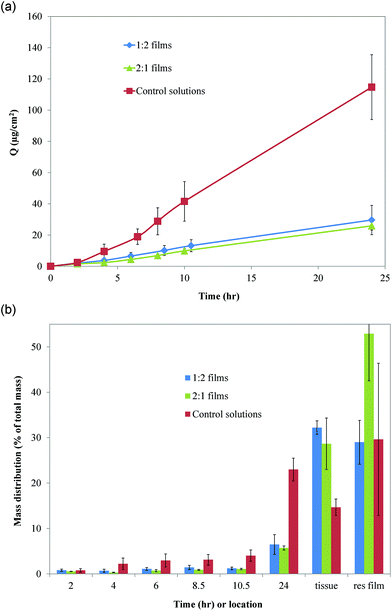 | ||
Fig. 8 (A) Cumulative drug permeation as a function of time for control solution (imiquimod solubilized in acetate buffer) and imiquimod-loaded PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC mucoadhesive films. (B) Percentage of total amount of imiquimod found in receptor compartment at increasing times, retained in tissue, and residual (res) film after 24 h. Data are mean ± standard deviation (n ≥ 3). CMC mucoadhesive films. (B) Percentage of total amount of imiquimod found in receptor compartment at increasing times, retained in tissue, and residual (res) film after 24 h. Data are mean ± standard deviation (n ≥ 3). | ||
Discussion
Mucoadhesive films loaded with immune response modulators can provide a localized and non-invasive approach to treatment of oral precancerous lesions. Although previous work showed that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PVP
2 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC mucoadhesive films lasted 4 h submerged in sink conditions in vitro and achieved sustained release for up to 3 h,7 several other film properties were not reported, such as adhesiveness of film to mucosal surface, mechanical properties for better handling, and swelling, which determines the release mechanism. All of these characteristics will play key roles in the design of a successful, bioerodible, mucoadhesive drug delivery system. The versatility of the present system allows modification and tailoring of multiple properties by simply changing composition of the films.
CMC mucoadhesive films lasted 4 h submerged in sink conditions in vitro and achieved sustained release for up to 3 h,7 several other film properties were not reported, such as adhesiveness of film to mucosal surface, mechanical properties for better handling, and swelling, which determines the release mechanism. All of these characteristics will play key roles in the design of a successful, bioerodible, mucoadhesive drug delivery system. The versatility of the present system allows modification and tailoring of multiple properties by simply changing composition of the films.
The film fabrication process involves drying polymer-drug solutions at 60 °C to evaporate the remaining solvent, mainly water. The amount of solvent retained in the films depends on the drying time and film composition. Prior studies of the PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC delivery system showed that residual water content significantly affected the drug release profile.7 Qualitative observation of films also showed differences in tackiness, strength, and flexibility with varying water content. Hence, it was important to control this variable for an accurate comparison of other film properties. Based on an analysis of mass changes as a function of time, the drying time for all film compositions was selected to maintain around 39% of residual water content in films. Further drying of films resulted in loss of flexibility and brittleness, and under-drying of films results in a soft gel that was difficult to handle.
CMC delivery system showed that residual water content significantly affected the drug release profile.7 Qualitative observation of films also showed differences in tackiness, strength, and flexibility with varying water content. Hence, it was important to control this variable for an accurate comparison of other film properties. Based on an analysis of mass changes as a function of time, the drying time for all film compositions was selected to maintain around 39% of residual water content in films. Further drying of films resulted in loss of flexibility and brittleness, and under-drying of films results in a soft gel that was difficult to handle.
