Influence of structure and properties of colloidal biomaterials on cellular uptake and cell functions
Zhengwei Maoa, Xiangyan Zhoua and Changyou Gao*ab
aMOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China
bState Key Laboratory of Diagnosis and Treatment for Infectious Diseases, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, China. E-mail: cygao@mail.hz.zj.cn; Fax: +86-571-87951108
First published on 15th July 2013
Abstract
The molecular structure and physicochemical properties of nano- and colloidal materials have a big impact on their intracellular uptake and cell functions. Elucidation of the cellular uptake and cytotoxicity of the colloidal materials has been recognized recently as one of the paramount prerequisites for their biomedical applications. In this review, the influence of important parameters of colloidal biomaterials such as size, shape, chemical and mechanical properties on cellular uptake and its pathways is summarized. The intracellular distribution and transportation of the colloidal particles are then correlated with their physiochemical properties. The impacts on cytotoxicity and cell functions are also discussed. Finally, more attention is suggested to be paid on the basic understanding of cellular uptake and subsequent influence on cells, which is of great significance for the function implementations and safer applications of nano- and colloidal materials.
 Zhengwei Mao | Zhengwei Mao is currently an associate professor of materials science at Zhejiang University. He obtained his PhD in materials science in 2007 under the supervision of Prof. Changyou Gao and Prof. Jiacong Shen at Zhejiang University, China. His main scientific interests are in the areas of functional colloids and their interactions with cells and tissues. |
 Changyou Gao | Changyou Gao is currently a professor of materials science at Zhejiang University, a winner for the National Science Fund for Distinguished Young Scholars of China, and a Cheung Kong Scholar of Ministry of Education of China. He obtained his PhD in polymer chemistry and physics in 1996 under the supervision of Prof. Jiacong Shen at Jilin University, China. His research interests include self-assembled microcapsules, nano and colloid biomaterials and their interaction with cells, and biomaterials for tissue regeneration and cell migration. |
1. Introduction
Colloidal particles are characterized by a length scale ranging from 1 nm to microns (typically 1 μm).1 They have spherical, tubular, or irregular shapes and may further fuse, aggregate or agglomerate with each other. Many engineered colloidal particles have already been used as fillers, sensors and carriers for catalysts in industry.2,3 Along with the rapid development of nanotechnology and nanomedicine, these tiny particles are used in biological field as detection probes, contrast agents and sensors for disease diagnosis and bio-imaging, especially for the early diagnosis of cancer.4 The most important application of colloidal particles, however, is acting as carriers for drugs and therapeutic biomacromolecules to endow the functions of sustained release and targeted delivery with enhanced efficiency and reduced toxicity.5–12The huge potential of colloidal particles makes it essentially necessary to carry out an in-depth study of their interactions with cells, which are the basic structural and functional units of all known living organisms.13 Due to their small enough size and/or their functionalities, colloidal particles are inevitably ingested into cells when they contact with each other.14,15 On the one hand, many of the biological applications of the particles are dependent on the cellular uptake, such as cell labeling, gene delivery and intracellular drug delivery. In these applications, the particles should be transported through cell membrane and located into specific cellular organelles such as cytosols and nuclei. On the other hand, the uptake of particles may bring some effects such as cytotoxicity and alternation of cell functions. Therefore, it is urgent to understand the uptake process of particles, including their uptake mechanism and intracellular fate as well as their subsequent influence on cell viability and functions.
In the endocytosis process the particles are firstly attached to the cell membrane through specific or nonspecific interactions, and then are wrapped by the cell membrane to form membrane-bounded vesicles. The particles can enter cells by several different endocytic pathways, such as phagocytosis, clathrin-mediated endocytosis, caveolae-mediated endocytosis, macropinocytosis and clathrin- and caveolin-independent endocytosis.16 Phagocytosis is conducted primarily by specialized cells, including macrophages, monocytes and neutrophils, which can clear out large particles in the blood such as pathogens and debris of dead cells. In contrast, pinocytosis can operate in all mammalian cells. Clathrin-mediated, caveolin-mediated, macropinocytosis, and clathrin- and caveolin-independent endocytosis are four major processes of pinocytosis involving different types of receptor–ligand interactions, which are mainly dependent on particle size and surface chemistry. Afterwards the particles can be transported to different cellular organelles such as cytoplasm, mitochondria, Golgi complex and even nucleus (Fig. 1).17–22 Some of them can escape from the cells by metabolism and some of them are accumulated in the cells.23 The interaction between the particles and cellular organs may influence some cellular functions such as intracellular reactive oxygen species (ROS), mitochondrial activity, inflammation, cytoskeleton, cell adhesion, cell migration, cell cycle and integrity of DNA,24–29 leading to the so-called cytotoxicity.21,30
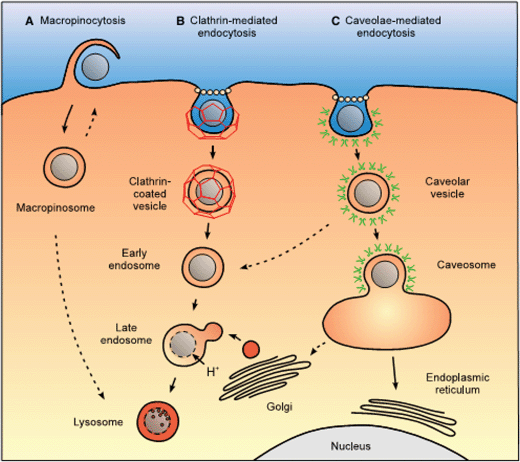 | ||
| Fig. 1 Intracellular nanocarrier trafficking following macropinocytosis, clathrin-mediated endocytosis and caveolae-mediated endocytosis.38 (A) Macropinocytosis leads to the formation of a macropinosome, which is thought to eventually fuse with lysosomes or recycle its content to the surface. (B) Clathrin-mediated endocytosis of a nanocarrier leads to the formation of an early endosome, which is acidified and fuses with prelysosomal vesicles containing enzymes (in red) to give rise to a late endosome and finally a lysosome, an acidic and enzyme-rich environment prone to nanocarrier and drug degradation. Unless a lysosomal delivery is desired, strategies for a cytosolic drug delivery by this route will focus on the drug escape from the endosome as early as possible. (C) Caveolae-mediated endocytosis of a nanocarrier gives rise to a caveolar vesicle that can be delivered to caveosome, avoiding a degradative acidic and enzyme-rich environment. Reprinted with permission from ref. 38. Copyright 2009, Springer. | ||
The particle uptake and subsequent influence on cells are largely dependent on the physiochemical properties of the particles including bulk and surface chemical compositions, sizes, shapes, mechanical properties, and so on.31,32 This review starts with the introduction of some key parameters of cellular uptake of colloidal particles and its pathways, and is followed by the distribution and transportation of the colloidal particles, finally ending up with the impact on cytotoxicity and cell functions. In our opinion, more attention should be paid to the basic research of cellular uptake and subsequent influence on cells, which is of great significance for the functional implementations and safer use of the colloidal particles.
2. Cellular uptake
Understanding the relationship between the physiochemical properties of colloidal particles and the cellular uptake amount and rate is one of the most important issues for designing ideal colloidal materials with a desirable biological performance. It is known that the efficiency of intracellular delivery of bioactive substrates, for instance, genes and chemotherapeutics, is critically dependent on the internalization rate and amount of the carriers. So far many results have been gathered to show that some parameters, such as size, shape, surface chemistry and rigidity of the colloidal particles can play decisive roles in the cell uptake kinetics and pathways.2.1. Size
Cellular uptake can take place when cells are exposed to colloidal particles with a size ranging from several nanometers to microns. Cells have highly tuned and precisely defined functions to regulate the uptake and transportation of biological components in this range.33–37 Some scale rules exist within the cell too. Fig. 2 illustrates the most crucial sizes involved in different ways of cellular uptake and transportation.38 These natural size-restricted structures execute their barrier functions when the cell internalizes particles, determining the uptake pathways and mechanisms for different particles. As acknowledged previously, ingestion of microparticles (>0.5 μm) is usually through a phagocytosis route and that of smaller ones (<0.2 μm) is usually through pinocytosis.34,35 The cellular uptake amount and rate are also largely dependent on particle size.34,39 It is known that the cellular uptake process is strongly dependent on the membrane-wrapping time, which is based on the diffusion rate of receptors on the cell membrane surface.40 Extremely small or large colloidal particles would yield an inefficient uptake.36,37 | ||
| Fig. 2 The cellular uptake of particles is dominated by the natural size rules and gatekeepers within a mammalian cell.33 The thickness of the plasma membrane bilayer is typically 4–10 nm. The sizes of endocytic vesicles in both phagocytosis and pinocytosis pathways for particle internalization are also introduced.193 Phagocytes can take up large particles (or nanoparticle aggregates), opsonized nanoparticles, or particles with certain ligand modification via phagocytosis. Particle internalization in a nonphagocytic mammalian cell is mainly through pinocytosis or direct penetration. With different surface modifications, particles may be taken up via specific (receptor-mediated) endocytosis or nonspecific endocytosis. The heterogeneity of particle surfaces and dispersion always requires multiple uptake pathways to be involved. Reprinted with permission from ref. 33. Copyright 2012, American Chemical Society. | ||
Theoretical considerations based on membrane deformation have been implemented in specific receptor-mediated interactions, so that the critical radius can be predicted to achieve full wrapping and internalization.35–37,41–46 All models converge on the same conclusion: the particles ought to have a minimum radius between 20 and 30 nm to achieve effective cellular uptake.35–37,41–43 Decuzzi and Ferrari proposed a similar theoretical approach that the contribution of nonspecific interactions (such as van der Waals and electrostatic forces) is as important as that of the specific receptor–ligand interaction.44 As the repulsive interaction between particles and cell increases, the wrapping time, the threshold and the optimal particle radius increase too, leading to hindrance of endocytosis. In contrast, an attractive non-specific interaction would favor particle endocytosis. These theoretical calculations are fitted with some experimental results. For example, Aoyama et al. demonstrated that 25 nm quantum dots (the size of core QDs) enter cells more effectively than the 7.5 nm and 2.5 nm ones.47 Chan et al. studied the internalization of pristine and protein-coated gold nanoparticles (NPs) with a size ranging from a few to hundreds of nanometers, and found 20–25 nm to be the optimal radius for uptake.34
However, the experimental observations are not always consistent with these theoretical rules. A case by case study reveals that the size influence may depend on both the chemical structures of colloidal particles and cell types. Zhang et al. found that 500 nm silica particles were taken up by human dermal fibroblasts with a larger amount through macropinocytosis and clathrin-mediated endocytosis pathways, whereas the uptake of 80 nm silica particles was mediated jointly by macropinocytosis, clathrin-mediated and caveolae-mediated endocytosis.48 Luo et al. found that silica particles with an average diameter of 200 nm have the largest uptake dosage by COS-7 cells (a cell line from the kidney of an African green monkey) compared to smaller ones.49 Polystyrene (PS) particles with the same size can be internalized into Caco-2 cells (a human colon carcinoma cell line) with the largest uptake dosage too.50 Chithrani et al. reported that 50 nm Au particles are more easily endocytosed by HeLa cells (human epithelial cells from a fatal cervical carcinoma) among those particles smaller than 100 nm.34 On the other hand, Natarajan et al. observed that 20 nm iron oxide particles are more quickly internalized with a final larger uptake dosage in comparison with the 100 nm ones.51 Qaddoumi et al. found that 100 nm poly(lactide-co-glycolide) (PLGA) particles have the highest uptake by rabbit conjunctival epithelial cells in comparison with larger ones (800 nm and 10 μm).52 Dawson and Halbert conducted a similar comparison by using PLGA particles with diameters from 155 nm to 600 nm, whose surfaces are decorated with bacterial invasin.53 They found that the larger particles (375 nm and 600 nm) are internalized by Hep2 (human Caucasian larynx carcinoma) cells with a larger amount than the smaller ones due to a higher surface density of invasin, which offers the particles a receptor-dependent uptake mechanism. Uptake of the particles may follow different mechanisms depending on the particle size and surface chemistry, and thereby may generate controversial results. Moreover, due to their smaller size and high surface–volume ratio, sedimentation and agglomeration of the colloidal particles may become a significant problem in some systems, especially in serum-containing media.54 This important impact, however, is rarely taken into consideration.
