Titanium dioxide nanoparticle-entrapped polyion complex micelles generate singlet oxygen in the cells by ultrasound irradiation for sonodynamic therapy†
Atsushi
Harada
*,
Masafumi
Ono
,
Eiji
Yuba
and
Kenji
Kono
Department of Applied Chemistry, Graduate School of Engineering, Osaka Prefecture University, 1-1 Gakuen-cho, Naka-ku, Sakai, Osaka 599-8531, Japan. E-mail: harada@chem.osakafu-u.ac.jp; Fax: +81 72 254 9328; Tel: +81 72 254 9328
First published on 26th September 2012
Abstract
A new modality of using ultrasound irradiation instead of photoactivation, as in photodynamic therapy (PDT), sonodynamic therapy, has emerged as a promising treatment for various types of cancer. Titanium dioxide (TiO2) has the ability to generate reactive oxygen species (ROS) by not only photo- but also ultrasound-irradiation. Here, the formation of core–shell type polyion complex micelles from TiO2 nanoparticles with polyallylamine bearing poly(ethylene glycol) grafts effectively improves the dispersion stability of the TiO2 nanoparticles under physiological conditions for therapeutic application. The TiO2 nanoparticles in the micelles can generate ROS including singlet oxygen (1O2) by sonication. Furthermore, the micelles are taken up into HeLa cells and the TiO2 nanoparticles generate 1O2, which is widely believed to be the main cytotoxic agent in PDT, even in the cells treated by sonication. This is the first result representing 1O2 generation of TiO2 nanoparticles in HeLa cells by sonication. Further, the micelles can selectively exhibit a cell-killing effect at only the ultrasound-irradiated area.
1. Introduction
Photodynamic therapy (PDT) is a minimally invasive therapeutic modality approved for the treatment of cancer diseases and non-oncological disorders.1–3 Extensive studies on the synthesis of various kinds of photosensitizers including porphyrin derivatives and the vehicles for photosensitizers have been reported for the development of effective PDT.4–7 Photo-irradiation with a wavelength specific to the photosensitizers induces reactions generating radicals such as hydroxyl (OH) and peroxy (HO2) radicals, as well as non-radical, but highly reactive singlet oxygen (1O2). The main cytotoxic agent in PDT is widely believed to be 1O2, and direct and indirect evidence supports a prevalent role for 1O2 in the molecular processes initiated by PDT.8 Also, photosensitizers such as hematoporphyrin and merocyanine 540 can be activated by sonication, and the modality using these compounds is frequently referred to as sonodynamic therapy (SDT).9–12Titanium dioxide (TiO2) has the ability to act as a photosensitizer and is also known to generate reactive oxygen species (ROS) including OH and HO2 radicals, superoxide anions (O2−), hydrogen peroxide (H2O2) and 1O2 by ultraviolet (UV) irradiation (less than 390 nm).13–16 It has been reported that UV-irradiated TiO2 nanoparticles (NPs) show a cell-killing effect toward HeLa cells.17 However, the implication of using TiO2 NPs in clinical situations is hampered: first, by the fact that UV light cannot penetrate deeply into human tissue, and second, that TiO2 NPs have poor dispersion stability at physiological pH.18,19 It was reported that TiO2 generates ROS by ultrasound irradiation (39 kHz),20 although the ultrasound frequency (39 kHz) was too low for clinical applications. Recently, sonication with a clinically appropriate frequency (1 MHz) of TiO2 NPs could also show an effective decrease in the cell viability and the inhibition of tumor growth in vivo, in which a TiO2 NP suspension was directly injected into a tumor.21 This indicates that the availability of TiO2 NPs in SDT and the development of a carrier system that can deliver TiO2 NPs into the cells through improvement of their dispersion stability under physiological conditions was strongly required for effective SDT.
We focused on the charge property of the surface OH groups of the TiO2 NPs. The isoelectric point of the TiO2 NPs having a crystal structure of anatase is 6.2, and the TiO2 NPs possess a negative charge at neutral pH.22 We have investigated the polyion complex (PIC) micelle system entrapping enzyme molecules in the core.23–25 By using polyallylamine bearing poly(ethylene glycol) grafts (PAA-g-PEG), anionic enzyme molecules (glucose oxidase) were successfully incorporated into the PIC micelles. The PEG grafts in the micelles provide good dispersion stability by surrounding the surface of the micelles.26 It is expected that the graft copolymers could electrostatically form the micelles with TiO2 NPs and the TiO2 NP-entrapped PIC micelles will be taken up into the cells so that the TiO2 NPs can exhibit their cell-killing effect through ROS generation by sonication (Fig. 1). From such motivation, we studied the preparation of PIC micelles from TiO2 NPs and PAA-g-PEG for use in SDT. Studies were also conducted on the ability of the TiO2 NP-entrapped PIC micelles to generate ROS including 1O2 by sonication. The cellular uptake, 1O2 generation in the cells and the selective cell-killing effect of the micelles by area-limited sonication were also studied.
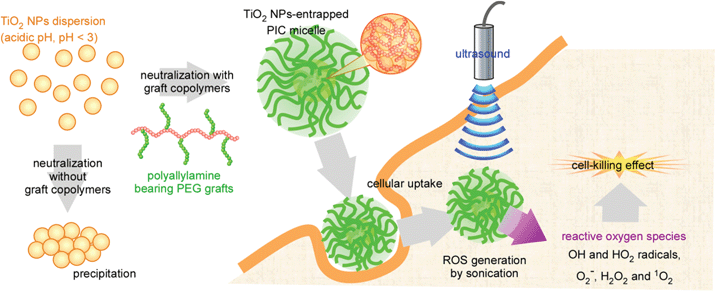 | ||
| Fig. 1 Schematic image of TiO2 NP-entrapped polyion compex micelles for sonodynamic therapy. | ||
2. Experimental methods
2.1. Materials
Four types of PAA-g-PEG having the same PAA main chain (DP = 160) and bearing PEGs of different molecular weight (Mn = 2k and 5k) and grafting densities (13 and 26 mol% for PEG2k, and 12 and 21 mol% for PEG5k), were synthesized according to our previous report.26 These graft copolymers are abbreviated as 2k13, 2k26, 5k12 and 5k21, respectively. The TiO2 NP dispersion with a crystal structure of anatase (10 nm, pH < 3) was purchased from Ishihara Sangyo Kaisha, Ltd. Fluorescence probe molecules for ROS detection (dihydrorhodamine 123 (DHR-123) and singlet oxygen sensor green (SOSG)) were purchased from Lambda and Invitrogen, respectively. L-Histidine was purchased from Wako Pure Chemical Industries, Ltd. Rhodamine B isothiocyanate, fluorescein 5-isothiocyanate and propidium iodide were purchased from Sigma-Aldrich. O-Phosphorylethanolamine was purchased from Tokyo Chemical Industry Co., Ltd. Calcein-AM was purchased from Nacalai Tesque, Inc. All reagents were used without further purification.2.2. Preparation of TiO2 NP-entrapped PIC micelles
PAA-g-PEG was dissolved in distilled water and added to the TiO2 NP dispersion, in which the weight ratio of polymer to TiO2 (polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2) was changed from 1 to 10. The mixing solutions (pH < 3) were neutralized using NaOH aq. Ultrafiltration was then performed using a USY-20 ultrafiltration unit (molecular weight cut off: 200
TiO2) was changed from 1 to 10. The mixing solutions (pH < 3) were neutralized using NaOH aq. Ultrafiltration was then performed using a USY-20 ultrafiltration unit (molecular weight cut off: 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; Toyo Roshi, Ltd.) for removal of free polymers, and the resulting solution was solvent exchanged with phosphate buffered saline (PBS). The final composition of the micelles, i.e. the weight ratio of polymer and TiO2, was determined using TG/DTA analysis.