Even though mucoadhesive films do not have load-bearing responsibility, understanding their tensile properties may be useful for better handling of films during the manufacturing process, while being applied to a mucosal surface, and when exposed to potentially demanding in vivo conditions. Selection of a 3 mm s−1 deformation rate was based on previous work with mucoadhesive films in which deformation rates ranged from 1 to 5 mm s−1.8–10 Modulus (1.8–6.8 MPa) and tensile strength (2.1–4.2 MPa) of the PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films decreased with increasing PVP content, which was contrary to results expected from PVP being considered the film-forming polymer.17 While the observed tensile strength and modulus were comparable to mucoadhesive films prepared from chitosan/copolymer of polyvinyl alcohol (PVA)/polyethylene glycol (PEG) (UTS = 3.5–5.4 MPa and modulus = 2.7–7.5 MPa)18 and copolymer of methylvinylether and maleic anhydride (UTS = 2.77 MPa),8 the UTS of PVP
CMC films decreased with increasing PVP content, which was contrary to results expected from PVP being considered the film-forming polymer.17 While the observed tensile strength and modulus were comparable to mucoadhesive films prepared from chitosan/copolymer of polyvinyl alcohol (PVA)/polyethylene glycol (PEG) (UTS = 3.5–5.4 MPa and modulus = 2.7–7.5 MPa)18 and copolymer of methylvinylether and maleic anhydride (UTS = 2.77 MPa),8 the UTS of PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC was 10 times lower than that for films made of hydroxypropylcellulose (20–110 MPa) and hydroxypropylmethylcellulose (HPMC; 40–150 MPa). The present mucoadhesive films exhibited substantial elongation before failure (150–300%) compared to 12–35, 55–125, and 66–130% reported for hydroxypropylcellulose, hydroxypropylmethylcellulose, and chitosan/PVA/PEG films, respectively.18,19
CMC was 10 times lower than that for films made of hydroxypropylcellulose (20–110 MPa) and hydroxypropylmethylcellulose (HPMC; 40–150 MPa). The present mucoadhesive films exhibited substantial elongation before failure (150–300%) compared to 12–35, 55–125, and 66–130% reported for hydroxypropylcellulose, hydroxypropylmethylcellulose, and chitosan/PVA/PEG films, respectively.18,19
Pull-off adhesion studies were performed on porcine buccal mucosa because of its resemblance to human buccal mucosa in terms of ultrastructure and composition.20,21 An initial force of 10 N was applied on films to imitate pressing a film onto a patient's cheek by a finger, as well as being based on similar research.12 Substantial variability was observed during pilot testing of multiple samples on a single tissue specimen. This was likely due to microscale adhesion of polymers on tissue and/or components of the tissue surface being modified with each test. Hence, care was taken to use fresh location on same tissue or a fresh tissue for each sample to reduce variability. Comparison of the present results with existing literature was difficult due to different experimental conditions, such as contact force, deformation rate, and contact time. The measured mucoadhesive forces, however, were comparable to several blends of polymers.17,18,22,23 While the adhesion force increased from 0.41 to 1.06 N cm−2 with increasing PVP content, several other polymer blends, including chitosan/PVA/PEG ranged from 0.33 to 0.41 N cm−2;18 copolymers of acrylic acid and 2-ethylhexyl acrylate ranged from 0.033 to 0.065 N cm−2 at different contact speeds and contact times;22 and plain films of hydroxyethylcellulose, chitosan, and polyvinyl alcohol recorded 0.58, 0.88 and 5.11 N cm−2, respectively.23
Shear stresses from the tongue, gums, and saliva may be more prominent than pull-off forces under actual oral conditions. The Wilhelmy plate method is commonly used to measure shear adhesion of polymers, often with mucin solution instead of tissues.1 Buccal mucosa is covered by mucus, which contains 4% mucin (glycoproteins).24 Several studies have proposed that the rate of diffusion of polymer chains into mucus and their interactions with mucin are the main factors responsible for mucoadhesion of polymeric films.2,24,25 Hence, 4% bovine mucin-coated membranes were used as a replacement of porcine buccal tissues for shear adhesion studies.
Variables, such as contact force, contact time, and volume of buffer used for hydration, play important roles in the performance of films in shear adhesion studies.15,22 Consequently, the parameters used for the adhesion experiments were based on pilot studies (data not shown). The difference in initial contact force for pull-off and shear adhesion was primarily attributed to the change of substrate. Furthermore, films were observed to tear during shear adhesion studies following application of 10 N contact force rather than desired sliding of films on mucin-coated membranes, which was why the initial contact force was reduced. However, both pull-off and shear maximum adhesive strength (force per unit area) were observed to increase with increasing PVP content, although CMC is well known as a mucoadhesive polymer.26
Changes in both mechanical and adhesive properties showed clear trends with increasing PVP content. Because measurements of mechanical properties showed that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 films were tough with high modulus and UTS and that 2
2 films were tough with high modulus and UTS and that 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 films were more adhesive, only 1
1 films were more adhesive, only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films were selected for better understanding of PVP and CMC effects on release, swelling, and erosion profiles. The close relationship between drug release and mass loss for 1
CMC films were selected for better understanding of PVP and CMC effects on release, swelling, and erosion profiles. The close relationship between drug release and mass loss for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 films suggests that release of imiquimod was controlled by erosion of the films. Comparison of mass loss and release profiles for 2
2 films suggests that release of imiquimod was controlled by erosion of the films. Comparison of mass loss and release profiles for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 films, however, suggests that release of imiquimod was controlled by both diffusion and erosion. This interpretation was also supported by Korsmeyer–Peppas mathematical modeling. According to this model, an ‘n’ value of greater than 1 suggests super case-2 relaxation, which involves erosion of films and swelling-controlled polymer relaxation, and the ‘n’ value of 0.89 suggests anomalous, non-Fickian diffusion.