The cell type also plays an important role in endocytic pathways, which in turn influences the uptake patterns. Phagocytosis is an endocytic pathway for professional phagocytes such as macrophages, monocytes and polymorphonuclear granulocytes, but usually not for other types of cells (Fig. 3).55,56 For example, Xia et al. presented that RAW 264.7 cells (mouse macrophages) ingest PS spheres through an endosomal–lysosomal route, while BEAS-2B cells (human bronchial epithelial cells) ingest the particles via a caveolar uptake mechanism.75 Zauner et al. found that HUVEC cells (human umbilical vein endothelial cells), ECV304 cells (derivative cells of the T24 bladder carcinoma) and HNX 14C cells (a human head and neck squamous cell carcinoma) can take up PS particles with a size up to 1010 nm.57 In contrast, HepG2 cells (human hepatoma cells) and Hepa1-6 cells (mouse hepatoma cells) do not take up particles larger than 93 nm. In addition, it has been shown that some types of cells such as epithelial cells, fibroblasts and endothelial cells can internalize larger particles,58–60 while HepG2 cells prefer to ingest smaller ones.61
 | ||
| Fig. 3 Different fates and effects of gold nanorods (Au NRs) in cancer cell, normal cell, and stem cell due to distinct pathways for cellular trafficking.55 Three kinds of endocytotic pathways regulate the process of internalization of Au NRs. However, large numbers of intracellular Au NRs transfer from lysosomes to mitochondria and cannot be excluded in cancer cells. Intracellular localization, not uptake pathway, determines the final fate of both Au NRs and cells. Due to the enhanced permeation of the lysosomal membrane after Au NR uptake, Au NRs are released into the cytoplasm of cancerous A549 cells and translocated from endosomes/lysosomes to mitochondria, inducing decreased mitochondrial membrane potentials, increased oxidation stress and finally reduced cell viability. However, Au NRs show almost no toxicity in normal cells since their lysosomal membranes remain more intact and Au NRs are localized in lysosomes. Reprinted with permission from ref. 55. Copyright 2011, American Chemical Society. | ||
2.2. Shape
The effect of particle shape is as important as that of the particle size on the cellular uptake. However, it is more complicated and thus is less tackled. So far some results have shown that cells can take up colloidal particles with various shapes such as spheres,62,63 rods,64 tubes65,66 and sheets.67,68 Chithrani et al. found that rod-shaped gold NPs are more difficultly endocytosed by HeLa cells than their spherical counterparts, regardless of the surface functionalization of targeting ligand transferrin.34 Qiu et al. also studied the effect of aspect ratio on the cellular uptake of gold nanorods. They demonstrated that the Au nanorods of a longer aspect ratio (ranging from 1 to 4 with the length × diameter of 33 × 30, 40 × 21, 50 × 17 nm, respectively) are internalized with a slower rate than those shorter ones. This is mainly attributed to the longer membrane-wrapping time required for the longer rod-shaped particles (Fig. 4).69 However, the shape effect can be varied when the size and chemical composition of the particles as well as the cell types are different. Geng et al. found that polymer spherical micelles and short filomicelles can be similarly internalized by the cells and tissues of a mouse.62 Trewyn et al. observed that the cellular uptake dosage of tubular mesoporous silica nanomaterials (MSN, width 80–150 nm, length 400–1000 nm) is larger than that of spherical ones (diameter 80–150 nm) in both cancerous CHO cells (Chinese hamster ovary cells) and normal fibroblasts.70 Huang et al. found that the MSNs with different shapes are firstly internalized in A375 cells by nonspecific cellular uptake and merged with endosomes, subsequently are released into cytoplasm. Long rod-shaped particles are more easily internalized by cells compared with short rod-shaped and sphere-shaped particles.71 Alemdaroglu et al. demonstrated that rod-like DNA block copolymer micelles are internalized 12 times more efficiently than their spherical counterparts.72 Gratton et al. demonstrated that crosslinked poly(ethylene glycol) (PEG) hydrogel cylinders with a diameter of 150 nm and a height of 450 nm (aspect ratio of 3) are internalized more effectively than similar size cylinders with a smaller aspect ratio.73 The contradictory results might be caused by some variables of the particles such as size and stiffness, leading to the difference in uptake mechanisms. | ||
| Fig. 4 Impact of aspect ratio of gold nanorods on cellular uptake.69 (A) Au nanorods of different aspect ratio of 1.0 (CTAB-1), 2.0 (CTAB-2), 2.9 (CTAB-3), and 4.2 (CTAB-4) (CTAB, cetyltrimethylammonium bromide); (B) numbers of Au nanorods within human breast adenocarcinoma (MCF-7) cells; (C) sketch map for how size and shape affect membrane wrapping kinetics in cell endocytosis. Changes in nanoparticle size may affect the surface ligand density, ligand conformation, surface curvature, and relative orientation during nanoparticle membrane docking. Changes in nanoparticle aspect ratio may affect the position of surface ligand and wrapping time.33 Reprinted with permission from ref. 69. Copyright 2010, Elsevier. | ||
Jin et al. observed the endocytosis and exocytosis of DNA-wrapped single-walled carbon nanotubes in NIH-3T3 (mouse embryonic fibroblast cell line) cells. The cellular uptake trend is bell-shaped as a function of the tube length, with 320 nm being the optimal length.74 However, some other studies demonstrate that the carbon nanotubes can enter cells via non-endocytic pathways.64,74 The shape and intrinsic hydrophobic nature of the carbon nanotubes enable a “needle-effect”, i.e. entering cells via physical penetration of the membrane.75,76 This behavior is likely dependent on the tube length.
In addition, different types of cells may also have different responses to the shape of colloidal particles. Hutter et al. studied the uptake of gold nanospheres, rods and urchins (i.e. spiky NPs) by two types of cells: phagocytic microglia cells and nonphagocytic neurons.77 The spiky particles are preferentially taken up by microglia, while only rods are ingested by neurons. Another example is that CHO cancer cells can ingest spherical and tube-like MSNs with similar and rapid rates, whereas noncancerous fibroblasts show less efficiency in internalization of tubular-shaped MSNs.70
2.3. Surface chemistry
The surface chemistry of colloidal particles is one of the most important factors for cellular uptake. In most cases the surface provides the direct driving forces (electrostatic, van der Waals’ force and hydrogen bonding) for cellular uptake, and determines the uptake pathways. Surface wettability, charge and ligands have paramount influence on the interactions between colloidal particles and cells too.Usually, the hydrophilic surface is not favorable for uptake. Zahr et al. reported that the cellular uptake decreases to 1/3 of the initial value when the particle surface is modified with hydrophilic polymers such as PEG.78 In contrast, a coating of poly(N-vinyl caprolactam) enhances the cellular uptake of PS particles.79 In addition, Torche et al. reported that a surface with the same molecules but different hydrophobicity (NPs prepared with 5% poly(vinyl alcohol) (PVA) are more hydrophilic compared to those prepared with 2% PVA) could have produced a difference in endocytic pathways.79 Even though poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC), a hydrophilic polymer, has been used for several years as a medical device coating with a similar performance to PEG, the PMPC polymersomes are internalized three orders of magnitude than the PEG ones.80–82 Similar results have been obtained on the uptake of PMPC-functionalized metal NPs, quantum dots, lipid nanoparticles, and polymeric micelles.83–87 Battaglia's group also observed that cellular uptake of PMPC is ubiquitous across many cell types including non-dividing cells such as neurons and certain immune cells.88,89 This phenomenon might be attributed to the zwitterionic nature of the PMPC-based polymers that may facilitate greater intercellular transportation.