000; Toyo Roshi, Ltd.) for removal of free polymers, and the resulting solution was solvent exchanged with phosphate buffered saline (PBS). The final composition of the micelles, i.e. the weight ratio of polymer and TiO2, was determined using TG/DTA analysis.
2.3. Physicochemical characterization of TiO2 NP-entrapped PIC micelles
Turbidity measurements were carried out using an ARVOSX multilabel counter (Perkin Elmer), and the absorbance at 595 nm was monitored to give the turbidity measurement. DLS and laser-Doppler electrophoresis measurements were carried out at 25 °C using an ELS-8000 (Otsuka Electronics Co., Ltd.) instrument equipped with a He–Ne ion laser (λ = 633 nm). The DLS measurements utilized a 90° detection angle: the average diameter was calculated using the Stokes–Einstein equation. Laser-Doppler electrophoresis was employed as a technique to measure particle velocity. Owing to the Doppler effect, the frequency of the scattered light was different from that of the original laser beam, and the electrophoretic mobility was determined from the frequency shifts caused by the Doppler effect. The zeta-potential was calculated using the Smoluchowski equation. TG/DTA measurements were carried out using TG8120 (Rigaku). The samples were measured under a N2 atmosphere from room temperature to 550 °C at a heating rate of 10 °C min−1 and calibrated using Al2O3 as a standard sample.2.4. Confirmation of ROS generation by sonication
Two kinds of fluorescence probe, DHR-123 and SOSG, were used for the detection of ROS. DHR-123 and SOSG were separately dissolved into PBS and methanol to concentrations of 5 mM for DHR-123 and 0.5 mM for SOSG, respectively. Stock solution (6 μL) was then added to 3 mL of the micelle solution including 45 μg mL−1 of TiO2, and the resultant solution was then sonicated by using a Sonitron2000V apparatus equipped with an ultrasound probe (ϕ 6 mm) (NEPA GENE Co., Ltd.). Samples were fractionated at various sonication times (1, 2, 4, 6, 8 and 10 min), and their fluorescence intensity was measured using a FP6500 spectrofluorometer (JASCO), in which the excitation and emission wavelengths (λex and λem) were λex 480 nm/λem 530 nm for DHR-123, and λex 485 nm/λem 525 nm, for SOSG. To support the confirmation of 1O2 generation, the above mentioned experiments using SOSG were also performed in the presence of L-histidine; a known quencher for 1O2.2.5. Cellular uptake of TiO2 NP-entrapped PIC micelles
To evaluate cellular uptake of TiO2 NP-entrapped PIC micelles, rhodamine-labeled PAA-g-PEG and fluorescein-labeled TiO2 NPs were prepared: PAA-g-PEG was labeled using rhodamine B isothiocyanate, in which PAA-g-PEG and rhodamine B isothiocyanate were reacted in 50 mM borate buffer (pH 8.5) and unreacted rhodamine B isothiocyanate was removed by dialysis against distilled water, and the amounts of labeled rhodamine were determined to be 3.0, 2.8, 2.9 and 3.5 mmol% of allylamine units, respectively, by using a calibration curve prepared by rhodamine B. The TiO2 NPs were labeled using fluorescein 5-carbamothioylaminoethoxy phosphonic acid, which was synthesized from the reaction between fluorescein 5-isothiocyanate and O-phosphorylethanolamine, through the formation of covalent bidentate bonds between the phosphate groups and TiO227,28 and purified through ultrafiltration using a USY-5 ultrafiltration unit. The labeled amount of fluorescein was determined from the concentration of fluorescein in the supernatant. HeLa cells were seeded in 0.5 mL of Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS) in 12-well culture plates at 1 × 105 cells per well the day before the uptake experiments. The cells were washed with PBS and then covered with DMEM (1 mL). The fluorescently-labeled micelle solutions, which included TiO2 NPs at the same concentration, were gently added to the cells and the solutions incubated at 37 °C for varying incubation times (2, 4, 8 and 24 hours). The cells were washed with PBS, and confocal laser scanning microscopic observation of the cells was performed using a laser scanning microscope (LSM 5 EXCITER, Carl Zeiss Co., Ltd). Also, the cells were detached from the surface of the dish using trypsin, and the cellular fluorescence was then evaluated by flow cytometry (EPICS XL, Beckman Coulter, Inc.).2.6. Evaluation of site-specific cell-killing effect
The site-specific cell-killing effect was evaluated by live/dead double staining assays using Calcein-AM and propidium iodide. HeLa cells were seeded in 2 mL of DMEM supplemented with 10% FCS in 35 mm dish at 2 × 105 cells for 1 day and 2 day for 4 hours and 24 hours incubation with micelles, respectively. The micelle solutions were gently added to the cells and incubated at 37 °C for 4 and 24 hours. The cells were washed with PBS, and 5 mL of DMEM supplemented with 10% FCS. Ultrasound irradiation was performed at the center of the dish for various sonication times (5, 10, 20, 30 and 40 min) using an ultrasound probe (ϕ 6 mm). For double staining, 1 mg mL−1 of propidium iodide in H2O and 1 mg mL−1 of Calcein-AM in dimethyl sulfoxide were prepared as stock solutions. Propidium iodide (15 μL) and Calcein-AM (10 μL) stock solutions were added to 15 mL of DMEM supplemented with 10% FCS. After sonication, the cells were washed with PBS and supplemented with 2 mL of double staining solution. After 30 min of incubation, the cells were washed with PBS. Then, fluorescence images of HeLa cells were observed using an IMT-2 microscope equipped with an IMT2-RFL fluorescence unit (OLYMPUS).2.7. Effect of sonication time and radical scavenger to cell viability
HeLa cells were seeded in 100 μL of DMEM supplemented with 10% FCS in each well of a 96-well plate at 1 × 104 cells for 1 day. The micelle solutions were gently added to the cells and incubated at 37 °C for 24 hours with and without 10 mM glutathione. The cells were washed with PBS, and 100 μL of DMEM supplemented with 10% FCS. Ultrasound irradiation was performed for various sonication times using an ultrasound probe (ϕ 6 mm), the size of which is almost the same as that of a well of a 96-well plate. After sonication, 6 mL of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] solution was added to each well, and the plates were incubated 37 °C for 3 hours, followed by the addition of 100 μL of 2-isopropanol containing 0.1 M HCl. The number of viable cells was determined by absorbance at 570 nm.3. Results and discussion
Four types of PAA-g-PEG having the same PAA main chain and bearing PEGs of different molecular weight (Mn = 2k and 5k) and grafting densities (13 and 26 mol% for PEG2k, and 12 and 21 mol% for PEG5k) were used in this study, and these graft copolymers are abbreviated as 2k13, 2k26, 5k12 and 5k21, respectively, from the Mn of PEG grafts and grafting densities. Turbidity measurements were performed to evaluate the complexation of TiO2 NPs and PAA-g-PEG (Fig. S1, ESI†). No change in the turbidity of the mixtures was observed at acidic pH (pH < 3). Since TiO2 NPs have positive zeta-potentials at acidic pH,29 the TiO2 NPs did not electrostatically interact with the cationic PAA-g-PEG and the mixtures did not induce changes in turbidity. The dispersion stability of the TiO2 NPs depends on pH, in which aggregates are formed at neutral pH.18,19 Indeed, neutralization of the TiO2 NP dispersion caused the immediate formation of a turbid solution and the subsequent precipitation of the TiO2 NPs. On the other hand, the mixtures of TiO2 NPs and polycations at neutral pH did not result in precipitation of the TiO2 NPs even after storing overnight. Meanwhile, neutralization of the mixtures of TiO2 NPs with PAA homopolymers provided almost no change in turbidity regardless of the mixing weight ratio. On the other hand, in the case of PAA-g-PEG, an increase in turbidity was observed at relatively low mixing weight ratios. When the TiO2 NP dispersion was mixed with a sufficient amount of the graft copolymer (a mixing weight ratio of more than 5), no change in turbidity was observed. These results indicate that the mixing of polycations with TiO2 NPs improved the dispersion stability of the TiO2 NPs at neutral pH through effective inhibition of their aggregation due to neutralization. Also, when the salt concentration was increased up to 0.5 M NaCl before neutralization, the polycations did not improve the dispersion stability of the TiO2 NPs and the mixtures were precipitated by neutralization, indicating that the polycations electrostatically interacted with the TiO2 NPs. Furthermore, the addition of polycations to the TiO2 NP aggregates at neutral pH did not induce re-dispersion of the TiO2 NPs. That is, the preparation process is important to improve the dispersion stability of the TiO2 NPs, and the mixing of polycations beforehand was required for effective inhibition of TiO2 NP aggregation.Dynamic light scattering (DLS) measurements were performed to evaluate the size of the obtained complexes (Fig. 2a). PAA homopolymers effectively inhibit the aggregation of TiO2 NPs even at low mixing weight ratios, and the mean diameters of the corresponding complexes were determined to be ∼40 nm (red open symbols in Fig. 2a). In the case of PAA-g-PEG, the mean diameters decreased with an increase in mixing weight ratio. Also, the complexes of smaller size were obtained for lower PEG weight fractions of PAA-g-PEG. While no significant difference in the turbidity measurements was observed at high mixing weight ratios (a polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 ratio of more than 5), as shown in Fig. S1,† a difference was observed in the mean diameters, as shown in Fig. 2a. The order of mean diameter was 5k21 > 5k12 = 2k26 > 2k13 > PAA, which agreed well with the order of PEG weight fraction in PEG-g-PAA. An increase in PEG weight fraction induced an increase in mean diameter, and PAA homopolymers bearing the lowest PEG fraction had the most effective inhibition ability of TiO2 NP aggregation. PEG grafts are expected to sterically disturb the electrostatic interaction between the PAA main chain and the TiO2 NPs. From these results, it was confirmed that the addition of sufficient amounts of polycations effectively inhibits the aggregation of TiO2 NPs at neutral pH and could result in the formation of small complexes (less than 100 nm; open symbols in Fig. 2a). However, in the case of preparing complexes at high mixing weight ratios, there is a possibility that the mixtures include free polycations. Therefore, ultrafiltration was performed on the mixtures for the removal of free polycations and the solvent was also exchanged with phosphate buffered saline (PBS). For the complexes prepared using PAA homopolymers at all mixing weight ratios except 5k21, and for the complexes prepared at a mixing weight ratio of 1 using PAA-g-PEG, precipitation occurred in the ultrafiltration process. The solubility of the PAA homopolymers in aqueous solution, supplemented with multivalent anions such as phosphate ions, decreased. Multivalent anions in PBS are expected to induce aggregation among the PAA complexes. Also, in the case of PAA-g-PEG complexes with low mixing weight ratios, the PAA main chain is expected to extend out from the surface and induce aggregation between complexes. On the other hand, the complexes prepared using PAA-g-PEG at high mixing weight ratios did not induce precipitation such that they maintained their dispersion stability even in PBS. This result suggests that the surface of the PAA-g-PEG complexes was surrounded by PEG grafts and the PAA main chain did not extend out from the surface of the complexes. While the mean diameters of the complexes in PBS (closed symbols in Fig. 2a) were higher than those before ultrafiltration (open symbols in Fig. 2a), the trend of the mean diameters was similar to those before ultrafiltration.
TiO2 ratio of more than 5), as shown in Fig. S1,† a difference was observed in the mean diameters, as shown in Fig. 2a. The order of mean diameter was 5k21 > 5k12 = 2k26 > 2k13 > PAA, which agreed well with the order of PEG weight fraction in PEG-g-PAA. An increase in PEG weight fraction induced an increase in mean diameter, and PAA homopolymers bearing the lowest PEG fraction had the most effective inhibition ability of TiO2 NP aggregation. PEG grafts are expected to sterically disturb the electrostatic interaction between the PAA main chain and the TiO2 NPs. From these results, it was confirmed that the addition of sufficient amounts of polycations effectively inhibits the aggregation of TiO2 NPs at neutral pH and could result in the formation of small complexes (less than 100 nm; open symbols in Fig. 2a). However, in the case of preparing complexes at high mixing weight ratios, there is a possibility that the mixtures include free polycations. Therefore, ultrafiltration was performed on the mixtures for the removal of free polycations and the solvent was also exchanged with phosphate buffered saline (PBS). For the complexes prepared using PAA homopolymers at all mixing weight ratios except 5k21, and for the complexes prepared at a mixing weight ratio of 1 using PAA-g-PEG, precipitation occurred in the ultrafiltration process. The solubility of the PAA homopolymers in aqueous solution, supplemented with multivalent anions such as phosphate ions, decreased. Multivalent anions in PBS are expected to induce aggregation among the PAA complexes. Also, in the case of PAA-g-PEG complexes with low mixing weight ratios, the PAA main chain is expected to extend out from the surface and induce aggregation between complexes. On the other hand, the complexes prepared using PAA-g-PEG at high mixing weight ratios did not induce precipitation such that they maintained their dispersion stability even in PBS. This result suggests that the surface of the PAA-g-PEG complexes was surrounded by PEG grafts and the PAA main chain did not extend out from the surface of the complexes. While the mean diameters of the complexes in PBS (closed symbols in Fig. 2a) were higher than those before ultrafiltration (open symbols in Fig. 2a), the trend of the mean diameters was similar to those before ultrafiltration.
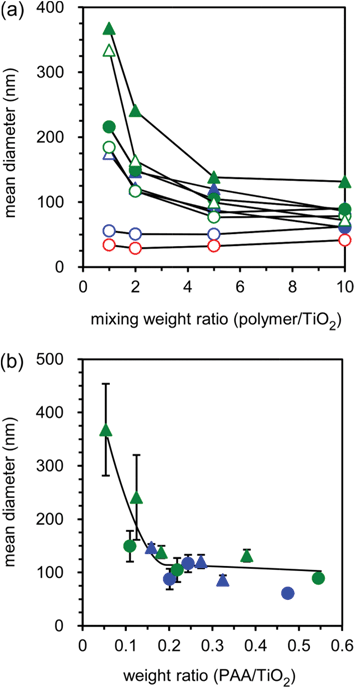 | ||
| Fig. 2 Change in mean diameter with various mixing weight ratios for the mixtures of TiO2 NPs and PAA-g-PEG before (open symbols) and after (closed symbols) ultrafiltration (a) and relationship between mean diameters and the final composition (weight ratio of PAA main chain against TiO2 NPs) of PIC micelles (b). The 2k13, 2k26, 5k12, 5k21 and PAA complexes are represented by blue circles, blue triangles, green circles, green triangles, and red circles, respectively. The mean diameters were determined by DLS measurements at 25 °C. The compositions of micelles were determined from TG/DTA analysis, and the weight ratios of the PAA main chain and TiO2 NPs were then calculated based on the composition of PAA-g-PEG. | ||
To confirm the composition of the finally obtained complexes, thermogravimetry/differential thermal analysis (TG/DTA) measurements were carried out (Fig. S2, ESI†). There was a two-step weight loss: slight decrease at 60–110 °C and steep decrease at 350–400 °C. These decreases were attributed to aquatic vaporization and polymer degradation, respectively. The composition of the complexes was determined from the ratio of weight loss at 150–550 °C and the remaining weight at 550 °C. The mean diameters of the complexes in PBS, i.e. after ultrafiltration, were plotted against the weight ratios of the PAA main chain and TiO2 NPs (PAA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2) in the final composition (Fig. 2b). Interestingly, these plots showed a correlation between the mean diameters and weight ratios of PAA and TiO2 NPs. At low PAA content (less than a 0.2 ratio of PAA
TiO2) in the final composition (Fig. 2b). Interestingly, these plots showed a correlation between the mean diameters and weight ratios of PAA and TiO2 NPs. At low PAA content (less than a 0.2 ratio of PAA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2), the mean diameters decreased with an increase in PAA
TiO2), the mean diameters decreased with an increase in PAA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 ratio. Also, in this region, the mean diameters had large error bars, indicating low reproducibility of complex preparation. However, when the PAA content was increased to more than 0.2 of PAA
TiO2 ratio. Also, in this region, the mean diameters had large error bars, indicating low reproducibility of complex preparation. However, when the PAA content was increased to more than 0.2 of PAA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2, the mean diameters of the complexes were almost constant (∼100 nm). Moreover, the error bars for these values showed a decrease in size indicating good reproducibility of complex preparation. These results indicate that the PAA content is a crucial factor in determining the size of the complexes. Further, the zeta-potentials of the complexes were near neutral in value, indicating that the electrostatically-neutral PEG grafts surrounded the surface of the complexes (Table S1, ESI†). The complexes had spherical shape in atomic force microscopic observation (Fig. S3, ESI†). From these results, the PAA-g-PEG complexes are expected to have a core–shell architecture sterically-stabilized by the PEG grafts, i.e. PIC micelles. Also, since there was small error bars in mean diameter of PIC micelles prepared at enough amounts of graft copolymers (Fig. 2b), PIC micelles were prepared at the mixing ratio of 10 (polymer
TiO2, the mean diameters of the complexes were almost constant (∼100 nm). Moreover, the error bars for these values showed a decrease in size indicating good reproducibility of complex preparation. These results indicate that the PAA content is a crucial factor in determining the size of the complexes. Further, the zeta-potentials of the complexes were near neutral in value, indicating that the electrostatically-neutral PEG grafts surrounded the surface of the complexes (Table S1, ESI†). The complexes had spherical shape in atomic force microscopic observation (Fig. S3, ESI†). From these results, the PAA-g-PEG complexes are expected to have a core–shell architecture sterically-stabilized by the PEG grafts, i.e. PIC micelles. Also, since there was small error bars in mean diameter of PIC micelles prepared at enough amounts of graft copolymers (Fig. 2b), PIC micelles were prepared at the mixing ratio of 10 (polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2) in feed for the following experiments.
TiO2) in feed for the following experiments.
The ability of ROS generation by ultrasound irradiation (sonication) was evaluated using the fluorescence probe, dihydrorhodamine 123 (DHR-123), which has often been used as an ROS detection probe. DHR-123 produces rhodamine 123, which exhibits a strong fluorescence through the reaction with ROS.30 An increase in the amount of rhodamine 123 by sonication was detected for not only the micelles but also for the PBS without micelles (Fig. S4, ESI†). The increase in the amount of rhodamine 123 without micelles is considered to be due to cavitational implosion, which generates solvent radicals; in the case of water, these are H and OH radicals that can combine to give hydrogen and hydrogen peroxide.31,32 The sonication of the micelles produced large amounts of rhodamine 123 compared with the sonication to PBS without micelles. Also, for both cases with and without micelles, the amounts of rhodamine 123 produced linearly increased with sonication time at varying sonication power, and the initial reaction rate was calculated from the slopes in Fig. S4a,b.†Fig. 3a shows the relationship between the initial reaction rates and sonication powers for the samples with and without micelles prepared from various types of PAA-g-PEG. All types of micelle showed an increase in the initial reaction rate depending on the sonication power, and there was no significant difference in the dependence of the initial reaction rate on the sonication power, indicating that the micelles showed the same ROS generation capability in this study. ROS generation by the sonication of TiO2 NPs was recently reported using aminophenyl fluorescein (APF), which produces fluorescein through its reaction with ROS as a fluorescence probe.33 Under the reported experimental conditions, the sonication (1 MHz) of APF solution without TiO2 NPs showed a comparable increase in fluorescence intensity to that of the sonication to sample solutions including TiO2 NPs, suggesting that APF is converted to fluorescein mainly through another reaction, such as cavitation. Although it is difficult to compare these results because of the difference in experimental conditions, the sonication of TiO2 NPs is found to exhibit more effective ROS generation. Considering the effect of cavitation, as determined from the slopes in Fig. 3a, ∼60% of the generated ROS was derived from the TiO2 NPs and ∼40% was derived from the cavitation.
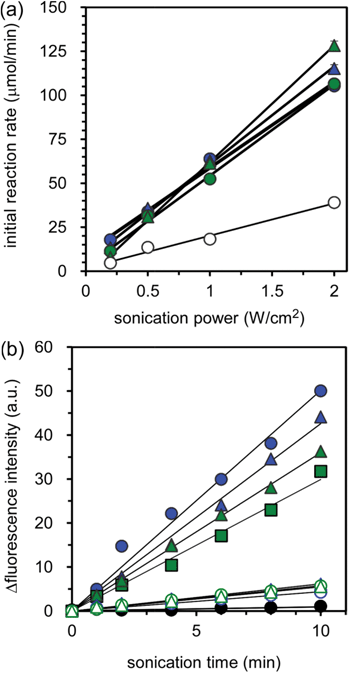 | ||
| Fig. 3 Plots of initial reaction rate generating ROS against sonication power for various types of PIC micelles (a) and singlet oxygen (1O2) generation by the sonication of TiO2 NP-entrapped micelles with and without L-histidine (b). In (a), the initial reaction rates were determined from the slopes in Fig. S4.† The 2k13, 2k26, 5k12 and 5k21 micelles and PBS without micelles are represented by blue circles, blue triangles, green circles, green triangles, and white circles, respectively. The concentration of DHR-123 and TiO2 NPs were 10 μM and 45 μg mL−1, respectively, and fluorescence measurements were performed at λex 480 nm/λem 530 nm. In (b), the 2k13, 2k26, 5k12, and 5k21 micelles are represented by blue circles, blue triangles, green circles and green triangles, respectively, and the closed and open symbols represent without and with L-histidine. SOSG in PBS without TiO2 NPs is represented as black circles. The concentrations of SOSG and TiO2 NPs were 1 μM and 45 μg mL−1, respectively. L-Histidine was added to the mixture to increase the concentration to a final concentration of 5 mM. Fluorescence measurements were performed at λex 485 nm/λem 525 nm. In (a) and (b), the sonication condition was fixed at the following parameters: a frequency of 1 MHz, a power of 0.5 W cm−2 and a duty cycle of 25%. | ||
It is well known that there are various species in the ROS including OH and HO2 radicals, O2−, H2O2 and 1O2, which are generated by photo-irradiation of TiO2. In PDT, the main cytotoxic agent is widely believed to be 1O2, in which direct and indirect evidence supports a prevalent role for 1O2 in the molecular processes inhibited by PDT.8 It was recently reported that ROS generated by the sonication of TiO2 NPs at 40 kHz might include 1O2 from the experiment using 1O2 quenchers such as L-histidine and sodium azide.34 On the other hand, although the range of ultrasound frequencies can be extended up to 100 MHz, it is customary to divide ultrasound into two distinct regions: conventional power ultrasound, up to 100 kHz, that especially affects chemical reactivity in liquids, and diagnostic ultrasound, above 1 MHz and up to 10 MHz, with applications in both medicine and materials processing.32 Diagnostic ultrasound at high frequency can reach deeply into human tissue. Therefore, it is important to evaluate whether 1O2 is present in the ROS generated by the sonication to TiO2 NPs at high frequency. However, there has been no report to date on 1O2 generation by the sonication of TiO2 NPs at high frequencies applicable to clinical situations. The generation of 1O2 by sonication (1 MHz) was confirmed using a singlet oxygen sensor green (SOSG), which is sensitive only to 1O2,30 as a fluorescence probe. SOSG exhibits green fluorescence through reaction with 1O2. While the exact structure of SOSG has not been disclosed, several spectral analyses suggest its structure as shown in Fig. S7.†35,36 Prior to the reaction with singlet oxygen, intramolecular electron transfer quenches the fluorescence. Upon the reaction with singlet oxygen and the formation of the endoperoxide, electron transfer is precluded.36Fig. 3b shows the change in fluorescence intensity with sonication time for the micelles prepared using various types of graft copolymer. It is obvious that all types of micelles show an increase in fluorescence intensity with an increase in sonication time, demonstrating the generation of 1O2 by sonication. Also, unlike the case of DHR-123 shown in Fig. S4b,† there is almost no change in fluorescence intensity following the sonication of PBS (black symbols in Fig. 3b). This is a reasonable result considering the nature of the fluorescence probes: DHR-123 and SOSG are specific probes for the detection of ROS and 1O2, respectively. As described above, the sonication of solvents generates the corresponding solvent radicals, and in the case of water these include ROS. Alternatively 1O2 is not generated following the sonication of water, and no change in fluorescence intensity was observed following the sonication of PBS in the presence of SOSG. Further, the 1O2 generation obtained following sonication of the micelles was also supported from the experimental results using SOSG and L-histidine (open symbols in Fig. 3b). The fluorescence intensities remarkably decreased in the presence of L-histidine, which is a quencher of 1O2,34 indicating the presence of 1O2 in the ROS generated by sonication of the micelles. These results demonstrate that the 1O2 generated by sonication of the micelles at high frequency (1 MHz) are crucial results for the design of therapeutic systems using TiO2 NPs, although the mechanism of 1O2 generation by sonication is still unclear.
To exhibit an effective therapeutic effect using the SDT system, the micelles are required to be taken up into the cells due to very short life time and diffusion distance of ROS. The cellular uptake of TiO2 NP-entrapped micelles was evaluated by flow cytometry and confocal laser scanning microscopic observation, in which fluorescein-labeled TiO2 NPs and rhodamine-labeled PAA-g-PEG were used (Fig. 4). The observed amount of rhodamine-labeled PAA-g-PEG corresponded to the difference in PAA-g-PEG content in the final compositions (polymer–TiO2). Therefore, sample solutions with fixed concentrations of TiO2 NPs were seeded in HeLa cells, i.e. the amount of fluorescein was fixed in this experiment. Fluorescence from the fluorescein-labeled TiO2 NPs was detected as an indication of uptake amount in the experiment using flow cytometry, and both fluorescein and rhodamine, i.e. TiO2 NPs and PAA-g-PEG, were observed using confocal microscopy (Fig. 4c,d,e). When the incubation time was prolonged, the cellular fluorescent distribution shifted to strong fluorescence intensity while maintaining unimodal distribution (Fig. 4a,b). This result suggested that the micelles were homogeneously taken up by the HeLa cells, and the amount of TiO2 NPs taken up increased with prolonged incubation times. When the micelles might be taken up via the endocytosis pathway, the local pH around the micelles might decrease to weak acidic pH in endosomes and lysosomes. In this case, the effect of pH on the stability of the micelles was a concern, since the isoelectric point of TiO2 NPs is 6.2. The stability of the micelles under weak acidic pH conditions was evaluated by light scattering measurements (Fig. S5, ESI†). There was no significant change in the mean diameter of the micelles, even when decreasing the pH from 7.4 to 5.0, indicating that the micelles were stable at the cellular uptake process. The micelles might be stabilized by not only electrostatic interaction but also by other intermolecular interactions including van der Waals forces and hydrogen bonding etc. Also, both green and red fluorescent dots were observed in the cytoplasm (Fig. 4c,d). Meanwhile, only yellow dots were observed in the confocal laser scanning microscopic image overlaid with the differential interference contrast image owing to complete overlap of the distribution of green and red fluorescent dots (Fig. 4e). This overlap corresponds to an equal distribution of TiO2 NPs and PAA-g-PEG, indicating that the micelles do not dissociate even in the cells.
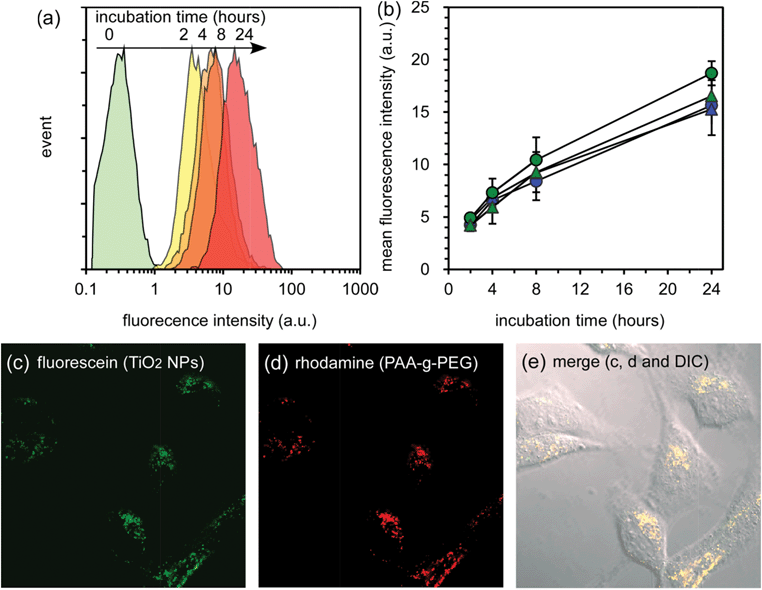 | ||
| Fig. 4 Cellular uptake of TiO2 NP-entrapped PIC micelles by HeLa cells. (a) Flow cytometry analysis of 2k13 micelles with varying incubation times. (b) Change in mean fluorescence intensity of HeLa cells treated by micelles with varying incubation time. The 2k13, 2k26, 5k12 and 5k21 micelles are represented by blue circles, blue triangles, green circles, and green triangles respectively. (c, d and e) Confocal laser scanning microscopic images and the confocal laser scanning microscopic images overlaid with the differential interference contrast image of HeLa cells treated with 2k13 micelles for 24 h incubation. | ||
The 1O2 generation of TiO2 NP-entrapped PIC micelles in the HeLa cells by sonication was evaluated using SOSG and confocal microscopy observation, since the uptake of the micelles by the HeLa cells was confirmed, as shown in Fig. 4. Yamaguchi et al. reported that the cytotoxicity of TiO2 NPs covalently modified with PEG by sonication to the cultured cells decreased with the co-incubation of a radical scavenger (glutathione), suggesting ROS generation in the cells by sonication.37 However, they did not confirm 1O2 generation of TiO2 NPs modified with PEG by sonication. The confocal observation was carried out on HeLa cells at both sonicated and non-sonicated areas (Fig. 5). The green fluorescence, which was produced by the reaction between SOSG and 1O2, was observed in the cytoplasm, and there was almost no fluorescence observed in the non-sonicated area. The TiO2 NP-entrapped micelles were observed to generate 1O2 by sonication even inside the cells. This is the first result revealing 1O2 generation by TiO2 NPs following sonication in the HeLa cells.
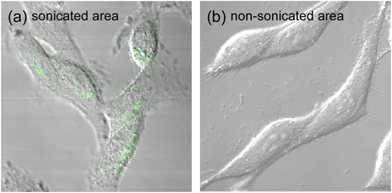 | ||
| Fig. 5 Confirmation of 1O2 generation by sonication of HeLa cells treated with PIC micelles. Confocal laser scanning microscopic image overlaid with the differential interference contrast image of HeLa cells treated with a mixture of 2k13 micelles and SOSG for 4 hours, and then sonicated for 5 min at a frequency of 1.0 MHz, a power of 0.5 W cm−2 and a duty cycle of 10%. | ||
Finally, the site-specific cell-killing effect of the TiO2 NP-entrapped PIC micelles was evaluated by live/dead double staining of the HeLa cells after ultrasound irradiation of the limited area. Fig. 6 shows the fluorescence microscopic images of the HeLa cells after treatment with the micelles for 4 and 24 hours and ultrasound irradiation for varying times, in which ultrasound was irradiated only at the center of the dish. The living cells are stained with Calcein-AM exhibiting green fluorescence after hydrolysis of the esterase in the cell, and the dead cells are stained with propidium iodide exhibiting red fluorescence through intercalation with DNA. When sonication was performed on the HeLa cells that were not treated with the micelles, all of the cells showed green fluorescence demonstrating their living state even after sonication for 40 min. Ultrasound irradiation at high frequency (1.0 MHz) did not provide the cell-killing effect and generate heat. Also, when the HeLa cells were treated with the micelles even for 24 hours, only green fluorescence was observed, suggesting that the micelles exhibited only negligible cytotoxicity. This might be due to the effect of the biocompatible PEG grafts surrounding the surface of the micelles. However, in the case of 4 hours incubation, by increasing the sonication time, red fluorescent cells started to be observed at the center of the cells after 20 min of sonication. The micelles were found to generate 1O2 even in the HeLa cells as shown in Fig. 5, and it is expected that other ROS might also be generated as a result of the sonication. The ROS generated inside the cells showed a cell-killing effect. The area of red fluorescent cells, i.e. dead cells, gradually spread with a prolonged sonication time. It should be noted that the area of dead cells produced after 20 min of sonication was smaller than the size of the ultrasound probe (ϕ 6 mm) used. In this study, ultrasound irradiation occurred radially from the probe, with the center being the most strongly irradiated. As a result, the cell-killing effect was observed initially at the center of the irradiated area, and the area of dead cells gradually spread with a prolonged sonication time. Further, in the case of 24 hours incubation, dead cells were observed from shorter sonication times. A larger amount of micelles were taken up into HeLa cells as shown in Fig. 4a,b, and the cell-killing effect was effectively observed. This suggests that the cell-killing effect could be controlled by not only the sonication time but also by the amount of TiO2 NPs in the cells. In addition, the change in the cell viability of HeLa cells treated by the micelles with varying sonication time was quantitatively evaluated by MTT assay (Fig. S6, ESI†). The cell viability of HeLa cells gradually decreased with a prolonged sonication time. Also, this decrease in cell viability was remarkably inhibited in the presence of a radical scavenger (glutathione). These results indicate that TiO2 NP-entrapped PIC micelles could show cytotoxicity only with sonication through the reaction of ROS generation.
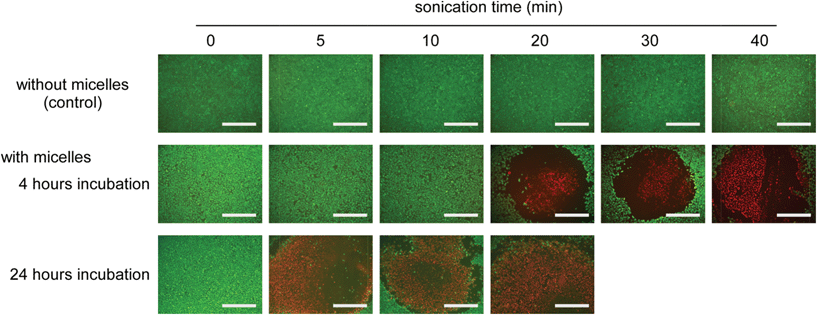 | ||
| Fig. 6 Live/dead double staining of HeLa cells treated with and without micelles at varying sonication times. HeLa cells were incubated with micelles for 4 and 24 hours, and the non-uptaken micelles were removed by PBS washing. PIC micelles were prepared using 2k13. Sonication was then performed for varying times around the center of each image by using a US probe with ϕ 6 mm (1.0 MHz of frequency, 0.5 W cm−2 of power and 10% of duty cycle). Living and dead cells were stained by Calcein-AM and propidium iodide, respectively. Scale bar is 1 mm. | ||
Conclusions
TiO2 NP-entrapped PIC micelles were successfully prepared by mixing a TiO2 NP dispersion and PAA-g-PEG. The micelles had a core–shell architecture, in which the PEG grafts sterically-stabilized the surface of the micelles. PEG grafts surrounding the surface of the micelles provided a biocompatible feature and the micelles had negligible cytotoxicity. The TiO2 NPs maintained their ability to generate ROS by sonication even after entrapment into the micelles. In addition, the generated ROS included 1O2, which is a major cytotoxic agent in PDT. Importantly, the micelles could generate 1O2 by sonication at a frequency appropriate for clinical situations even after uptake by HeLa cells, and this ability could lead to the cell-killing effect at a sonicated area. The results obtained here indicate that TiO2 NP-entrapped micelles have potential utility in SDT. In addition, TiO2 has versatile properties including chemical inertness toward biological systems, low cost, and easy preparation of stable suspensions with a nanoparticulate form.38 These properties are expected to become advantages compared with SDT using well-designed organic molecules such as hematoporphyrin and merocyanine 540. Further, the clinical introduction of high intensity focused ultrasound irradiation, which is a non-invasive thermal ablation method capable of targeting tissue without damaging normal cell structure,39 is expected to find application as an alternative method for locally irradiating tumors in combination with TiO2 NP-entrapped PIC micelles.Acknowledgements
The authors thank Dr A. Hayashi and Mr M. Nagao, Osaka Prefecture University, for the TG/DTA measurements. This research was partially supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Nanomedicine Molecular Science” (No. 24107519) and Scientific Research (B) (No. 23300179) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.Notes and references
- B. W. Henderson and T. J. Dougherty, Photochem. Photobiol., 1992, 55, 145 CrossRef CAS.
- D. E. J. G. J. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380 CrossRef CAS.
- E. Buytaert, M. Dewaele and P. Agostinis, Biochim. Biophys. Acta, 2007, 1776, 86 CAS.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795 CrossRef CAS.
- J. Lovell, T. W. B. Liu, J. Chen and G. Zheng, Chem. Rev., 2010, 110, 2839 CrossRef CAS.
- N. Nishiyama, Y. Morimoto, W. D. Jang and K. Kataoka, Adv. Drug Delivery Rev., 2009, 61, 327 CrossRef CAS.
- J. Kim, Y. Piao and T. Hyeon, Chem. Soc. Rev., 2009, 38, 372–390 RSC.
- M. Niedre, M. S. Patterson and B. C. Wilson, Photochem. Photobiol., 2002, 75, 382–391 CrossRef CAS.
- K. Tachibana, N. Kimura, M. Okumura, H. Higuchi and S. Tachibana, Cancer Lett., 1993, 72, 195 CrossRef CAS.
- K. Tachibana, T. Uchida, S. Hisano and E. Morioka, Lancet, 1997, 349, 325 CrossRef CAS.
- N. Yumita and S. Umemura, Cancer Chemother. Pharmacol., 2003, 51, 174 CAS.
- K. Hachimine, H. Shibaguchi, M. Kuroki, H. Yamada, T. Kinugasa, Y. Nakae, R. Asano, I. Sakata, Y. Yamashita and T. Shirakusa, Cancer Sci., 2007, 98, 916 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS.
- A. Mills and S. Le Hunte, J. Photochem. Photobiol., A, 1997, 108, 1 CrossRef CAS.
- P. F. Schwartz, N. J. Turro, S. H. Bossmann, A. M. Braum, A. M. A. A. Wahab and H. Durr, J. Phys. Chem. B, 1997, 101, 7127 CrossRef.
- R. Cai, Y. Kubota, T. Shuin, H. Sakai, K. Hashimoto and A. Fujishima, Cancer Res., 1992, 52, 2346 CAS.
- Z. M. Yaremko, N. H. Tkachenko, C. Bellmann and A. Pich, J. Colloid Interface Sci., 2006, 296, 565 CrossRef CAS.
- R. A. French, A. R. Jacobson, B. Kim, S. L. Isley, R. L. Penn and P. C. Baveye, Environ. Sci. Technol., 2009, 43, 1354 CrossRef CAS.
- N. Shimizu, C. Ogino, M. F. Dadjour and T. Murata, Ultrason. Sonochem., 2007, 14, 184 CrossRef CAS.
- Y. Harada, K. Ogawa, Y. Irie, H. Endo, L. B. Feruil Jr., T. Uemura and K. Tachibana, J. Controlled Release, 2011, 149, 190 CrossRef CAS.
- A. A. Keller, H. Wang, D. Zhou, H. S. Lenihan, G. Cherr, B. J. Cardinale, R. Miller and Z. Ji, Environ. Sci. Technol., 2010, 44, 1962 CrossRef CAS.
- A. Harada and K. Kataoka, Prog. Polym. Sci., 2006, 31, 949 CrossRef CAS.
- A. Harada and K. Kataoka, J. Am. Chem. Soc., 1999, 121, 9241 CrossRef CAS.
- A. Harada and K. Kataoka, J. Am. Chem. Soc., 2003, 125, 15306 CrossRef CAS.
- A. Kawamura, C. Kojima, M. Iijima, A. Harada and K. Kono, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3842 CrossRef CAS.
- A. T. Myller, J. J. Karhe and T. T. Pakkanen, Appl. Surf. Sci., 2010, 257, 1616 CrossRef CAS.
- P. H. Mutin, V. Lafond, A. F. Popa, M. Granier, L. Markey and A. Dereux, Chem. Mater., 2004, 16, 5670 CrossRef CAS.
- M. D. Chadwick, J. W. Goodwin, E. J. Lawson, P. D. A. Mills and B. Vincent, Colloids Surf., A, 2002, 203, 229 CrossRef CAS.
- N. M. Dimitrijevic, E. Rozhkova and T. Rajh, J. Am. Chem. Soc., 2009, 131, 2893 CrossRef CAS.
- G. Cravotto and P. Cintas, Chem. Soc. Rev., 2006, 35, 180 RSC.
- R. Silva, H. Ferreira and A. Cavaco-Paulo, Biomacromolecules, 2011, 12, 3353 CrossRef CAS.
- C. Ogino, N. Shibata, R. Sasai, K. Takaki, Y. Miyachi, S. Kuroda, K. Ninomiya and N. Shimizu, Bioorg. Med. Chem. Lett., 2010, 20, 5320 CrossRef CAS.
- J. Wang, Y. Guo, X. Jin, L. Liu, R. Xu, Y. Kong and B. Wang, Ultrason. Sonochem., 2011, 18, 177 CrossRef CAS.
- X. Ragas, A. Jimenez-Banzo, D. Sanchez-Garcia, X. Batllori and S. Nonell, Chem. Commun., 2009, 2920 RSC.
- A. Gollmer, J. Arnbjerg, F. H. Blaikie, B. W. Pedersen, T. Breitenbach, K. Daasbjerg, M. Glasius and P. R. Ogilby, Photochem. Photobiol., 2011, 87, 671 CrossRef CAS.
- S. Yamaguchi, H. Kobayashi, T. Narita, K. Kanehira, S. Sonezaki, N. Kudo, Y. Kubota, S. Terasaka and K. Houkin, Ultrason. Sonochem., 2011, 18, 1197 CrossRef CAS.
- E. Fabian, R. Landsiedel, L. Ma-Hock, K. Wiench, W. Wohlleben and B. van Ravenzwaay, Arch. Toxicol., 2008, 82, 151 CrossRef CAS.
- H. U. Ahmed, E. Zacharakis, T. Dudderidge, J. N. Armitage, R. Scott, J. Calleary, R. Illing, A. Kirkham, A. Freeman, C. Ogden, C. Allen and M. Emberton, Br. J. Cancer, 2009, 101, 19 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: turbidity and TG/DTA data for the complexes, AFM image, the data of DLS and ROS generation reaction, and MTT assay for the micelles. See DOI: 10.1039/c2bm00066k |
| This journal is © The Royal Society of Chemistry 2013 |