1 films, however, suggests that release of imiquimod was controlled by both diffusion and erosion. This interpretation was also supported by Korsmeyer–Peppas mathematical modeling. According to this model, an ‘n’ value of greater than 1 suggests super case-2 relaxation, which involves erosion of films and swelling-controlled polymer relaxation, and the ‘n’ value of 0.89 suggests anomalous, non-Fickian diffusion.
Two types of swelling studies were performed for better understanding of film behavior. While conventional swelling studies based on mass gain, the gold standard for swelling studies, show behavior of films in bulk solutions, the agar-based radial swelling studies can better mimic the conditions of a film applied to the mucosal surface, which is the intended application of this delivery system. CMC, which is known for its water-retaining properties, was observed to reach a swelling index of 3000 and still retain its integrity, unlike PVP films, which had a lower swelling index and eroded faster. Hence, the presence of more CMC in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PVP
2 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films caused more swelling compared to 2
CMC films caused more swelling compared to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films. Early erosion of 2
CMC films. Early erosion of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films at 60 min can also be attributed to the presence of more PVP. This early erosion of polymer chains from 2
CMC films at 60 min can also be attributed to the presence of more PVP. This early erosion of polymer chains from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films may have enhanced diffusion of eroded chains through agar, resulting in more radial swelling than was observed for 1
CMC films may have enhanced diffusion of eroded chains through agar, resulting in more radial swelling than was observed for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PVP
2 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films.
CMC films.
The combined findings from mathematical modeling and release, erosion, and swelling profiles indicate that drug release from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PVP
2 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films was controlled by swelling and slow erosion of films that resulted in sustained release. In contrast, earlier and faster erosion of chains and less swelling opened up the bulk of 2
CMC films was controlled by swelling and slow erosion of films that resulted in sustained release. In contrast, earlier and faster erosion of chains and less swelling opened up the bulk of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVP
1 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC film and resulted in burst release. This was then followed by continually decreasing concentrations of drug, which were governed by diffusion of drug from the residual polymeric matrix into buffer.
CMC film and resulted in burst release. This was then followed by continually decreasing concentrations of drug, which were governed by diffusion of drug from the residual polymeric matrix into buffer.
Because a potential application of the mucoadhesive films is for local treatment of oral dysplasia, studying the transport characteristics and permeability of imiquimod in vitro can give preliminary knowledge about the feasibility of this approach before initiating in vivo studies. The goal is to deliver and retain drug in the epithelium rather than penetration into vasculature for systemic distribution. Porcine tissue was chosen because of its close resemblance to human buccal mucosa and its extensive use in other permeability studies.20,21 The thickness of epithelium in human mucosa ranges from 250 to 400 μm.21 Prior studies showed that use of tissue sections ≤500 μm represents transport kinetics of a compound through epithelium, whereas connective tissue dominates transport through mucosa when tissue sections were >500 μm.21
Permeability studies of four different compounds encompassing hydrophilic and hydrophobic compounds on only the epithelial layer showed that permeation increased with lipophilicity.21 Other permeability studies of hydrophilic substances, such as mannitol and lidocaine hydrochloride, showed low permeability, which required use of used fatty acids, such as oleic acid, to increase permeation through the epithelium.31,32 Hydrophobic substances, such as carvedilol, had rapid permeation in the first few hours.19 Because imiquimod is also hydrophobic, it showed good permeability when used alone in a solution. The current PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC mucoadhesive films significantly decreased the flux of imiquimod and helped localize imiquimod within the epithelium. Interactions of mucoadhesive polymers with the epithelial tissue as well as the hydrophilicity and large size of the polymers may have resulted in their being trapped in the tissue, which created a transport barrier and reduced permeation. A brief literature review of other mucoadhesive systems did not reveal data about the amount of drug retained in tissue when films and control solutions were used;16,19,27,31,33,34 parameters such as flux and permeability coefficient were reported. Although 1
CMC mucoadhesive films significantly decreased the flux of imiquimod and helped localize imiquimod within the epithelium. Interactions of mucoadhesive polymers with the epithelial tissue as well as the hydrophilicity and large size of the polymers may have resulted in their being trapped in the tissue, which created a transport barrier and reduced permeation. A brief literature review of other mucoadhesive systems did not reveal data about the amount of drug retained in tissue when films and control solutions were used;16,19,27,31,33,34 parameters such as flux and permeability coefficient were reported. Although 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PVP
2 PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC films exhibited sustained release of imiquimod for up to 3 h in contrast to 2
CMC films exhibited sustained release of imiquimod for up to 3 h in contrast to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 films, for which burst release was observed in first 40 min with continuously decreased release, no significant difference was evident between both films in transport kinetics or absorption within epithelium. This may be attributed to inability of the polymers to permeate the tissue, which thereby acted as the rate-limiting step, rather than erosion of polymers, which controlled release of drug.