The influence of the surface charge of particles on cellular uptake is mainly driven by electrostatic interactions. Simply classifying internalization of NPs in terms of their surface charge, the neutral and anionic nanoparticles are less efficiently internalized than the cationic ones because the cell membrane is normally slightly negatively charged. Perumal et al. prepared poly(amidoamine) (PAMAM) 4th generation dendrimers with different functional end groups, including cationic primary amines, neutral hydroxyl groups and anionic carboxylic groups.90 They found that the dendrimers with cationic groups enter into cells with a faster rate than both neutral and anionic ones. Yu et al. also demonstrated that positively charged PLGA particles are taken up by human endothelial cells with a larger amount.91 Lorenz et al. reported that the cellular uptake increases along with the increase of positive charge density to some extent.92 However, a high positive charge can promote aggregation of the particles in cell culture medium, and thus inhibit the cellular uptake.
The surface charge not only governs the uptake rate and amount but also the uptake mechanism. Harush-Frenkel et al. found that positively charged PEGylated polylactide (PLA) particles show a rapid uptake by the clathrin-mediated pathway, while negatively charged ones show an inferior uptake rate and do not utilize the same endocytosis pathway.93 Moreover, the positively charged particles cause nonspecific adhesion to the cell membrane, but the negatively and near-neutral charged ones decrease the non-specific adsorption and favor specific cell recognition and internalization, for example, ligand-mediated uptake.94
Various ligands can be conjugated to the colloidal particle surface for targeting cancer cells,95 intracellular organelles and even the nucleus.96 The targeting ligands not only facilitate the uptake process by receptor-mediated endocytosis, but also reduce the adverse effects by direct transportation of the particles to the desired sites. The frequently used ligands include antibodies, proteins, aptamers, peptides, cell growth factors, and other small molecules.97 For example, decoration of paclitaxel-loaded PLGA NPs with human epidermal growth factor receptor-2 (HER2) antibody, trastuzumab, obtains multifunctional carriers that target cancer cells, such as Caco-2 cells and SK-BR-3 cells (human Caucasian breast adenocarcinoma cells).98 Some proteins such as transferrin are widely used to target NPs to those cells that over express transferrin receptors on cell membranes, e.g. HeLa K44A cells (expression of K44A-dynamin in the HeLa cells).99,100 Silica particles decorated with Tat peptide, a cell penetrating peptide (CPP) derived from HIV-1 protein, has the capability to penetrate the non-phagocytic cell membrane and target the nucleus.101 A cyclic arginine–glycine–aspartic acid (cRGD) peptide can bind to integrins, which are abundant on the membranes of various cells, and thus can be used to specifically deliver the colloidal particles.100 Recently, aptamers, which are oligonucleic acids or peptide molecules that bind to specific target molecules on cancer cells, have been used to decorate colloidal particles and show potential applications in cancer therapy.102 For example, AS1411, a 26-mer DNA aptamer with high binding affinity to nucleolin, which is over expressed on the plasma membrane of tumor cells, was immobilized on multilayer-modified bovine serum albumin (BSA) NPs, leading to liver cancer cell targeting and enhanced cellular uptake.103 Folic acid is a well known targeting molecule for many kinds of cancer cells in which folate receptors are frequently over expressed. Therefore, folic acid decoration can significantly promote the uptake of PLGA particles by HepG2 cells.104 Recently, anchorage of two or more targeting ligands to obtain multifunctional particles has attracted much attention. Tkachenko et al. decorated gold colloidal particles with a cell-targeting peptide (from an adenoviral receptor-mediated endocytosis peptide) and a nucleus-targeting peptide (from an adenoviral nuclear localization signal peptide).96 The gold colloidal particles bearing these two types of peptides have a greater efficiency for nuclear targeting than any single peptide explored up to now.
2.4. Mechanical properties
Recent studies have demonstrated that cells can sense external physical cues including substrate stiffness, showing different cell behaviors.105 Since endocytosis involves several mechanical steps that can induce deformation on the cargo, mechanical properties of the particles may also influence on the cell uptake. Banquy et al. studied the uptake of ∼200 nm hydrogel particles of N,N-diethyl acrylamide and 2-hydroxyethyl methacrylate copolymers with a different modulus by macrophages.106 The stiffness of the particles affects both the uptake efficiency and the entry pathways. Softer and stiffer particles are internalized via macropinocytosis and a clathrin-dependent mechanism, respectively, while those with an intermediate Young's modulus are taken up via multiple mechanisms. Liu et al. demonstrated that the stiffness of particles with a size of ∼800 nm influences the uptake efficiency as well.107 In a recent work, Yi et al. touched on this problem using a theoretical model. Their calculation shows that stiffer particles can achieve full wrapping more easily than softer ones, and thereby experience smaller energy changes during wrapping.43 The effect on mechanical properties is still in its infant stage and needs to be consolidated further.3. Intracellular distribution and transportation
In general, colloidal particles are internalized into early endosomes created by endocytosis after cellular uptake.108 In some cases, these early endosomes can rapidly mature to late endosomes and then fuse with either other late endosomes or lysosomes, leading to degradation of the particles due to an acidic environment in the presence of various enzymes.109–112 In other cases, the colloidal particles can escape from endosomes/lysosomes and then interact with other cell organelles.17 Understanding of the intracellular transportation of the colloidal particles is strongly tied with their specific applications such as gene and drug delivery as well as impacts on cell functions. For example, cell damage is induced by NPs accumulation in the lysosomal compartment. Typical lysosomal pathologies upon accumulation include phospholipidosis and lysosomal overload with morphological changes and autophagy, leading to cell apoptosis or necrosis.113–116 Release of starch-coated silver NPs into cytosol and their interactions with mitochondrion and the nucleus are responsible for the mitochondrial toxicity and DNA damage.117The intracellular distribution of particles is strongly affected by the entry routes. Certain internalization pathways such as clathrin-mediated endocytosis may lead to a complete degradation of colloidal particles at the later lysosomal stage.118 Since particle properties have a large influence on the uptake pathways, they are supposed to impact on intracellular distribution and fate as well. Results from the recent researches prove that the properties of colloidal particles, including chemical composition, size, surface charge, hydrophobicity and even the ligand arrangement, can influence their subcellular distribution and transportation.36,41,44,119–121 The common intracellular compartments are early endosomes, late endosomes, lysosomes, mitochondria, endoplasmic reticulum (ER) and the Golgi complex.16
3.1. Size
Particle size has a large impact on the intracellular distribution, especially the entrance of cell nucleus, which is a dominant step for gene delivery. Oh et al. examined PEG-functionalized gold NPs (AuNPs) whose diameter ranges from 2.4 nm to 89 nm. Only the 2.4 nm AuNPs are localized in the nucleus of the COS-1 cell. The 5.5 and 8.2 nm AuNPs are partially delivered into the cytoplasm, and partially remained at the cell membrane, whereas the 16 nm and larger AuNPs are located at the cellular periphery.121 Chithrani et al. showed that 50 nm colloidal gold NPs are clustered exclusively within either endosomes or lysosomes with an average diameter of 300–500 nm.122 Muñoz Javier et al. reported that microparticles cannot enter the nucleus of NIH 3T3 (the mouse embryonic fibroblast cell line) cells and MCF7 (human breast adenocarcinoma cell line) cells, but can be found inside the endosomes and lysosomes as well as on the cell membranes.110 Hu et al. found that silica particles with a diameter ranging from 60 nm to 600 nm distribute inside the cytoplasms and lysosomes, and on the cell membranes.97 Although carbon nanotubes always have a larger length, Pantarotto et al. demonstrated that they can penetrate into the cell nucleus because of their small diameter.123 The functional size of the nuclear pores (or more specifically the central transport channel) is variable from 10 nm to about 30 nm depending on the physiological state of the cells.124 Therefore, it is reasonable that only those extremely small particles can penetrate into the nucleus. However, sometimes surface-modified particles with a larger size can also be found inside the cell nucleus. For example, Mao et al. observed 200 nm silica particles decorated with Tat peptide can enter into the nucleus of HepG2 cells.101 The particles might enter the cell nucleus during the cell duplication process.3.2. Surface chemistry
The surface chemistry can influence the intracellular distribution and fate of the particles (Fig. 5).125 Nam et al. showed that hydrophobically modified glycol chitosan (HGC) particles can be entrapped in lysosomes and late endosomes, but less in the endoplasmic reticulum region.112 Nativo et al. found that the majority of citrate- or CALNN (cysteine-alanine-leucine-asparagine-asparagine) pentapeptide-protected gold particles ends in the endosomes, with only a few ones dispersed freely in the cytosol.126 However, starch-coated silver NPs distribute throughout the cytoplasm and lysosomes, and can co-localize inside the mitochondria and nuclei of normal human lung fibroblasts and human glioblastoma cells.117 Panyam et al. showed that PLGA particles can escape endo-lysosomal rapidly because of their selective surface charge reversal in the acidic endo-lysosomes, and then deliver their payload in the cytoplasm.127 In another case, pH sensitive, membrane disruption polymers such as poly(propylacrylic acid) (PPAA) have been used to enhance endosomal escape.128–130 | ||
| Fig. 5 Use of electron microscopy to determine the uptake and subcellular localization of NPs. (A) Untreated RAW 264.7 cells. Cells were treated with 10 μg mL−1 (B) COOH-PS nanospheres, and (C) NH2-PS nanospheres for 16 h.125 Labels: M = mitochondria, P = particles. Anionic and cationic PS nanospheres are responsible for contrasting morphological changes. Carboxylated PS nanospheres were taken up in loose-fitting phagosomes, with preservation of the mitochondria architecture. In contrast, NH2-PS nanospheres could be seen to collect in large membrane-bound vacuoles and the nuclei of cells that showed the disappearance of mitochondria. This suggests that the cationic particles could enter an endocytic compartment that targets mitochondria. Reprinted with permission from ref. 125. Copyright 2006, American Chemical Society. | ||
Another more widely acknowledged endosomal escape strategy is to use cationic polymers in particular polyamines such as poly(ethylene imine) (PEI). They present a high density of amine groups which can absorb protons generated by ATPase in the endosomes/lysosomes. This buffering effect can slow down the process of acidification, with a consequent increase of the endosomal osmotic pressure. Such an effect is commonly known as the “proton sponge effect”, i.e. the increase of ions within the endosomes causes osmolysis and consequent release of their contents into the cytosol.131,132 This strategy is widely used in the design of gene delivery vectors. Duan and Nie showed that luminescent quantum dots coated with hyperbranched copolymer ligands, PEI-g-PEG, are rapidly internalized by endocytosis, and are initially stored in vesicles, followed by endosomal escape and release into the cytoplasm.133
Several groups have demonstrated that surface decoration of liposomes or cell-penetrating peptides on colloidal particles can avoid the endosomal pathway.126 For example, the positively charged lipids can not only interact with DNA to form a complex, but also enable the binding and fusion of the complex to the cell and the plasma membrane.134 The introduction of Tat peptide or nuclear location signal (NLS) peptide enables the penetration of particles into the cell nucleus.135,136
Besides the accumulation of particles inside cells, a fraction of particles might be exocytosized from cells. Luciani et al. showed that magnetic NPs are released by monocytes or macrophages with membrane vesicles upon stress or activation.137 Panyam and Labhasetwar demonstrated that exocytosis of 100 nm PLGA NPs by vascular smooth muscle cells occurs when the extracellular NPs concentration gradient is removed, with about 65% of the internalized fraction undergoing exocytosis in 30 min.138 Although the exocytosis process is dependent on the size and shape of the NPs, it yields a different trend compared to the endocytosis process.63 Smaller gold NPs are exocytosized at a faster rate and at a higher percentage than their larger counterparts. A few numbers of proteins and receptors on smaller NPs facilitate faster processing, leading to a higher rate of exocytosis compared to larger ones. Slowing et al. also demonstrated that HeLa and HUVEC cells have significant differences in expelling MSNs.139
4. The influences of colloidal particles on cell functions
4.1. Acute toxicity
Internalization of the colloidal particles may cause various impacts on cells (Fig. 6).140 One of the greatest concerns is their toxicity, which is decisive for many biomedical applications. The cellular uptake of particles may bring changes on cell cycle, proliferation, ROS production and mitochondrion health, cell skeleton organization and morphology, protein synthesis, and even cell death. | ||
| Fig. 6 Mechanism of cytotoxicity induced by industrial nanoparticles.194 Reprinted with permission from ref. 194. Copyright 2012, American Chemical Society. | ||
One reason for the adverse effects is the chemical composition of the particles, which may release toxins when interacting with cells. Peters et al. evaluated the effects of five different nano-scaled particles (PVC, TiO2, SiO2, Co, Ni) on endothelial cells, and found that only the Co particles possess cytotoxic effects.141 The QDs can release Cd2+ ions and subsequently produce ROS to induce cytotoxicity in a biological environment.142 Mitochondria are the main sources of cellular ROS and the basis of the ROS metabolism. Maintenance of ROS homeostasis depends on the respiratory chain and the membrane potential.143,144 Damage to the mitochondrial inner membrane increases ROS production, which in turn causes lysosomal disruption.145 The ZnO nanowires are found to release Zn2+ ions in cells and thus enhance the intracellular ROS level, destroying mitochondrial integrity and inducing cell death.146 In addition, the chemicals adsorbed on their surfaces may affect the reactivity of particles. Fractions isolated from particulate air pollutants (diesel exhaust particles) exert toxic effects on cells in vitro.147 Particles in ambient air can have very complex compositions. For example, metallic irons are able to potentiate the effect of carbon black NPs, resulting in enhanced reactivity including oxidative stress.148 Despite the great achievements, more effort should be made to clarify the various effects.
The most important reason for the adverse effects is the small size of the particles, since in many cases the bulk materials are bioinert and even biocompatible. It is well known that during the internalization process the particles will disrupt the integrity of the cellular membrane barriers.120 For example, during internalization of gelatin NPs the membrane of human fibroblasts is disrupted and the cytoskeleton is disorganized, although the cell viability and morphology are not apparently altered.149 Another consequence of the small size is the relatively huge surface area, which dominates the interactions between particles and cells. Magrez et al. demonstrated that carbon-based nanomaterials have size-dependent toxicity to H596 lung tumor cells.150 Pan et al. showed that gold NPs with a diameter of 1.4 nm and capped with triphenylphosphine monosulfonate (TPPMS) are much more cytotoxic than 15 nm ones by increasing intracellular oxidative stress.151 Besides size, the shape of the particles also plays an important role in determining their effects on cells. Results show that the CNTs with a smaller aspect ratio have less cytotoxicity.150
The toxicity of the particles can be mediated by surface chemistry. Some previous studies have shown that the particles with cationic surface coatings can disrupt the cell plasma membrane by generating transient holes, leading to cell death because of losing membrane polarization and/or leakage of ions and molecules.152 For example, Zhu et al. found that poly(L-lysine) (PLL)-modified silica particles increased the cytotoxicity for human nasopharyngeal carcinoma cells.153 However, the particles decorated with biological membrane-penetrating molecules (for example, cell-penetrating peptides) can penetrate cell membranes but do not elicit overt membrane poration and cell death.154,155 Colloidal particles with surface modifications by biocompatible polymers or proteins reduce their harmful effects to cells.156 Mao et al. demonstrated that bare gold particles cause higher toxicity and disorganization of the cytoskeleton of ECV-304 cells, while poly(ε-caprolactone)-coated gold particles with the same dosage do not bring an apparent influence on cells.157 Gupta and Gupta compared the toxicity of superparamagnetic iron oxide NPs (SPION) and pullulan-coated SPION (Pn-SPION) to human fibroblasts. The former ones are toxic and their internalization results in disruption of the cytoskeleton organization by disrupting the microtubule structures in the central and peripheral domains of the cells, whereas the latter are non-toxic but also induce the formation of free tubulin compared to the control cells.158
It should be further mentioned that the level of cytotoxicity is dependent on the cell types too. Xia et al. found that 60 nm NH2-labeled PS nanospheres are highly toxic to macrophages and epithelial cells, but do not influence the viability of human microvascular endothelial, hepatoma, and pheochromocytoma (PC-12) cells.75
The pro-inflammatory responses induced by colloidal particles have been regarded as one of the toxic mechanisms. Baierl et al. found that fullerenes reduce the viability of bovine alveolar macrophages and enhance the production of tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6) and interleukin 8 (IL-8).159 Silica NPs can induce the mRNA expression of pro-inflammatory cytokines such as IL-1β, TNF-α and IL-6 or other inflammatory-related enzymes such as inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) in a time-dependent manner in peritoneal macrophage.160 Shibata et al. showed that chitin particles and mannan-coated latex beads induce the production of IL-12, TNF-α and INF-α by spleen cells, while other particles such as laminarin-coated latex beads and soluble mannan not, because this production is linked to the recognition and ingestion of particles through the mannose receptor.161 In contrast, when the gold NPs are capped with lysine and polylysine, they are not cytotoxic to RAW 264.7 macrophage cells. The production of ROS and nitrite species is reduced, and the pro-inflammatory cytokines such as TNF-α and IL1-β are not secreted.162
4.2. Substantial influence on cell functions
The adverse effects of uptake of colloidal particles do not necessarily cause acute toxicity such as cell death. They may induce more substantial impacts on cell functions such as alternation of cell adhesion and migration abilities, cell differentiation, long term genotoxicity, and so on.The cell adhesion and migration are important cellular events related to the cytoskeleton.148,163 Since the cytoskeleton organization may be influenced by the ingested particles, these two cellular events may also be influenced. Qi et al. reported that the HepG2 cells incubated with silicon nanowires exhibit a weaker adherence ability to the substrates.164 This impact is dominated by several parameters of the particles such as size, shape, stiffness and surface chemistry. Zhang et al. compared the impacts of 80 nm and 500 nm silica particles on the adhesion ability of human dermal fibroblasts, and demonstrated that particles with a larger size cause more impedance on the cell adhesion and spreading (Fig. 7A).48 Huang et al. found that cell adhesion is greatly weakened as a result of nonspecific cellular uptake of short and long rod-shaped MSNs, but is not affected by the stimulus of sphere-shaped MSNs.71 Recently, Liu et al. suggested that the cell adhesion ability is significantly affected after ingestion of poly(2-hydroxyethyl methacrylate) (PHEMA) hydrogel particles, especially after uptake of the stiffer ones.107 Moreover, Yu et al. found that the positively charged PLGA particles have a larger impedance on the cell adhesion ability.91
 | ||
| Fig. 7 (A) Relative cell adhesion ability, (B) cell migration ability, and (C) gene expression of fibronectin, laminin and FAK of human dermal fibroblasts. The data are normalized to that of the control.48 The fibroblasts were cultured without or with RITC–SiO2 particles of different diameters for 10 h. Asterisk indicates significant difference at p < 0.05. Reprinted with permission from ref. 48. Copyright 2010, Elsevier. | ||
Pan et al. demonstrated that TiO2 particles can significantly inhibit the mobility of human dermal fibroblasts.165 Zhang et al. showed that silica particles also have this effect due to the alternation of gene expression of adhesion and migration-related proteins (Fig. 7).48 Besides, this impact is dependent on the surface charge of particles. Yu et al. suggested that the positively charged PLGA particles can cause a larger impedance on the mobility of human endothelial cells.91 However, no inhibitory effect is observed for A375 human melanoma cells after treatment with MSNs. On the contrary, the migration rate of cells treated with sphere-shaped MSNs is remarkably faster compared to that of the untreated cells.71 The cell adhesion, migration and cytoskeleton organization after uptake of the particles are not only closely tied with but also influenced by each other. However, they are not well addressed up to now, and thereby more systematic studies should be performed to thoroughly elucidate the cellular behaviors and intrinsic mechanisms.
The cell phonotype may also be affected after being exposed to colloidal particles. Exposure of MC3T3-E1 (mouse osteoblast-like cells) to polymethylmethacrylate (PMMA) particles can significantly reduce their mineralization ability.166 Pisanic et al. found that the influence of anionic iron oxide on PC-12 cells is dose-dependent. Uptake of the particles decreases the cells’ capacity of neurites in response to the nerve growth factor.167 Kostura et al. observed that uptake of SPION inhibits the adipogenic and osteogenic differentiation of human mesenchymal stem cells.168
Since the colloidal particles can penetrate subcellular structures such as mitochondria169 and cell nuclei,170 they may cause potential genotoxicity too. Kim et al. found that silica NPs can cause the breakage of DNA-double strands of human neuronal cells.171 Rim et al. demonstrated that the genotoxicity of carbon blacks are elevated when their size becomes smaller or after their surface is decorated with methylcyclohexane.172
More and more evidence suggests that the influence on cell functions by the particle uptake should be more carefully studied. Jiang et al. demonstrated that gold and silver NPs coated with antibodies can regulate the process of membrane receptor internalization in a particle size-dependent manner. The binding and activation of membrane receptors in turn alters the downstream signaling and subsequent cellular responses.40,173 Moreover, different cell types may respond differently to the same particles, making the problem more complicated. Expression array analysis and phenotypic measurements of exposed cell populations may yield high throughput and systematic evaluation of the changes of cell functions, and thereby provide insight into the mechanisms responsible for adverse effects.40 Ding et al. reported on whole-genome expression analysis of human fibroblasts exposed to multi-walled carbon nano-onions versus multi-walled carbon nanotubes.174 Exposure to these nanomaterials brings perturbation of multiple cellular pathways, and determines material-specific toxicogenomic profiles. Waters et al. conducted whole-genome microarray analyses of the RAW 264.7 murine macrophage cells exposed to amorphous silica NPs, and found that cellular responses are highly conserved across particle sizes.175 Wang et al. found that 886 genes were down-regulated and 758 genes were up-regulated more than 2 folds after the smooth muscle cells were exposed to multilayer microcapsules.176 Such “genomic footprinting” approaches, combined with proteomics-based safety evaluation of the nanomaterials, can ultimately aid in the assessment and prediction of the impacts of colloidal particles on cells.177,178
5. Perspectives of the cellular interaction
The cellular uptake, intracellular distribution and cell responses provide a practical way to explore the interactions between colloidal particles and biological systems, which in turn can guide the design and applications of functional colloidal particles. Although many results have been obtained, some important issues are remained to be clarified and substantiated.Very little attention has been paid to the alternation of particles inside cells, although it is of paramount importance for the biological performance of most types of delivery carriers. For example, the surface and bulk chemistry of the particles might be altered due to the adsorption of proteins and partial degradation in the presence of various enzymes.144 Besides, the interactions between cellular organelles, intracellular molecules and the particles are not thoroughly studied, leading to an insufficient understanding of the impacts on cell functions and related mechanisms. Therefore, novel detection methodologies, such as confocal Raman microscopy, fluorescence resonance energy transfer (FRET),179 total internal reflection microscopy (TIRM)180 and fluorescence correlation spectroscopy181,182 are encouraged to be used for real-time imaging of intracellular events. For example, the intracellular distribution and degradation of PLGA particles inside cells can be followed by confocal Raman microscopy.183,184 FRET is achieved between cy®5-labeled gene medicine and PEI-conjugated QDs in the complex.185 From confocal microscopic analysis, intracellular uptake and release of genes from the complex are visualized as a function of incubation time.186
The fate of colloidal particles after cellular uptake is another important issue. Generally, the colloidal particles have three possible fates after cellular uptake. The first one is degradation in cell lysosomes, which mostly occurs for those biodegradable particles. Another one is exocytosis, the reverse process of endocytosis. The particles can be partially degraded to make them smaller, and then escape from the cells via exocytosis.187,188 However, the non-degradable colloidal particles cannot be degraded or expelled easily due to their excellent stability and relatively large sizes, leading to the third fate of the particles, i.e. accumulation inside cells and in turn adverse effects on cell functions. For example, the accumulation of gold NPs can inhibit the proliferation of multiple myeloma cells through cell-cycle arrest in the G1 phase by up-regulation of cell-cycle inhibitor proteins.189 Therefore, long term cytotoxicity should be evaluated and the comprehensive effects of colloidal particles should be elucidated. Another important issue is where the particles are accumulated when the cells proliferate: whether they will divide equally into two daughter cells as other cell organelles or just remain in one daughter cell.
Finally, the widely applied 2D cell culture models may not adequately represent the functions of 3D tissues that have extensive cell–cell and cell–matrix interactions, as well as markedly different diffusion/transport conditions.190 Therefore, a cytotoxicity assay in 2D culture may not accurately reflect the actual toxicity of colloidal biomaterials in the body. To obtain more adequate and detailed information about colloidal biomaterials–tissue interactions, a 3D cell culture-based toxicology evaluation system is very superior.191 Moreover, an important obstacle to understand the in vivo toxicity of the colloidal biomaterials is that the direct measurement of dosage of particles in different tissues is often difficult or costly. By contrast, quantification of the cellular uptake amount of particles in vitro is much easier, allowing the correlation of the uptake ratio with the particle characteristics (particle composition, size, morphology, concentration, and surface properties). This strategy facilitates to clarify the dose-dependent effect, enriching the basis for comparative toxicity assessment of particles.192
Acknowledgements
Financial support by the Natural Science Foundation of China (no. 51120135001, 20934003 and 51003094), the National Basic Research Program of China (2011CB606203), the Frame Work Program 7 of European Commission (HINAMOX NMP4-SL-2009-228825), Open Project of State Key Laboratory of Supramolecular Structure and Materials (sklssm201303) and Research Project of Department of Education of Zhejiang Province (Z201018687).References
- J. R. Lead and K. J. Wilkinson, Environ. Chem., 2006, 3, 159 CrossRef CAS
.
- C. Graf and A. van Blaaderen, Langmuir, 2002, 18, 524 CrossRef CAS
.
- M. G. Lines, J. Alloys Compd., 2008, 449, 242 CrossRef CAS
.
- Y. Hong, C. Gao, Y. Xie, Y. Gong and J. Shen, Biomaterials, 2005, 26, 6305 CrossRef CAS
.
- H. Park, J. Yang, J. Lee, S. Haam, I. H. Choi and K. H. Yoo, ACS Nano, 2009, 3, 2919 CrossRef CAS
.
- G. Yordanov, N. Kaneva and C. Dushkin, Colloid Polym. Sci., 2009, 287, 733 CAS
.
- G. Lambert, E. Fattal, H. Pinto-Alphandary, A. Gulik and P. Couvreur, Pharm. Res., 2000, 17, 707 CrossRef CAS
.
- K. A. Janes, P. Calvo and M. J. Alonso, Adv. Drug Delivery Rev., 2001, 47, 83 CrossRef CAS
.
- K. Sokolov, M. Follen, J. Aaron, I. Pavlova, A. Malpica, R. Lotan and R. Richards-Kortum, Cancer Res., 2003, 63, 1999 CAS
.
- I. H. El-Sayed, X. Huang and M. A. El-Sayed, Cancer Lett., 2006, 239, 129 CrossRef CAS
.
- L. Fan, F. Li, H. Zhang, Y. Wang, C. Cheng, X. Li, C. h. Gu, Q. Yang, H. Wu and S. Zhang, Biomaterials, 2010, 31, 5634 CrossRef CAS
.
- M. V. Yezhelyev, X. Gao, Y. Xing, A. Al-Hajj, S. Nie and R. M. O'Regan, Lancet Oncol., 2006, 7, 657 CrossRef CAS
.
- H. F. Krug and P. Wick, Angew. Chem., Int. Ed., 2011, 50, 1260 CrossRef CAS
.
- B. Nowack and T. D. Bucheli, Environ. Pollut., 2007, 150, 5 CrossRef CAS
.
- J. Pan and S. S. Feng, Biomaterials, 2008, 29, 2663 CrossRef CAS
.
- S. D. Conner and S. L. Schmid, Nature, 2003, 422, 37 CrossRef CAS
.
- M. S. Cartiera, K. M. Johnson, V. Rajendran, M. J. Caplan and W. M. Saltzman, Biomaterials, 2009, 30, 2790 CrossRef CAS
.
- D. Maysinger, J. Lovrić, A. Eisenberg and R. Savić, Eur. J. Pharm. Biopharm., 2007, 65, 270 CrossRef CAS
.
- J. Panyam and V. Labhasetwar, Adv. Drug Delivery Rev., 2003, 55, 329 CrossRef CAS
.
- D. Garry, R. Sorenson and H. Coulter, Diabetologia, 1987, 30, 115 CAS
.
- J. Lovrić, H. S. Bazzi, Y. Cuie, G. R. A. Fortin, F. M. Winnik and D. Maysinger, J. Mol. Med., 2005, 83, 377 CrossRef
.
- L. Lacerda, A. Bianco, M. Prato and K. Kostarelos, J. Mater. Chem., 2008, 18, 17 RSC
.
- W. Meng, T. L. Parker, P. Kallinteri, D. A. Walker, S. Higgins, G. A. Hutcheon and M. C. Garnett, J. Controlled Release, 2006, 116, 314 CrossRef CAS
.
- A. N. Zelikin, K. Breheney, R. Robert, E. Tjipto and K. Wark, Biomacromolecules, 2010, 11, 2123 CrossRef CAS
.
- S. M. Hussain, L. K. Braydich-Stolle, A. M. Schrand, R. C. Murdock, K. O. Yu, D. M. Mattie, J. J. Schlager and M. Terrones, Adv. Mater., 2009, 21, 1549 CrossRef CAS
.
- C. M. Sayes, R. Wahi, P. A. Kurian, Y. Liu, J. L. West, K. D. Ausman, D. B. Warheit and V. L. Colvin, Toxicol. Sci., 2006, 92, 174 CrossRef CAS
.
- H. Yin, P. S. Casey, M. J. McCall and M. Fenech, Langmuir, 2010, 26, 15399 CrossRef CAS
.
- P. Sanpui, A. Chattopadhyay and S. S. Ghosh, ACS Appl. Mater. Interfaces, 2011, 3, 218 CAS
.
- P. H. Danielsen, P. Møller, K. A. Jensen, A. K. Sharma, H. K. Wallin, R. Bossi, H. Autrup, L. Mølhave, J.-L. Ravanat, J. J. Briedé, T. M. de Kok and S. Loft, Chem. Res. Toxicol., 2011, 24, 168 CrossRef CAS
.
- H. Hillaireau and P. Couvreur, Cell. Mol. Life Sci., 2009, 66, 2873 CrossRef CAS
.
- A. França, P. Aggarwal, E. V. Barsov, S. V. Kozlov, M. A. Dobrovolskaia and Á. González-Fernández, Nanomedicine, 2011, 6, 1175 CrossRef
.
- M. Rabinovitch, Trends Cell Biol., 1995, 5, 85 CrossRef CAS
.
- M. Zhu, G. Nie, H. Meng, T. Xia, A. Nel and Y. Zhao, Acc. Chem. Res., 2013, 46, 622 CrossRef CAS
.
- B. D. Chithrani, A. A. Ghazani and W. C. W. Chan, Nano Lett., 2006, 6, 662 CrossRef CAS
.
- H. Yuan, J. Li, G. Bao and S. Zhang, Phys. Rev. Lett., 2010, 105, 138101 CrossRef
.
- S. Tzlil, M. Deserno, W. M. Gelbart and A. Ben-Shaul, Biophys. J., 2004, 86, 2037 CrossRef CAS
.
- G. Bao and X. R. Bao, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 9997 CrossRef CAS
.
- H. Hillaireau and P. Couvreur, Cell. Mol. Life Sci., 2009, 66, 2873 CrossRef CAS
.
- D. L. Thorek and A. Tsourkas, Biomaterials, 2008, 29, 3583 CrossRef CAS
.
- W. Jiang, B. Y. Kim, J. T. Rutka and W. C. Chan, Nat. Nanotechnol., 2008, 3, 145 CrossRef CAS
.
- S. Zhang, J. Li, G. Lykotrafitis, G. Bao and S. Suresh, Adv. Mater., 2009, 21, 419 CrossRef CAS
.
- C. Abhishek, B. Giuseppe and G. Ramin, Phys. Biol., 2011, 8, 046002 CrossRef
.
- X. Yi, X. Shi and H. Gao, Phys. Rev. Lett., 2011, 107, 098101 CrossRef
.
- P. Decuzzi and M. Ferrari, Biomaterials, 2007, 28, 2915 CrossRef CAS
.
- T. Nakai, T. Kanamori, S. Sando and Y. Aoyama, J. Am. Chem. Soc., 2003, 125, 8465 CrossRef CAS
.
- D. A. Giljohann, D. S. Seferos, P. C. Patel, J. E. Millstone, N. L. Rosi and C. A. Mirkin, Nano Lett., 2007, 7, 3818 CrossRef CAS
.
- F. Osaki, T. Kanamori, S. Sando, T. Sera and Y. Aoyama, J. Am. Chem. Soc., 2004, 126, 6520 CrossRef CAS
.
- Y. Zhang, L. Hu, D. Yu and C. Gao, Biomaterials, 2010, 31, 8465 CrossRef CAS
.
- D. Luo, E. Han, N. Belcheva and W. M. Saltzman, J. Controlled Release, 2004, 95, 333 CrossRef CAS
.
- K. Yin Win and S. S. Feng, Biomaterials, 2005, 26, 2713 CrossRef
.
- A. Natarajan, C. Gruettner, R. Ivkov, G. L. DeNardo, G. Mirick, A. Yuan, A. Foreman and S. J. DeNardo, Bioconjugate Chem., 2008, 19, 1211 CrossRef CAS
.
- M. Qaddoumi, H. Ueda, J. Yang, J. Davda, V. Labhasetwar and V. Lee, Pharm. Res., 2004, 21, 641 CrossRef CAS
.
- G. F. Dawson and G. W. Halbert, Pharm. Res., 2000, 17, 1420 CrossRef CAS
.
- E. C. Cho, Q. Zhang and Y. Xia, Nat. Nanotechnol., 2011, 6, 385 CrossRef CAS
.
- L. Wang, Y. Liu, W. Li, X. Jiang, Y. Ji, X. Wu, L. Xu, Y. Qiu, K. Zhao, T. Wei, Y. Li, Y. Zhao and C. Chen, Nano Lett., 2011, 11, 772 CrossRef CAS
.
- K. Furumoto, K. Ogawara, M. Yoshida, Y. Takakura, M. Hashida, K. Higaki and T. Kimura, Biochim. Biophys. Acta, Gen. Subj., 2001, 1526, 221 CrossRef CAS
.
- W. Zauner, N. A. Farrow and A. M. Haines, J. Controlled Release, 2001, 71, 39 CrossRef CAS
.
- R. R. Nepomuceno and A. J. Tenner, J. Immunol., 1998, 160, 1929 CAS
.
- R. Goldman, Exp. Cell Res., 1977, 104, 325 CrossRef CAS
.
- U. S. Ryan, D. R. Schultz, J. D. Goodwin, J. M. Vann, M. P. Selvaraj and M. A. Hart, Infect. Immun., 1989, 57, 1356 CAS
.
- Y. Hu, J. Xie, Y. W. Tong and C. H. Wang, J. Controlled Release, 2007, 118, 7 CrossRef CAS
.
- Y. Geng, P. Dalhaimer, S. Cai, R. Tsai, M. Tewari, T. Minko and D. E. Discher, Nat. Nanotechnol., 2007, 2, 249 CrossRef CAS
.
- B. D. Chithrani and W. C. Chan, Nano Lett., 2007, 7, 1542 CrossRef CAS
.
- K. Kostarelos, L. Lacerda, G. Pastorin, W. Wu, S. Wieckowski, J. Luangsivilay, S. Godefroy, D. Pantarotto, J. P. Briand, S. Muller, M. Prato and A. Bianco, Nat. Nanotechnol., 2007, 2, 108 CrossRef CAS
.
- B. C. Heng, X. Zhao, E. C. Tan, N. Khamis, A. Assodani, S. Xiong, C. Ruedl, K. W. Ng and J. S. Loo, Arch. Toxicol., 2011, 85, 1517 CrossRef CAS
.
- Y. Okamura, Y. Fukui, K. Kabata, H. Suzuki, M. Handa, Y. Ikeda and S. Takeoka, Bioconjugate Chem., 2009, 20, 1958 CrossRef CAS
.
- H. Yue, W. Wei, Z. Yue, B. Wang, N. Luo, Y. Gao, D. Ma, G. Ma and Z. Su, Biomaterials, 2012, 33, 4013 CrossRef CAS
.
- Q. Mu, G. Su, L. Li, B. O. Gilbertson, L. H. Yu, Q. Zhang, Y.-P. Sun and B. Yan, ACS Appl. Mater. Interfaces, 2012, 4, 2259 CAS
.
- Y. Qiu, Y. Liu, L. Wang, L. Xu, R. Bai, Y. Ji, X. Wu, Y. Zhao, Y. Li and C. Chen, Biomaterials, 2010, 31, 7606 CrossRef CAS
.
- B. G. Trewyn, J. A. Nieweg, Y. Zhao and V. S. Y. Lin, Chem. Eng. J., 2008, 137, 23 CrossRef CAS
.
- X. Huang, X. Teng, D. Chen, F. Tang and J. He, Biomaterials, 2010, 31, 438 CrossRef CAS
.
- F. E. Alemdaroglu, N. C. Alemdaroglu, P. Langguth and A. Herrmann, Macromol. Rapid Commun., 2008, 29, 326 CrossRef CAS
.
- S. E. A. Gratton, P. A. Ropp, P. D. Pohlhaus, J. C. Luft, V. J. Madden, M. E. Napier and J. M. DeSimone, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11613 CrossRef CAS
.
- H. Jin, D. A. Heller and M. S. Strano, Nano Lett., 2008, 8, 1577 CrossRef
.
- T. Xia, M. Kovochich, M. Liong, J. I. Zink and A. E. Nel, ACS Nano, 2008, 2, 85 CrossRef CAS
.
- S. M. Moghimi, P. Symonds, J. C. Murray, A. C. Hunter, G. Debska and A. Szewczyk, Mol. Ther., 2005, 11, 990 CrossRef CAS
.
- E. Hutter, S. Boridy, S. Labrecque, M. Lalancette-Hébert, J. Kriz, F. O. M. Winnik and D. Maysinger, ACS Nano, 2010, 4, 2595 CrossRef CAS
.
- A. S. Zahr, C. A. Davis and M. V. Pishko, Langmuir, 2006, 22, 8178 CrossRef CAS
.
- A. M. Torche, P. Le Corre, E. Albina, A. Jestin and R. Le Verge, J. Drug Targeting, 2000, 7, 343 CrossRef CAS
.
- M. Massignani, C. LoPresti, A. Blanazs, J. Madsen, S. P. Armes, A. L. Lewis and G. Battaglia, Small, 2009, 5, 2424 CrossRef CAS
.
- C. LoPresti, M. Massignani, C. Fernyhough, A. Blanazs, A. J. Ryan, J. Madsen, N. J. Warren, S. P. Armes, A. L. Lewis, S. Chirasatitsin, A. J. Engler and G. Battaglia, ACS Nano, 2011, 5, 1775 CrossRef CAS
.
- H. Lomas, I. Canton, S. MacNeil, J. Du, S. P. Armes, A. J. Ryan, A. L. Lewis and G. Battaglia, Adv. Mater., 2007, 19, 4238 CrossRef CAS
.
- Y. C. Chung, I. H. Chen and C. J. Chen, Biomaterials, 2008, 29, 1807 CrossRef CAS
.
- Y. Goto, R. Matsuno, T. Konno, M. Takai and K. Ishihara, Biomacromolecules, 2008, 9, 3252 CrossRef CAS
.
- M. Ukawa, H. Akita, T. Masuda, Y. Hayashi, T. Konno, K. Ishihara and H. Harashima, Biomaterials, 2010, 31, 6355 CrossRef CAS
.
- N. M. Zaki, A. Nasti and N. Tirelli, Macromol. Biosci., 2011, 11, 1747 CrossRef CAS
.
- I. C. a. G. Battaglia, Chem. Soc. Rev., 2012, 41, 2718 RSC
.
- M. Massignani, I. Canton, T. Sun, V. Hearnden, S. MacNeil, A. Blanazs, S. P. Armes, A. Lewis and G. Battaglia, PLoS One, 2010, 5, e10459 Search PubMed
.
- C. Murdoch, K. J. Reeves, V. Hearnden, H. Colley, M. Massignani, I. Canton, J. Madsen, A. Blanazs, S. P. Armes, A. L. Lewis, S. MacNeil, N. J. Brown, M. H. Thornhill and G. Battaglia, Nanomedicine, 2010, 5, 1025 CrossRef CAS
.
- O. P. Perumal, R. Inapagolla, S. Kannan and R. M. Kannan, Biomaterials, 2008, 29, 3469 CrossRef CAS
.
- D. Yu, Y. Zhang, X. Zhou, Z. Mao and C. Gao, Biomacromolecules, 2012, 13, 3272 CrossRef CAS
.
- M. R. Lorenz, V. Holzapfel, A. Musyanovych, K. Nothelfer, P. Walther, H. Frank, K. Landfester, H. Schrezenmeier and V. Mailander, Biomaterials, 2006, 27, 2820 CrossRef CAS
.
- O. Harush-Frenkel, N. Debotton, S. Benita and Y. Altschuler, Biochem. Biophys. Res. Commun., 2007, 353, 26 CrossRef CAS
.
- C. J. Sunderland, M. Steiert, J. E. Talmadge, A. M. Derfus and S. E. Barry, Drug Dev. Res., 2006, 67, 70 CrossRef CAS
.
- C. Sun, R. Sze and M. Zhang, J. Biomed. Mater. Res., Part A, 2006, 78, 550 CrossRef
.
- A. G. Tkachenko, H. Xie, D. Coleman, W. Glomm, J. Ryan, M. F. Anderson, S. Franzen and D. L. Feldheim, J. Am. Chem. Soc., 2003, 125, 4700 CrossRef CAS
.
- L. Hu, Z. W. Mao and C. Y. Gao, J. Mater. Chem., 2009, 19, 3108 RSC
.
- B. Sun, B. Ranganathan and S. S. Feng, Biomaterials, 2008, 29, 475 CrossRef CAS
.
- C. Tekle, B. v. Deurs, K. Sandvig and T. G. Iversen, Nano Lett., 2008, 8, 1858 CrossRef CAS
.
- X. Pan, J. Guan, J. W. Yoo, A. J. Epstein, L. J. Lee and R. J. Lee, Int. J. Pharm., 2008, 358, 263 CrossRef CAS
.
- Z. Mao, L. Wan, L. Hu, L. Ma and C. Gao, Colloids Surf., B, 2010, 75, 432 CrossRef CAS
.
- O. C. Farokhzad, J. Cheng, B. A. Teply, I. Sherifi, S. Jon, P. W. Kantoff, J. P. Richie and R. Langer, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 6315 CrossRef CAS
.
- L. Xie, W. Tong, D. Yu, J. Xu, J. Li and C. Gao, J. Mater. Chem., 2012, 22, 6053 RSC
.
- J. Zhou, G. Romero, E. Rojas, L. Ma, S. Moya and C. Gao, J. Colloid Interface Sci., 2010, 345, 241 CrossRef CAS
.
- F. Rehfeldt, A. J. Engler, A. Eckhardt, F. Ahmed and D. E. Discher, Adv. Drug Delivery Rev., 2007, 59, 1329 CrossRef CAS
.
- X. Banquy, F. Suarez, A. Argaw, J. M. Rabanel, P. Grutter, J. F. Bouchard, P. Hildgen and S. Giasson, Soft Matter, 2009, 5, 3984 RSC
.
- W. Liu, X. Zhou, Z. Mao, D. Yu, B. Wang and C. Gao, Soft Matter, 2012, 8, 9235 RSC
.
- Y. Yan, A. P. Johnston, S. J. Dodds, M. M. Kamphuis, C. Ferguson, R. G. Parton, E. C. Nice, J. K. Heath and F. Caruso, ACS Nano, 2010, 4, 2928 CrossRef CAS
.
- S. De Koker, B. G. De Geest, S. K. Singh, R. De Rycke, T. Naessens, Y. Van Kooyk, J. Demeester, S. C. De Smedt and J. Grooten, Angew. Chem., Int. Ed., 2009, 48, 8485 CrossRef CAS
.
- A. Muñoz Javier, O. Kreft, M. Semmling, S. Kempter, A. G. Skirtach, O. T. Bruns, P. del Pino, M. F. Bedard, J. Rädler, J. Käs, C. Plank, G. B. Sukhorukov and W. J. Parak, Adv. Mater., 2008, 20, 4281 CrossRef
.
- B. G. De Geest, R. E. Vandenbroucke, A. M. Guenther, G. B. Sukhorukov, W. E. Hennink, N. N. Sanders, J. Demeester and S. C. De Smedt, Adv. Mater., 2006, 18, 1005 CrossRef CAS
.
- H. Y. Nam, S. M. Kwon, H. Chung, S. Y. Lee, S. H. Kwon, H. Jeon, Y. Kim, J. H. Park, J. Kim, S. Her, Y. K. Oh, I. C. Kwon, K. Kim and S. Y. Jeong, J. Controlled Release, 2009, 135, 259 CrossRef CAS
.
- B. W. Neun and M. A. Dobrovolskaia, Methods Mol. Biol., 2011, 697, 237 CAS
.
- U. T. Brunk, J. Neuzil and J. W. Eaton, Redox Rep., 2001, 6, 91 CrossRef CAS
.
- X. Ma, Y. Wu, S. Jin, Y. Tian, X. Zhang, Y. Zhao, L. Yu and X. J. Liang, ACS Nano, 2011, 5, 8629 CrossRef CAS
.
- O. Zabirnyk, M. Yezhelyev and O. Seleverstov, Autophagy, 2007, 3, 278 CAS
.
- P. V. AshaRani, G. Low Kah Mun, M. P. Hande and S. Valiyaveettil, ACS Nano, 2009, 3, 279 CrossRef CAS
.
- A. M. Alkilany, P. K. Nagaria, C. R. Hexel, T. J. Shaw, C. J. Murphy and M. D. Wyatt, Small, 2009, 5, 701 CrossRef CAS
.
- V. C. Mosqueira, P. Legrand, A. Gulik, O. Bourdon, R. Gref, D. Labarre and G. Barratt, Biomaterials, 2001, 22, 2967 CrossRef CAS
.
- A. Verma, O. Uzun, Y. Hu, H. S. Han, N. Watson, S. Chen, D. J. Irvine and F. Stellacci, Nat. Mater., 2008, 7, 588 CrossRef CAS
.
- E. Oh, J. B. Delehanty, K. E. Sapsford, K. Susumu, R. Goswami, J. B. Blanco-Canosa, P. E. Dawson, J. Granek, M. Shoff, Q. Zhang, P. L. Goering, A. Huston and I. L. Medintz, ACS Nano, 2011, 5, 6434 CrossRef CAS
.
- B. D. Chithrani, J. Stewart, C. Allen and D. A. Jaffray, Nanomed.: Nanotechnol., Biol. Med., 2009, 5, 118 CrossRef CAS
.
- D. Pantarotto, J. P. Briand, M. Prato and A. Bianco, Chem. Commun., 2004, 16 RSC
.
- C. M. Feldherr, D. Akin and R. J. Cohen, J. Cell Sci., 2001, 114, 4621 CAS
.
- T. Xia, M. Kovochich, J. Brant, M. Hotze, J. Sempf, T. Oberley, C. Sioutas, J. I. Yeh, M. R. Wiesner and A. E. Nel, Nano Lett., 2006, 6, 1794 CrossRef CAS
.
- P. Nativo, I. A. Prior and M. Brust, ACS Nano, 2008, 2, 1639 CrossRef CAS
.
- J. Panyam, W. Z. Zhou, S. Prabha, S. K. Sahoo and V. Labhasetwar, FASEB J., 2002, 16, 1217 CrossRef CAS
.
- T. R. Kyriakides, C. Y. Cheung, N. Murthy, P. Bornstein, P. S. Stayton and A. S. Hoffman, J. Controlled Release, 2002, 78, 295 CrossRef CAS
.
- C. Kusonwiriyawong, P. van de Wetering, J. A. Hubbell, H. P. Merkle and E. Walter, Eur. J. Pharm. Biopharm., 2003, 56, 237 CrossRef CAS
.
- R. A. Jones, C. Y. Cheung, F. E. Black, J. K. Zia, P. S. Stayton, A. S. Hoffman and M. R. Wilson, Biochem. J., 2003, 372, 65 CrossRef CAS
.
- M. L. Patil, M. Zhang, O. Taratula, O. B. Garbuzenko, H. He and T. Minko, Biomacromolecules, 2009, 10, 258 CrossRef CAS
.
- O. Boussif, F. Lezoualc'h, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix and J. P. Behr, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 7297 CrossRef CAS
.
- H. Duan and S. Nie, J. Am. Chem. Soc., 2007, 129, 3333 CrossRef CAS
.
- P. L. Felgner, T. R. Gadek, M. Holm, R. Roman, H. W. Chan, M. Wenz, J. P. Northrop, M. R. Gordon and M. Danielsen, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 7413 CrossRef CAS
.
- J. S. Suk, J. Suh, K. Choy, S. K. Lai, J. Fu and J. Hanes, Biomaterials, 2006, 27, 5143 CrossRef CAS
.
- T. Shiraishi, R. Hamzavi and P. E. Nielsen, Bioconjugate Chem., 2005, 16, 1112 CrossRef CAS
.
- N. Luciani, C. Wilhelm and F. Gazeau, Biomaterials, 2010, 31, 7061 CrossRef CAS
.
- J. Panyam and V. Labhasetwar, Pharm. Res., 2003, 20, 212 CrossRef CAS
.
- I. I. Slowing, J. L. Vivero-Escoto, Y. Zhao, K. Kandel, C. Peeraphatdit, B. G. Trewyn and V. S. Y. Lin, Small, 2011, 7, 1526 CrossRef CAS
.
- M. Horie, H. Kato, K. Fujita, S. Endoh and H. Iwahashi, Chem. Res. Toxicol., 2012, 25, 605 CrossRef CAS
.
- K. Peters, R. E. Unger, C. J. Kirkpatrick, A. M. Gatti and E. Monari, J. Mater. Sci.: Mater. Med., 2004, 15, 321 CrossRef CAS
.
- Y. Zhang, J. He, P. N. Wang, J. Y. Chen, Z. J. Lu, D. R. Lu, J. Guo, C. C. Wang and W. L. Yang, J. Am. Chem. Soc., 2006, 128, 13396 CrossRef CAS
.
- T. P. Cash, Y. Pan and M. C. Simon, Free Radical Biol. Med., 2007, 43, 1219 CrossRef CAS
.
- T. Cedervall, I. Lynch, M. Foy, T. Berggård, S. C. Donnelly, G. Cagney, S. Linse and K. A. Dawson, Angew. Chem., Int. Ed., 2007, 46, 5754 CrossRef CAS
.
- A. Terman, T. Kurz, B. Gustafsson and U. T. Brunk, IUBMB Life, 2006, 58, 531 CrossRef CAS
.
- K. H. Müller, J. Kulkarni, M. Motskin, A. Goode, P. Winship, J. N. Skepper, M. P. Ryan and A. E. Porter, ACS Nano, 2010, 4, 6767 CrossRef
.
- T. Xia, P. Korge, J. N. Weiss, N. Li, M. I. Venkatesen, C. Sioutas and A. Nel, Environ. Health Perspect., 2004, 112, 1347 CrossRef CAS
.
- M. R. Wilson, J. H. Lightbody, K. Donaldson, J. Sales and V. Stone, Toxicol. Appl. Pharmacol., 2002, 184, 172 CrossRef CAS
.
- A. K. Gupta, M. Gupta, S. J. Yarwood and A. S. G. Curtis, J. Controlled Release, 2004, 95, 197 CrossRef CAS
.
- A. Magrez, S. Kasas, V. Salicio, N. Pasquier, J. W. Seo, M. Celio, S. Catsicas, B. Schwaller and L. Forro, Nano Lett., 2006, 6, 1121 CrossRef CAS
.
- Y. Pan, A. Leifert, D. Ruau, S. Neuss, J. Bornemann, G. Schmid, W. Brandau, U. Simon and W. Jahnen-Dechent, Small, 2009, 5, 2067 CrossRef CAS
.
- P. R. Leroueil, S. Hong, A. Mecke, J. R. Baker Jr., B. G. Orr and M. M. Banaszak Holl, Acc. Chem. Res., 2007, 40, 335 CrossRef CAS
.
- S. G. Zhu, J. J. Xiang, X. L. Li, S. R. Shen, H. B. Lu, J. Zhou, W. Xiong, B. C. Zhang, X. M. Nie, M. Zhou, K. Tang and G. Y. Li, Biotechnol. Appl. Biochem., 2004, 39, 179 CrossRef CAS
.
- T. Takeuchi, M. Kosuge, A. Tadokoro, Y. Sugiura, M. Nishi, M. Kawata, N. Sakai, S. Matile and S. Futaki, ACS Chem. Biol., 2006, 1, 299 CrossRef CAS
.
- P. E. Thoren, D. Persson, P. Isakson, M. Goksor, A. Onfelt and B. Norden, Biochem. Biophys. Res. Commun., 2003, 307, 100 CrossRef CAS
.
- J. K. Jaiswal, H. Mattoussi, J. M. Mauro and S. M. Simon, Nat. Biotechnol., 2003, 21, 47 CrossRef CAS
.
- Z. Mao, B. Wang, L. Ma, C. Gao and J. Shen, Nanomed.: Nanotechnol., Biol. Med., 2007, 3, 215 CrossRef CAS
.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 1565 CrossRef CAS
.
- T. Baierl, E. Drosselmeyer, A. Seidel and S. Hippeli, Exp. Toxicol. Pathol., 1996, 48, 508 CrossRef CAS
.
- E. J. Park and K. Park, Toxicol. Lett., 2009, 184, 18 CrossRef CAS
.
- Y. Shibata, W. James Metzger and Q. N. Myrvik, J. Immunol., 1997, 159, 2462 CAS
.
- R. Shukla, V. Bansal, M. Chaudhary, A. Basu, A. Bhonde and M. Sastry, Langmuir, 2005, 21, 10644 CrossRef CAS
.
- W. Z. D. R. Absolom and A. W. Neumann, J. Biomed. Mater. Res., 1987, 21, 161 CrossRef
.
- S. Qi, C. Yi, W. Chen, C. C. Fong, S. T. Lee and M. Yang, ChemBioChem, 2007, 8, 1115 CrossRef CAS
.
- Z. Pan, W. Lee, L. Slutsky, R. A. F. Clark, N. Pernodet and M. H. Rafailovich, Small, 2009, 5, 511 CrossRef CAS
.
- J. H. Lee, H. H. Ahn, K. S. Kim, J. Y. Lee, M. S. Kim, B. Lee, G. Khang and H. B. Lee, J. Tissue Eng. Regener. Med., 2008, 2, 288 CrossRef CAS
.
- T. R. Pisanic II, J. D. Blackwell, V. I. Shubayev, R. R. Fiñones and S. Jin, Biomaterials, 2007, 28, 2572 CrossRef
.
- L. Kostura, D. L. Kraitchman, A. M. Mackay, M. F. Pittenger and J. W. M. Bulte, NMR Biomed., 2004, 17, 513 CrossRef
.
- T. A. Haas and E. F. Plow, Curr. Opin. Cell Biol., 1994, 6, 656 CrossRef CAS
.
- M. Chen and A. von Mikecz, Exp. Cell Res., 2005, 305, 51 CrossRef CAS
.
- Y. J. Kim, M. Yu, H. O. Park and S. Yang, Mol. Cell. Toxicol., 2010, 6, 336 CrossRef CAS
.
- K. T. Rim, S. J. Kim, J. H. Han, M. G. Kang, J. K. Kim and J. S. Yang, Mol. Cell. Toxicol., 2011, 7, 415 CrossRef CAS
.
- N. Feliu and B. Fadeel, Nanoscale, 2010, 2, 2514 RSC
.
- L. Ding, J. Stilwell, T. Zhang, O. Elboudwarej, H. Jiang, J. P. Selegue, P. A. Cooke, J. W. Gray and F. F. Chen, Nano Lett., 2005, 5, 2448 CrossRef CAS
.
- K. M. Waters, L. M. Masiello, R. C. Zangar, B. J. Tarasevich, N. J. Karin, R. D. Quesenberry, S. Bandyopadhyay, J. G. Teeguarden, J. G. Pounds and B. D. Thrall, Toxicol. Sci., 2009, 107, 553 CrossRef CAS
.
- B. Wang, Y. Zhang, Z. Mao and C. Gao, Macromol. Biosci., 2012, 12, 1534 CrossRef CAS
.
- H. Haniu, Y. Matsuda, K. Takeuchi, Y. A. Kim, T. Hayashi and M. Endo, Toxicol. Appl. Pharmacol., 2010, 242, 256 CrossRef CAS
.
- T. L. Lee, W. Y. Chan and O. M. Rennert, ACS Nano, 2009, 3, 3830 CrossRef CAS
; author reply 3830.
- Y. Sun, H. Wallrabe, S.-A. Seo and A. Periasamy, ChemPhysChem, 2011, 12, 462 CrossRef CAS
.
- G. D. Byrne, M. C. Pitter, J. Zhang, F. H. Falcone, S. Stolnik and M. G. Somekh, J. Microsc., 2008, 231, 168 CrossRef CAS
.
- T. Ohrt, J. Muetze, P. Svoboda and P. Schwille, Curr. Top. Med. Chem., 2012, 12, 79 CrossRef CAS
.
- N. Ruthardt, D. C. Lamb and C. Brauchle, Mol. Ther., 2011, 19, 1199 CrossRef CAS
.
- U. Reibetanz, M. H. A. Chen, S. Mutukumaraswamy, Z. Y. Liaw, B. H. L. Oh, S. Venkatraman, E. Donath and B. R. Neu, Biomacromolecules, 2010, 11, 1779 CrossRef CAS
.
- A. A. van Apeldoorn, H. J. van Manen, J. M. Bezemer, J. D. de Bruijn, C. A. van Blitterswijk and C. Otto, J. Am. Chem. Soc., 2004, 126, 13226 CrossRef CAS
.
- Y. Wang and L. Chen, Nanomed.: Nanotechnol., Biol. Med., 2011, 7, 385 CrossRef CAS
.
- H. Lee, I. K. Kim and T. G. Park, Bioconjugate Chem., 2010, 21, 289 CrossRef CAS
.
- H. C. Fischer, L. Liu, K. S. Pang and W. C. W. Chan, Adv. Funct. Mater., 2006, 16, 1299 CrossRef CAS
.
- X. H. Xu, W. J. Brownlow, S. V. Kyriacou, Q. Wan and J. J. Viola, Biochemistry, 2004, 43, 10400 CrossRef CAS
.
- R. Bhattacharya, C. R. Patra, R. Verma, S. Kumar, P. R. Greipp and P. Mukherjee, Adv. Mater., 2007, 19, 711 CrossRef CAS
.
- J. Lee, G. D. Lilly, R. C. Doty, P. Podsiadlo and N. A. Kotov, Small, 2009, 5, 1213 CAS
.
- A. F. Panasci, C. A. Ohlin, S. J. Harley and W. H. Casey, Inorg. Chem., 2012, 51, 6731 CrossRef CAS
.
- J. G. Teeguarden, P. M. Hinderliter, G. Orr, B. D. Thrall and J. G. Pounds, Toxicol. Sci., 2007, 95, 300 CrossRef CAS
.
- M. A. Dobrovolskaia and S. E. McNeil, Nat. Nanotechnol., 2007, 2, 469 CrossRef CAS
.
- M. Horie, H. Kato, K. Fujita, S. Endoh and H. Iwahashi, Chem. Res. Toxicol., 2012, 25, 605 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2013 |