1 films, for which burst release was observed in first 40 min with continuously decreased release, no significant difference was evident between both films in transport kinetics or absorption within epithelium. This may be attributed to inability of the polymers to permeate the tissue, which thereby acted as the rate-limiting step, rather than erosion of polymers, which controlled release of drug.
A variety of polymers are being used to develop mucoadhesive films for delivery of different drugs. Some of the more extensively used polymers include HPMC, chitosan, hydroxyethylcellulose (HEC), Carbopol, Eudragit RL PO, gelatin, CMC, PVA, polyethylene (PE) and PVP (K30 and K90 variations).8,16–19,22,23,27–31 In addition, new copolymers are being developed, such as copolymers of methylvinylether and maleic anhydride8 and acrylic acid and 2-ethylhexyl acrylate.22 All these polymers and blends of different compositions have advantages and disadvantages. For example, while chitosan is a natural, adhesive polymer, the resulting films can be brittle.28 Addition of other polymers, such HEC,28 PVP K30,29 copolymer of PVA and PE,18 to chitosan increased the film-forming ability, UTS, and percentage of elongation. In the present work, however, a range of properties can be achieved simply by adjusting the ratio of PVP to CMC.
The measured range of adhesive, mechanical, and drug release properties of mucoadhesive films can be attributed to the combined properties of PVP and CMC. The hygroscopic nature and tackiness of PVP increased adhesive properties, while the excessive swelling and slower erosion of CMC aided film retention during the release studies. The ability of PVP to absorb moisture (up to 40% of its weight) resulted in decreased modulus and UTS because it enabled PVP chains to move and reposition more easily under load. With increasing CMC content, however, chain entanglement and decreased mobility likely increased modulus and UTS and decreased elongation. Swelling studies showed the early erosion of pure PVP films beginning at 60 min, but the presence of CMC helped control erosion, thereby providing sustained release for 3 h.
Conclusion
The present mucoadhesive drug delivery system based on CMC and PVP offers a wide range of tensile, adhesive, degradation, and release properties without addition of new polymers/excipients. Controlled release and increased localization of imiquimod within the epithelium provided by PVP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CMC mucoadhesive films may increase the potential of these films for local treatment of oral dysplasia. Further bioactivity studies in vivo will be important for determining the best combination of properties and appropriate film type for treatment of dysplastic lesions.
CMC mucoadhesive films may increase the potential of these films for local treatment of oral dysplasia. Further bioactivity studies in vivo will be important for determining the best combination of properties and appropriate film type for treatment of dysplastic lesions.
Acknowledgements
This work was supported in part by the National Institutes of Health (DE019645) and Kentucky NASA EPSCoR (NNX08BA13A).References
- A. Ahuja, J. Ali and K. R. Khar, Drug Dev. Ind. Pharm., 1997, 23, 489–515 CrossRef CAS.
- N. Salamat-Miller, M. Chittchang and T. P. Johnston, Adv. Drug Delivery Rev., 2005, 57, 1666–1691 CrossRef CAS.
- V. V. Khutoryanskiy, Macromol. Biosci., 2011, 11, 748–764 CrossRef CAS.
- J. Glaholm, in Oral Cancer, ed. J. P. Shah, N. W. Johnson and J. G. Batsakis, Martin Dunitz, New York, 2003, pp. 339–366 Search PubMed.
- S. T. Sonis, J. Support. Oncol., 2007, 5, 3–11 CAS.
- National Cancer Institute (U.S.), Chemotherapy and you: a guide to self-help during treatment, NIH Publication No. 11-7156, National Institutes of Health, Bethesda, MD, 2007 Search PubMed.
- S. K. Ramineni, L. L. Cunningham Jr., T. D. Dziubla and D. A. Puleo, J. Pharm. Sci., 2013, 102, 593–603 CrossRef CAS.
- R. F. Donnelly, P. A. McCarron, A. A. Zawislak and A. D. Woolfson, Int. J. Pharm., 2006, 307, 318–325 CrossRef CAS.
- J. S. Boateng, H. N. Stevens, G. M. Eccleston, A. D. Auffret, M. J. Humphrey and K. H. Matthews, Drug Dev. Ind. Pharm., 2009, 35, 986–996 CrossRef CAS.
- R. K. Mishra, M. Datt and A. K. Banthia, AAPS PharmSciTech, 2008, 9, 395–403 CrossRef CAS.
- K. K. Peh and C. F. Wong, J. Pharm. Pharm. Sci., 1999, 2, 53–61 CAS.
- P. Wu, L. Lucchesi, J. Guo, S. A. Prahl and K. Gregory, Society for Biomaterials 30th Annual Meeting Transactions, 2005 Search PubMed.
- M. A. Alam, F. J. Ahmad, Z. I. Khan, R. K. Khar and M. Ali, AAPS PharmSciTech, 2007, 8, E109 CrossRef.
- D. S. Jones, M. L. Bruschi, O. de Freitas, M. P. Gremiao, E. H. Lara and G. P. Andrews, Int. J. Pharm., 2009, 372, 49–58 CrossRef CAS.
- C. F. Wong, K. H. Yuen and K. K. Peh, Int. J. Pharm., 1999, 180, 47–57 CrossRef CAS.
- K. G. Desai, S. R. Mallery, A. S. Holpuch and S. P. Schwendeman, Pharm. Res., 2011, 28, 2599–2609 CrossRef CAS.
- L. Perioli, V. Ambrogi, F. Angelici, M. Ricci, S. Giovagnoli, M. Capuccella and C. Rossi, J. Controlled Release, 2004, 99, 73–82 CrossRef CAS.
- P. Mura, G. Corti, M. Cirri, F. Maestrelli, N. Mennini and M. Bragagni, J. Pharm. Sci., 2010, 99, 3019–3029 CAS.
- Y. V. Vishnu, K. Chandrasekhar, G. Ramesh and Y. M. Rao, Curr. Drug Delivery, 2007, 4, 27–39 CrossRef CAS.
- P. W. Wertz and C. A. Squier, Crit. Rev. Ther. Drug Carrier Syst., 1991, 8, 237–269 CAS.
- U. Kulkarni, R. Mahalingam, S. I. Pather, X. Li and B. Jasti, J. Pharm. Sci., 2009, 98, 471–483 CrossRef CAS.
- A. H. Shojaei, J. Paulson and S. Honary, J. Controlled Release, 2000, 67, 223–232 CrossRef CAS.
- N. A. Nafee, M. A. Boraie, F. A. Ismail and L. M. Mortada, Acta Pharm., 2003, 53, 199–212 CAS.
- L. Serra, J. Domenech and N. A. Peppas, Eur. J. Pharm. Biopharm., 2009, 71, 519–528 CrossRef CAS.
- N. V. Madhav, A. K. Shakya, P. Shakya and K. Singh, J. Controlled Release, 2009, 140, 2–11 CrossRef CAS.
- J. D. Smart, I. W. Kellaway and H. E. Worthington, J. Pharm. Pharmacol., 1984, 36, 295–299 CrossRef CAS.
- R. K. Averineni, S. G. Sunderajan, S. Mutalik, U. Nayak, G. Shavi, K. Armugam, S. R. Meka, S. Pandey and U. Nayanabhirama, Pharm. Dev. Technol., 2009, 14, 199–207 CrossRef CAS.
- K. Luo, J. Yin, O. V. Khutoryanskaya and V. V. Khutoryanskiy, Macromol. Biosci., 2008, 8, 184–192 CrossRef CAS.
- S. S. Shidhaye, N. S. Saindane, S. Sutar and V. Kadam, AAPS PharmSciTech, 2008, 9, 909–916 CrossRef CAS.
- F. Cui, C. He, M. He, C. Tang, L. Yin, F. Qian and C. Yin, J. Biomed. Mater. Res., Part A, 2009, 89, 1063–1071 CrossRef.
- R. Abu-Huwaij, S. Assaf, M. Salem and A. Sallam, Drug Dev. Ind. Pharm., 2007, 33, 437–448 CrossRef CAS.
- J. Lee, S. K. Lee and Y. W. Choi, Arch. Pharm. Res., 2002, 25, 546–549 CrossRef CAS.
- M. I. Mohamed, M. Haider and M. A. Mohamed Ali, J. Chem. Pharm. Res., 2011, 3, 665–686 CAS.
- I. Diaz del Consuelo, F. Falson, R. H. Guy and Y. Jacques, J. Controlled Release, 2007, 122, 135–140 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |