A bio-inspired neural environment to control neurons comprising radial glia, substrate chemistry and topography†
Paul
Roach‡
a,
Terrance
Parker
b,
Nikolaj
Gadegaard
c and
Morgan R.
Alexander
*a
aLaboratory of Biophysics and Surface Analysis, School of Pharmacy, University of Nottingham, University Park, Nottingham NG7 2RD, UK. E-mail: morgan.alexander@nottingham.ac.uk; Fax: +44 (0) 115 9515102; Tel: +44 (0) 115 9515119
bSchool of Biomedical Sciences, Medical School, Queen's Medical Centre, University of Nottingham, Nottingham NG7 2UH, UK
cDivision of Biomedical Engineering, Rankine Building, University of Glasgow, Glasgow G12 8LT, UK
First published on 3rd October 2012
Abstract
Achieving alignment of cells is key to the success of regenerative strategies of neural tissue. We report a high-throughput method to investigate neural cell response to surface chemistry overlaid orthogonally onto a gradient of gradually changing groove widths. Using a bio-inspired approach wherein radial glial cells, which naturally guide neurons in the developing brain, enhance the attachment and directional outgrowth of neurons, we show the differences in the interaction and cellular response of glia, neurons and co-cultured cells. Radial glia were found to preferentially reside in grooves of width 6–35 μm with greater alignment to grooves <10 μm on the hydrophobic and hydrophilic extremes of chemistry. When neurons were sequentially cultured after radial glia, they showed enhanced alignment compared to when they were cultured alone, for all chemistries and groove widths. This is not dependent on co-localisation of the neurons with glia suggesting the radial glial cells pre-condition the substrate giving rise to enhanced attachment and alignment of subsequently cultured neurons. The results indicate a dependence of both primary radial glia and neuron responses on surface chemistry and micro-groove width. Grooved surfaces (width 5–10 μm) of mid-range wettability show the greatest potential to significantly enhance axonal alignment and, therefore, potential regeneration, when pre-conditioned by radial glia, highlighting the importance of surface engineering for neural scaffolds.
Introduction
Cells, in vivo, interact with a complex microenvironment comprised of many cell types and other structural components from the extracellular matrix (ECM). The milieu experienced by cells is dynamic due to the cascade of cell–cell signalling that is constantly occurring to mediate the environment, regulating cellular functions, such as adherence, migration, proliferation and differentiation.1,2 Tissue engineering strategies for transplant therapies may benefit from approaches that mimic in vivo environments, using multiple cell types as a potential means to replicate a natural environment for cells cultured in vitro, which moves away from isolated/purified cell populations on tissue culture plasticware.3 The inter-relationship of different cell types is particularly important when considering the nervous system, wherein many different cell types act in synergy to develop and sustain the system. The co-culture of cells in vitro has, therefore, become of much interest in order to further understand, and make use of, natural cell–cell signalling cues.4Tissue engineering for the replacement or repair of damaged nerve tissues involves the manipulation of highly complex processes, with many factors affecting the reconstruction of aligned and functional neuronal pathways. Regeneration of the nervous system therefore remains a clinical challenge, with many researchers looking towards natural materials for ‘bio-inspired’ approaches to improve cell alignment, connectivity and maintenance.
The orientation of neurons is of the utmost importance in order to attain signal transduction along a nerve fibre or within a network. The plethora of cell types present in the nervous system have specific functions to guide neurons during development, insulate axonal extensions, support established neuronal networks and generally preserve the complex environment. Schwann cells and oligodendrocytes insulate axons by depositing a myelin sheath. Loss of these cells causes neurological impairment associated with disorders such as multiple sclerosis. The close connectivity between neurons and these supporting cells has been investigated in attempts to guide nerve growth. The success of such methods in vivo, however limited, has been attributed to the presence of the topographic and neurotrophic factors presented by the synthetic scaffold5 or supporting cells delivered within the graft construct.6
Many different types of (bio)material have been investigated for nerve repair, exhibiting varying synthetic chemical functionalities and natural signalling molecules, and have been presented in numerous construct designs. A good example of nerve conduit design, taking into account both topographic and surface chemical functionality to improve cell attachment and alignment, was shown by Haycock et al., with micro-scale electrospun polymer fibres being modified by plasma polymerisation to steer Schwann cell growth in vitro.7 More recently a more detailed study has also been carried out showing the effects of electrospun fibre dimensions on neurite outgrowth (of neuronal, Schwann cell and dorsal root ganglia alone and in co-culture).8 The development of such scaffolds has led only to the limited repair of short nerve gaps (of the order of 30 mm) in the clinic.9
Surface cues presented to cells upon adhesion have been of interest for decades.10 Surface chemistry alone or in combination with topography has been shown to provide a degree of control over cell attachment, morphology and migration, with control over differentiation also being demonstrated.11,12 Topographical cues are often used in order to direct neurite outgrowth, with additional biochemical signals (including the use of growth factors or the co-culture of differing cell types) being used to mimic natural cues presented in vivo.13 The directional growth of neurites is of key importance for the optimal function of nerve tissue due to the necessity for linear interconnections between neighbouring neurons such that an electrical signal can be carried along the nerve fibre. A deeper understanding of the developing nervous system may, therefore, aid in the reconstruction of damaged or diseased neural tissue, both of the peripheral (PNS) and central nervous systems (CNS), with bio-inspired approaches making use of natural signalling factors becoming more widely investigated. Numerous review articles have been published covering the area of biomaterial guidance cues for nerve repair.9,10,14,15
Unlike Schwann cells or oligodendrocytes that closely inter-connect with neurons, providing neuro-protection and insulation, Bergmann glial cells or ‘radial glia’ primarily guide the migration of neurons in the developing brain.16,17 Radial glia are ubiquitous in the development of all vertebrate brains, being found in their highest concentration in the cerebella in developing rat models.18 These cells are believed to differentiate to form neurons or glial cells with some remaining as Bergmann glia in the adult brain and optic nerve fibers.19,20 Neurons actively migrate along the long radial glia processes, possibly directed by chemical signals excreted by the cells as well as the topographical cue presented by the cell morphology.21 Such characteristics may be harnessed to provide supporting cues for nerve reconstruction. Although radial glia provide guidance cues and serve as neural progenitors in all regions of the CNS,22 the close connectivity of these cells with neurons might lead to advances in tissue engineering and regenerative medicine across both the CNS and PNS.
The capability to design specific surfaces to dictate cell responses is critical for the advancement of regenerative medicine strategies. Cell directional control is particularly crucial for neural cell control, where directional communication between neighbouring cells is necessary. The application of surface control may have broad ranging impact, not only on the development of artificial nerve conduits for future therapies of damaged or diseased nervous tissue, but also for the study of model neurological diseases in vitro. Lab-on-a-chip devices are often used to culture model systems,23,24 with close connectivity of many differing cell types (and communication between them) being key for neural models.
A substrate having a gradient in chemistry presented orthogonally to a series of microgrooves with gradually changing dimensions has previously proved useful in assessing the attachment and alignment of cells to a wide range of groove topographies with a selection of surface chemistry.25 This approach allows a high-throughput analysis of cell–surface interactions since all the combinations of chemistry and topography are on the same sample. Here, we explore the cell–surface response of neural cells alone and in sequential culture using a substrate fabricated with grooves ranging in width from 5 to 95 μm with surface chemistry varying from a hydrocarbon to a nitrogen-containing polymer. We demonstrate that the role of radial glia in neurite outgrowth can be harnessed in vitro by first establishing an adhered glial layer. The potential for biomimetic surfaces to enhance desired cellular responses may lead to advanced materials and implantable surface engineered constructs.
Materials and methods
Micro-patterned polymer substrates
Substrates having parallel grooves, with widths gradually increasing across the substrate, were prepared in poly(methyl methacrylate) (PMMA) via hot embossing against a silicon master, Fig. 1.25 The topographical patterns, covering an area of 10 × 10 mm2, consisted of repeating 5 μm wide ridges separated by grooves ranging from 5 to 95 μm, being ∼3.4 μm deep.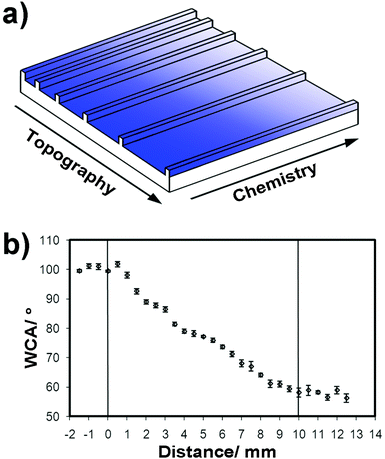 | ||
| Fig. 1 (a) Gradient platform schematic with changing chemistry represented by the gradient colour; the groove width varied on a log scale 5–95 μm, with a constant ridge depth of 3.4 μm; (b) wettability of a gradient ppHex–ppAAm layer. The error bars relate to the standard deviation of six repeats at each relative position. | ||
Chemical modification
A thin plasma polymer overlayer (∼1–2 nm) was applied across the grooved substrate to impart a gradient in chemistry at the surface. This chemical gradient was oriented orthogonal to the groove direction, giving rise to a platform on which a range of sized topographic features were presented, all having the full range of chemistries. Plasma polymer coatings were fabricated in a T-shaped borosilicate chamber closed with stainless steel endplates. External copper band electrodes situated at either end of the chamber were used to initiate the plasma. A 13.56 MHz radio frequency power source (Coaxial Power System Ltd) was used with the power being matched manually to maintain 20 W input and <1 W reflected power. Allylamine and hexane monomers were obtained from Sigma–Aldrich and degassed thoroughly via freeze pump thaw cycles immediately prior to use. Plasma polymerisation was carried out at a working pressure of 300 mtorr. Plasma-polymerised allylamine (ppAAm) was deposited across the whole sample surface before a subsequent layer of plasma-polymerised hexane (ppHex) was deposited. A glass coverslip positioned 40 μm above the sample surface was used as a mask, giving rise to a shallow thickness gradient of ppHex. An internalised thickness shear monitor allowed reproducibility of the deposited polymer films.Wettability analysis
Wettability measurements on flat chemical gradient surfaces were performed on a DSA100 instrument (Krüss, Germany) using picolitre ultrapure water droplets (∼18 M Ohms). Evaporation of such small droplets shows an apparent reduction in the water contact angle (WCA), so a repeating frame capture set at 18 ms was used with images taken over 1 s.26 The first stable droplet in contact with the surface was used for WCA determination using tangential lines to the droplet at the three phase boundary. An average of six measurements were taken from each sample position.Cell isolation
All animal experiments were approved by the Home Office and were in accordance with the animal handling guidelines of the University of Nottingham. Primary radial glia and neurons were isolated from E14–16 cerebella and P1 cortex, respectively, from Wistar rats. The brains were removed and placed in ice cooled Hank's buffered saline solution (HBSS). Cerebella and cortices were then separated for radial glia and neuron preparations, respectively. The brain regions were finely chopped before incubating in 0.1% trypsin (Worthington Biochem.), 0.5% DNase (Worthington Biochem.) in HBSS at 37 °C, 5% CO2 for 30 minutes. The tissue was then washed with HBSS and then carefully triturated to dissociate cells in HBSS containing 0.5% DNase. After centrifuging at ∼800 rpm for 3 minutes, cells were re-suspended in culture media consisting of 1% L-glutamine (Sigma) and 10% foetal bovine serum (FBS) (Sigma) in Dulbecco's modified eagle medium (DMEM) (Sigma) and transferred into 75 cm2 flasks at a density of 5 × 105 cells mL−1. Cells were incubated in a humidified atmosphere at 37 °C, 5% CO2. As glia adhere relatively rapidly to tissue culture plastic compared to neurons the two cell types can be separated by differential adhesion. Cells were allowed to rest for 4 hours in between passages, with 5 differential adhesion cycles being used to obtain enriched fractions. Cell populations were assessed through optical and fluorescence microscopy. Radial glial cells were further enriched by fluorescence-activated cell sorting (FACS) using a specific marker, 3CB2 (Developmental Studies Hybridoma Bank) conjugated to a fluorophore (goat-anti-mouse Alexa Fluor 488) (Invitrogen). This enrichment step was carried out immediately prior to seeding radial glia.Neurons were isolated from cerebral tissue in a similar manner to that described above. Cortex tissue was removed from P1 rat frontal lobes, digested and differentially cultured to separate neurons. Neuron enrichment was carried out via five differential adhesion cycles, wherein the non-adhered cells were taken forward from step to step. After this procedure, almost all cells stained positive for neurofilament indicating that the enrichment was successful (∼20 fold enrichment).
Cell culture
After isolation of the primary material, cell cultures were kept in a humidified atmosphere at 37 °C, 5% CO2. Enriched neuron fractions were passaged every 4 days by enzymatic digestion using 0.1% (v/v) trypsin in phosphate buffered saline (PBS, pH 7.4, Dabco). Neurons of passage number 3 were used for this study. The single cell suspensions were used for seeding and cell counting was performed using a haemocytometer, adjusting the seeding density to 104 cells cm−2 on the substrates housed in 6 well plates. Enriched fractions of radial glia were seeded directly from FACS analysis onto prepared gradient platforms 2–3 days after plasma deposition. Due to lower cell numbers of the radial glia, all cells collected were diluted in media and seeded onto all surfaces at a density of ∼5 × 103 cells cm−2, using an adequate volume of media to cover the surface (∼100 μL). After initial attachment over 2 hours the media volume was increased to 2 mL. Cells were cultured for an allotted time on the substrates, after which they were rinsed gently three times with warmed PBS to remove any unbound cells and proteinaceous material and fixed in 1% paraformaldehyde (PFA, Sigma) in PBS at room temperature for 15 minutes. The substrates were then rinsed again in PBS three times before being stained with appropriate antibodies and fluorescent markers for further analysis.Antibody and fluorescent labeling
Alexa Fluor 488 phalloidin (Invitrogen) and DAPI (Vector Shield) were used to stain cytoskeleton F-actin and nuclei, respectively. Co-localisation of mouse-anti-3CB2 (DSHB) and anti-nestin (Millipore) was used to confirm the identity of radial glia populations27 with specific radial glial marker 3CB2 being used along with anti-neurofilament (Millipore) to visualise co-cultures of radial glia and neurons. Secondary Alexa Fluor antibodies were used for complementary staining. All antibodies were diluted in PBS.Image analysis
Cells were visualised via manual optical microscopy or using a confocal microscope fitted with an automated stage. Cell attachment and alignment with substrate groove direction were assessed using ImageJ software. Confocal fluorescent images of the cells on gradient substrates were segmented into 100 equal units, each being processed individually. Raw images were manually manipulated to adjust contrast/brightness and threshold levels, allowing reliable and reproducible data acquisition. Images were taken of three repeat samples per time period and the data processed for each image individually. Fluorescent labels allowed visualisation of the long cell axis to be easily identifiable. Cells presenting an angle between the groove direction and long cell axis of ±10° or less were noted as ‘aligned’ (20° aligned data is shown in the ESI†). Heat plots were constructed using Origin v8.5 showing the total cell number and numbers of cells aligned per section of platform. An area around the grooved pattern was also assessed to give information about cell interaction with the surface chemical gradient alone. Averages of 3 sample repeats are shown, with all data for all individual samples presented in the ESI.†Results
Surface characterization of gradients
Samples with gradually changing chemistry and groove width were prepared by deposition of conformal plasma polymers on topographically patterned PMMA employing a mask under which deposited species could diffuse to form a chemical gradient. The surface chemistry of these materials has been thoroughly characterised using X-ray photoelectron spectroscopy (XPS), time of flight secondary ion mass spectrometry (ToF-SIMS) and water contact angle (WCA) measurements and presented in previous publications.25,28 To ensure that the production of the ppHex to ppAAm chemical gradient surfaces was comparable to these previous studies, wettability measurements were conducted using sessile drop WCA. The WCA was measured on a flat border along the edge of the grooved area, as shown in Fig. 1. The maximum and minimum water contact angles associated with the ppHex end of the gradient (unmasked) and the ppAAm end, respectively, compare well with those previously reported.25 A linear transition between these two values over a distance of 10 mm was also observed, consistent with these previous works. This is formed as a result of the diffusion limited ingress of species that form the ppHex deposit on the pre-deposited ppAAm surface.Neural cell enrichment
E14–E16 Wistar rats were used to obtain the largest population of radial glia employing isolation and purification steps. Glial cells are known to adhere more rapidly to tissue culture plastic compared to neurons, allowing differential adhesion to be used to separate the varying cell types. Isolation of the specific cell types was carried out by 5 differential adhesion cycles, during which glia were progressively enriched by removal of other cell types. A large fraction of cells after this stage were observed to have a high degree of co-localisation of nestin and 3CB2 (ESI,†Fig. 1a). Fluorescence microscopy using co-staining of nestin, 3CB2 and DAPI highlighted that not all cells were radial glia following differential adhesion. Fluorescence-activated cell sorting was employed to further purify radial glia, using 3CB2 as the radial glial marker.29 FACS analysis revealed an increase in the number of cells positively stained for 3CB2 after 5 differential adhesion cycles (∼2% of the total population compared to ∼15%). Tight restrictions were placed when gating the FACS collection of positive cells, resulting in low final cell numbers but, importantly, high confidence in the cell type selected. After FACS almost all cells showed co-localisation of nestin and 3CB2 (ESI,†Fig. 1b and 2). Similarly an enriched neuronal population was obtained from cortical tissue, showing high fractions of neurons by immunohistochemistry.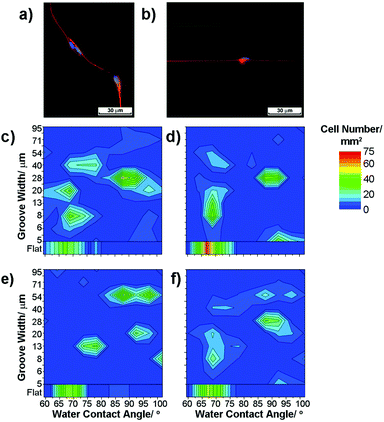 | ||
| Fig. 2 Fluorescence images of radial glia on (a) flat and (b) ppAAm grooved substrates (red – 3CB2 cytoskeletal marker for radial glia, green – nestin and auto-fluorescence from the PMMA substrate, blue – DAPI nuclei stain). (c–f) Heat plots of radial glia cell numbers cultured for 3 days; ‘hotter’ colours denote higher cell numbers. Note that (c–e) are individual repeats, shown as a mean average in (f). | ||
Cell response to chemical and topographical gradients
Substrates presenting gradients in both surface chemistry and orthogonally aligned grooves were used to investigate the cell responses of neurons and radial glia alone and in sequential culture. Such a substrate allowed a multiplexed investigation of chemical and topographical effects, ranging from hydrophilic wide grooves to more hydrophobic narrow grooves and all combinations in between.Radial glia response
After enrichment and isolation, radial glia were seeded onto gradient substrates from a homogeneous suspension in media. To ensure uniform exposure of the cells to the substrate, an adequate cell density and media volume were used to allow seeding across the whole surface. Cells were then cultured for 1, 3 and 15 days, with the media changed after the first day and every 2 days thereafter. During the media change any unbound cells were removed, although the surfaces were not washed so any loosely bound cells remained. Fixation using PFA was carried out after 1, 3 and 15 days to allow fluorescence microscopy to be used to visualise cells in order to quantify their number and morphology. Radial glia were found initially to attach and spread well on areas across the substrates, extending projections in random directions on flat areas and becoming aligned with groove walls within the patterned area, Fig. 2a–b.Using an automated microscope stage to capture images from the whole area of the substrate, cell responses were correlated to specific locations and, in turn, to specific surface characteristics. The images captured from 1 mm2 sections were analysed individually to map out cell response characteristics in relation to surface wettability and groove width. Cell numbers within a mm2 region are shown as the cell density, with cell alignment being recorded as the number of cells aligning their projections within ±10° of the groove direction (±20° is also given in the ESI†) or normalised to the total number of cells mm−2 and shown as a percentage aligned in Fig. 2c–e.
Data from three replicates of each surface were analysed separately to assess reproducibility. As an example of the reproducibility observed, Fig. 2c–e show the number of radial glia attached to the gradient substrate after 3 days in culture on three separate samples. The cell number within a specific region of the platform is presented using a colour intensity scale where the ‘hotter’ colours convey a higher cell density. Some variation between samples can be observed, although the main ‘hotspots’ of cell attachment and alignment were largely consistent and averages of data clearly show the trends observed in the individual samples, Fig. 2f.
On flat surfaces (Fig. 3) glia were found to be present at a higher density in the mid-point of wettability tested (WCA ∼ 75–80°, p < 0.05) at day 1, becoming more dense towards more hydrophilic nitrogen-containing surfaces (WCA ∼ 60–75°) by day 3. This may be expected as it is well known for many cell types to attach more readily to amine-containing surfaces compared to hydrophobic hydrocarbon surfaces.28 On samples analysed after 15 days, a slightly higher number of cells were observed residing towards the more hydrophobic end of the chemical gradient (WCA ∼ 90–95°). Although cells on flat surfaces were not in contact with the grooved regions, their ‘alignment’ was assessed for completeness. Cells were found to be randomly oriented on flat surfaces.
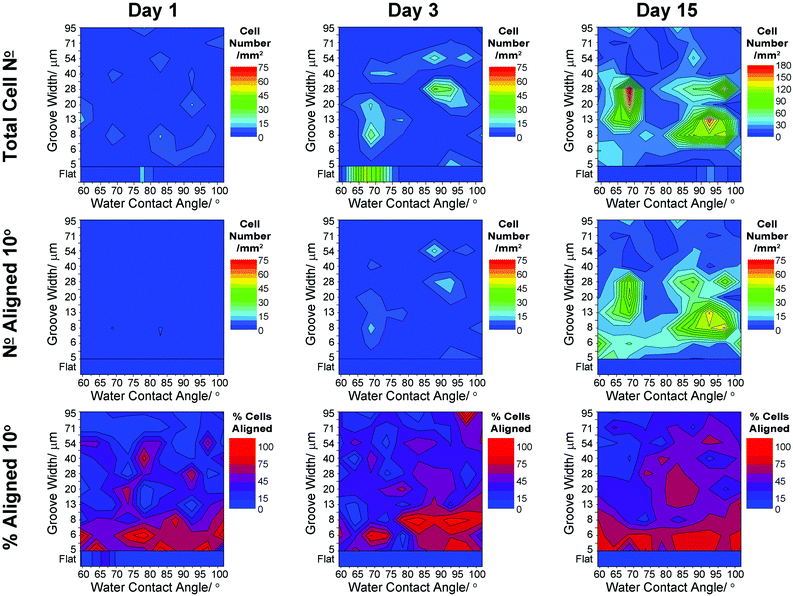 | ||
| Fig. 3 Heat scale plots showing variation in radial glia responses to surface wettability and groove width: cell density, cell number and percentage cells aligned to within 10° of the groove direction. Averages shown for all data (n = 3) from 1, 3 and 15 days in culture. | ||
On gradient surfaces the radial glial cell density was observed to vary, with regions of high density highlighted by the heat plots in Fig. 3. After 1 day in culture there was little variation in cell number, suggesting that seeding and initial attachment was fairly even. At day 3, localised regions of higher cell density were observed: one region being at WCA ∼ 65–70°, groove width 6–25 μm and the other being at WCA ∼ 85–95°, groove width of 20–35 μm. After 15 days in culture these regions remained, presenting higher cell densities. Neural alignment is known to be difficult to retain over long periods of time, although this characteristic is certainly a requirement for any material designed for neural repair or regeneration. Cells aligned within 10° of the groove direction were mostly limited to grooves having widths 8–35 μm, with a high degree of radial glia alignment still presented after 15 days in culture on groove widths <35 μm.
Heat plots clearly show variance of the cell response with respect to the surface chemistry and topography, although reducing the data down to a 2D format is useful also to highlight general trends associated only to either chemical or topographic effects. The data presented in Fig. 4 are summed data across each characteristic surface cue. Normalisation of the aligned local cell number data to the total number of cells present, allows comparison of the alignment. This is important when considering the overall degree of cell alignment in response to the substrate cues. Each data point represents an average of three independent samples, each being cultured, fixed and assessed at the relative time point. Fig. 4a–b show the average percentage of cells aligned with respect to (a) grooved areas and (b) the gradient in wettability for radial glia; lines are included in the plots to highlight the general trends.
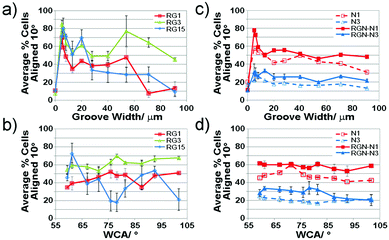 | ||
| Fig. 4 Line plots showing the average percentage cells aligned with the groove direction with respect to (a) and (c) the groove width; (b) and (d) the wettability gradient; (a) and (b) refer to radial glial cells, whereas (c) and (d) refer to neurons alone (dashed lines) and neurons co-cultured with radial glia (solid lines). RG – radial glia; N – neurons; RGN – neurons co-cultured with radial glia. Error bars show the standard error for three replicates of each sample point. Connecting lines are shown to highlight the trends only. | ||
Interrogation of the data in this format highlights that a higher fraction of radial glial cells align to grooves of width 5–10 μm after 1 day in culture, with little variation apparent with increasing culture time. As a general trend, some alignment was also observed after 1 and 3 days in culture around 50 μm grooves. Radial glia alignment was affected by surface wettability, generally indicating a higher proportion of aligned cells at mid-ranging wettability. After 15 days in culture, the summed average of data for each WCA showed higher alignment of radial glia at each end of the wettability gradient.
Neuron response
Neurons were found to attach and spread well on the substrates. On flat surfaces, slightly higher densities were observed on areas of mid-ranging wettability (WCA ∼ 80–85°) after 1 day in culture, Fig. 5. After 3 days in culture, neurons were somewhat more evenly distributed, with slightly higher cell densities tending towards more hydrophilic surfaces (WCA ∼ 60–80°). Neurons were randomly oriented on the flat surfaces, showing no significant alignment to the chemical gradient.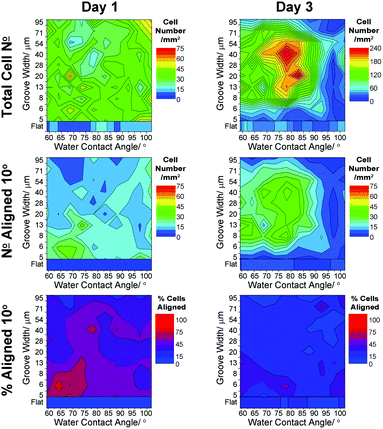 | ||
| Fig. 5 Heat scale plots showing variation in neuron responses to surface wettability and groove width: cell density, cell number and percentage cells aligned to within 10° of the groove direction. Averages shown for all data (n = 3) from 1 and 3 days in culture. | ||
Neurons cultured on grooved surfaces showed quite an even distribution after 1 day in culture, indicating that initial seeding and attachment of cells was even across the whole substrate. After 3 days of culture, the cell density increased significantly at mid-ranging wettability (WCA ∼ 75–85°) and groove width 15–55 μm. This increase was concurrent with a slight decrease in density towards surfaces of higher wettability (WCA ∼ 92–102) and smaller groove width (<7 μm). The number of neurons aligned in the groove direction followed a similar pattern, with a slight indication of cells being aligned more after 1 day on hydrophilic surfaces of groove width 5–7 μm (Fig. 4c–d, dotted lines; ESI† and Fig. 3). After 3 days, the percentage of aligned neurons decreased considerably across the whole surface, with the majority of neurons remaining aligned found to reside towards the more hydrophilic end of the gradient (WCA < 90°) within grooves having widths 7–55 μm. The reduction in cell alignment after only 3 days in culture showed that even in areas where high numbers of cells were found to be aligned, there were also many unaligned cells.
Neuron response when sequentially cultured on radial glia
In this investigation, the alignment of neurons was of particular interest. After 15 days of glial culture on the gradient substrates, neurons were added and cultured for a further 1–3 days. Immunostaining with fluorescent markers allowed clear differentiation of neurons and glia in co-culture. Data obtained from these co-cultures gives insight into the effects of cell–cell interactions. Neurons were found to initially adhere across the gradient platform, preferentially co-localising with pre-adhered islands of radial glia, as highlighted in Fig. 6 (white arrowheads). Co-localisation of neurons with pre-adhered radial glia was independent of the size of the radial glia island (ESI,†Fig. 4). On flat surfaces, neurons in co-culture were found to adhere in slightly higher numbers on hydrophilic surfaces after 1 day in culture; whereas, after 3 days, a slightly higher density was observed towards intermediate wettability (Fig. 7).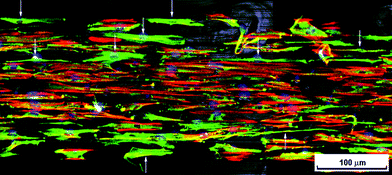 | ||
| Fig. 6 Fluorescence microscopy image of neurons seeded onto radial glia after 1 day in co-culture. Region of platform having WCA ∼ 85–90° and groove width ∼10 μm. Arrow heads indicate neurons (red – 3CB2 cytoskeletal marker for radial glia, green – neurofilament and auto-fluorescence from the PMMA substrate, blue – DAPI nuclei stain). | ||
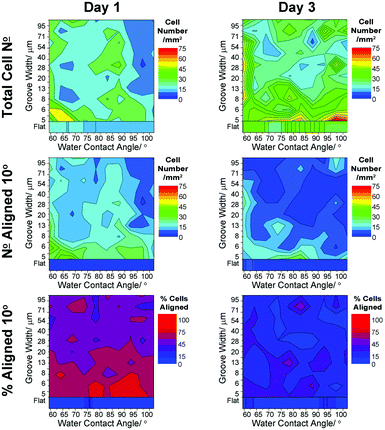 | ||
| Fig. 7 Heat scale plots showing variation in neuron density and alignment when co-cultured with a mature radial glial population: neuron cell density, cell number and percentage neurons aligned to within 10° of the groove direction. Averages shown for all data (n = 3) from 1 and 3 days in culture. | ||
On dual gradient surfaces, where both substrate chemistry and topography were present, along with radial glia chemical and morphological cues, neurons were found to behave differently compared to those in mono-cultures. In this study, these co-culture samples showed the most differences between repeats, possibly resulting from compounded variations observed due to the initial radial glia–surface interaction and sequential neuron–glia interaction (all supporting data are shown in the ESI†). However, the trends observed in the same regions in all repeats are clearly represented in the averaged data.
After 1 day in co-culture, neurons were observed to have a higher density localised in the area presenting a more hydrophilic ppAAm surface (WCA ∼ 60–70°) and grooves of width 5–8 μm. After 3 days in co-culture, the neuron density was found to be evenly spread with higher densities found across the range of wettabilities on grooves <5 μm. A slightly higher density was also apparent across a wider range of groove widths (7–54 μm) on the most hydrophilic edge of the platform. This was not related to an artefactual collection of cells at the edge of the platform as only minimal cell density was observed outside the grooved area.
In co-culture, aligned neurons were observed to be more abundant on areas reflecting a higher cell density. After 1 day, aligned neurons were found at higher densities within narrow grooves (<8 μm) (Fig. 4c and 7) with a localised hotspot at WCA ∼ 60–70°. After 3 days, lower localised alignment was observed, with higher neuron density found at the most hydrophilic edge of the platform and on the smaller grooved areas <6 μm. Normalised data also indicates a higher percentage of aligned neurons on small grooves to a much greater extent after only 1 day in culture compared to 3 days (Fig. 4c–d).
Discussion
Cell culture on substrates presenting dual chemical and topographical gradients acted as a screening tool to highlight ‘hotspots’ of cell density and alignment with respect to specific surface cues. Here, we discuss the response of radial glia and neurons in mono-culture with respect to surface chemistry and topography (both independently and in combination) and further the sequential culture of neurons onto pre-adhered radial glia on gradient substrates.Neural cell response on chemical surface gradients
Interestingly, many more cells, both radial glia and neurons, were found attached on grooved areas of the substrate compared to flat regions, possibly indicating a connection between alignment and adhesion, which are factors of importance for nerve regeneration. Since no negative responses, such as cell death, were observed for cells initially adhering to the gradient substrates, any responses in terms of varying cell density at differing time periods may be attributed to either cell proliferation or migration.Radial glia were found to initially adhere onto flat surfaces presenting mid-ranging wettability (WCA ∼ 75–85°, p < 0.05), although, after 3 days in culture, a higher cell density was observed towards more amine-rich hydrophilic surfaces (p < 0.04) and, by day 15, hydrophobic (WCA ∼ 90–95°, p < 0.03) portions of the chemical gradient were favoured, Fig. 3. In grooved areas of the platform, radial glia were found to preferentially attach in islands at either end of the chemical gradient, a pattern observed with initial 1 day attachment, which became more prominent by 15 days in culture.
In contrast to glia, neurons were found to respond somewhat differently to the gradient surfaces. After 1 day in culture, neurons were evenly distributed across the whole platform, Fig. 5, indicating a lack of significant initial surface cue-directed attachment. Where radial glia were observed at high densities in two zones surrounding the area of mid-ranging wettability, neurons were observed to cluster in this area. Similar clustering characteristics have been observed for other cell types.25,30
On flat surfaces, radial glia, as well as neurons, were observed to be randomly orientated, having no topographical guidance cues to steer their migration or extensions. Others investigating cell–surface interactions have shown that surface chemistry can quite readily steer the attachment of cells to specific areas, such as cell spreading being hindered on hydrophobic areas, whilst migration and attachment is promoted on hydrophilic or charged regions.25,30
Neural guidance using a combination of chemical and topographical surface cues
The alignment of cells can be assessed by the degree of orientation of cell processes in relation to specific surface properties. There is little consistency between research groups with a wide range of angles being used to classify a cell as being aligned. Largely, this fits into within 30° of the feature direction. In this study, we focused on highly aligned cells having their long axis within 10° of the groove direction, although we also looked more broadly at 20° (data shown in the ESI†). As could be expected, the number of cells aligned increased where clusters of cells were observed. For this reason, data were normalised with the corresponding percentage of cells aligned per unit area.Radial glia
Even after only 1 day in culture, radial glia more closely aligned with smaller grooves ∼5–10 μm wide, Fig. 3. 60–90% of cells within this region, spanning the whole chemical gradient, were found to be aligned, which persisted over 15 days. The region on which alignment was observed, however, did not generally expand to larger groove widths, suggesting that the surface topographical cue dominated cell elongation irrespective of where cells adhered. This effect has been observed by others demonstrating similar axonal extension along both chemical31 and topographical patterns.13On gradient platforms, we can consider effects of surface chemistry and topography alone or in combination. A much higher fraction of radial glia were found to align on narrow groove widths compared to flat and larger grooved areas, Fig. 4a. The edge density presented to cells by surface topography has previously been shown to impact on cell adhesion.32,33 Smaller grooves present much higher edge density compared to flat surfaces or areas presenting wider grooves, which impacts on the degree of interaction between cells and the surface topography. Interestingly, in all samples analyzed, radial glia showed an increase in alignment on 20 μm width grooves. The percentage of aligned radial glia onto grooves of this size increased with the culture time, possibly due to the size of the groove matching more closely with the cell body. A higher degree of radial glia alignment was observed within grooves >40 μm, reaching 60–80% across a range of groove dimensions by day 3, Fig. 4a. This again may be attributed to cells becoming associated with the groove walls over time and therefore becoming aligned. After 15 days in culture, however, this degree of alignment decreased to values initially observed at day 1. Such a change may again be attributed to cell migration. At early time points migration around the surface would allow contact of cells with groove edges. Conditioning of the surface by adsorbing species from the media, and by cell secreted molecules, is a highly dynamic process. After prolonged culture, the surface is likely to be (bio)chemically very different. It is possible that the domination of alignment dictated by contact guidance becomes the lesser controlling effect compared to the cell interaction with conditioned surfaces.
Surface chemistry, considered alone, was found to impact on radial glia alignment. Alignment after 1 and 3 days in culture was higher in areas of mid-ranging wettability, Fig. 4b. After 15 days, alignment decreased significantly in this region with greater remaining alignment being observed on more amine rich, hydrophilic surfaces. This trend may again be attributed to the ability of cells to move on the surface.
Neurons
Some alignment of neurons along the direction of grooves was observed after only 1 day in culture, as with the radial glia (ESI,†Fig. 3), suggesting that the cells were able to locate onto the groove walls. A higher fraction of neurons were found to align onto fine grooves (<8 μm) at day 1, with a similar trend observed at day 3, Fig. 4c. Neurons located in fine grooves presenting hydrophilic chemistry were found to have the highest alignment, Fig. 5. Neuron alignment was not found to follow any significant trends with respect to surface chemistry alone, although slightly higher alignment was found for neurons residing in grooved areas presenting a WCA ∼ 70°, Fig. 4d. This indicates that both topographical and chemical cues play an important role in determining neuron alignment.Where radial glia were observed to generally remain aligned during the 15 days in culture, fractional neuron alignment decreased significantly by day 3 (max ∼90% within 1 mm2 at day 1, decreasing to ∼40%). This loss of alignment is indicative of neuron culture over long periods.13 Neurons cultured on dual gradient surfaces showed very different responses compared to radial glia, indicating that optimal surface cues for attachment and cell guidance are cell-type dependant.
Neuron interaction with radial glial-conditioned surfaces
Using radial glia to pre-condition surfaces allows for a more natural ‘bio-inspired’ surface to be presented to subsequently cultured neurons. Conditioned by proteins from the media and cell-secreted factors, as well as the cell bodies presented by the glia themselves, neurons are exposed to a mix of cues and respond accordingly. For the purposes of this report, a hydrophilic to hydrophobic chemical gradient will be used to denote the regional specificity of the platform, although it is clear that, after 15 days in culture, the native chemical functionality of the fabricated surfaces may no longer be available for the neurons to observe directly due to accumulation of adsorbed proteins.Neuron densities were found to differ when cultured in mono- and co-culture with radial glia, Fig. 5 and 7. After only 1 day in co-culture, a small region of neuron high density was found towards the hydrophilic portion of the gradient (WCA ∼ 60–70°) having <8 μm grooves; the degree of this alignment was higher than that observed for neurons cultured alone. Radial glial cells were found to be present in high numbers in this region, possibly indicating an effect to encourage neuron attachment.
After 3 days in co-culture, neuron numbers in this region fell with an increase in their number in adjacent areas, where glial numbers were less. This suggests that the presence of radial glia may promote attachment neurons through conditioning of the surface. Radial glia-secreted factors may act as a chemo-attractant for migrating cells after initial attachment—a process akin to that which naturally occurs in the developing brain.
Co-localisation of neurons with islands of adhered radial glia was observed, although groups of neurons were also found in areas without radial glia. Those co-localised were found to attach adjacent to, rather than on top of, groups of glia, Fig. 6. This possibly suggests a preference of neurons to form a monolayer rather than to attach directly onto the radial glia bed. The spread of neurons across the whole gradient platform demonstrates a change in surface chemical properties during glial cell culture, which alters the specificity of neurons to attach onto native surfaces of mid-ranging wettability. Others have shown similar responses in terms of cell–surface interactions, with surface chemical functionality becoming masked by protein/cell conditioning.34
Examining the effects of surface cues independently on the degree of neuron alignment shows little summed variation with respect to either chemistry or topography, Fig. 4. Neuron alignment, however, was significantly enhanced across both gradients when cultured with radial glia. Separating out the effect of groove width (Fig. 4c, solid vs. dashed lines), it is clear that the same general trends are observed for neurons cultured alone and those cultured with radial glia, although a dramatic enhancement in neuron alignment was observed for the latter. After 1 day in culture, 20% more neurons were found to be aligned to grooves <8 μm wide. Alignment was still increased at day 3 although to a lesser extent. Similar observation was found considering the surface chemistry gradient, Fig. 4d, with ∼10% enhancement in neuron alignment.
Higher fractions of neurons across the whole platform were more closely aligned with the groove direction when cultured with radial glia, suggesting a synergistic effect of surface conditioning and topographical cues. Regions with the highest alignment were found across the chemical gradient on groove widths ∼5–10 μm, covering a much larger area on the platform compared to neurons cultured alone, Fig. 4a and 7. This data matches closely with that found for radial glia, suggesting a strong effect of communication between the cell types. Interestingly, after 3 days in culture, a much lower degree of neuron alignment was observed.
Mechanism of neuron guidance by radial glial co-culture
It is clear that cells can be guided to reside in specific locations on substrates in vitro, having some control over their attachment and alignment using chemical and topographical cues. Amine-containing polymer surfaces are well known to support cell attachment and viability, often rationalised as resulting from the electrostatic interaction of the cell membranes with positively charged amine groups presented at the surface.35 Cell–surface interactions can also be rationalised by cells interacting with the layer of protein molecules pre-adsorbing from solution at shorter timescales than those required for cell binding events. Conditioning of the surfaces by proteins is understood to be influenced by surface chemistry34 and nano-topography,36 with protein orientation, conformation and adsorbed amount, as well as the underlying surface wettability, playing vital roles in the subsequent cell adhesion.37The specific chemical functionality presented by the gradient surface will consequently be coated by adhering proteins and will no longer be available for direct interaction during the timescales involved for cell culture. The surface chemistry will, however, impact on the characteristics of the protein layer and will, therefore, indirectly impact on subsequently adhering cells. It is likely that the composition of the protein layer changes across the chemical gradient and with time due to protein adsorption and surface conditioning being a dynamic system. Furthermore, proteins secreted by the radial glia themselves (rather than those delivered in the culture media) would constitute a better environment for these cells and may take some time to build up to an adequate concentration at the surface. We hypothesise that the differences in neuron responses, particularly with respect to temporal variation, may be attributed to the surface becoming conditioned by secreted factors. The investigation of this may lead to new avenues of material/surface engineering for neural control, although this lies outside the scope of the current study.
Contact guidance with physically presented micro-topographical features is well documented, being related to actin nucleation in cell cytoskeletons.32,33 This mechanism of guiding cell orientation and outgrowth is also well documented and is synergistic with surface chemistry. The interaction of a cell with a physical edge can be further controlled depending on the ability of the cell, firstly, to migrate along the surface to meet the edge and, secondly, by the strength of cell attachment impacting on the ability of cells to closely interact along the length of the surface features.
Radial glia and neurons display a close interconnectivity in vivo, being found in close proximity in the developing brain, with neuronal migration steered by radial glia cell morphology and signalling proteins.38 Such close connectivity has been shown to be essential for axon conduction and normal function.39 Glia can be used to guide neuronal outgrowth,40 with radial glia specifically being shown to migrate rapidly in damaged tissues, suggesting a capability to improve neuro-protection or increase re-growth.41
Although no previously reported investigations have focused on radial glia responses to surface cues, other researchers have shown attachment of cerebella-derived neurons onto both hydrophilic and hydrophobic surfaces, suggesting the presence of a pre-adsorbing proteinaceous layer as a major factor in mediating cell attachment.42 The influence of radial glia on neurons may, therefore, extend past a topographical and chemical cue presented by the glial body, to include the conditioning of the surface by secreted molecules. The findings of this study support this hypothesis, with the neuron response being influenced by the presence of radial glia. Neurons found to localize away from areas of radial glia were still more closely aligned to groove direction compared to those neurons cultured alone. Increased alignment of neurons was also observed in areas neighbouring high densities of radial glia, further indicating that chemical conditioning acts together with the micro-scale topography to support neuron alignment.
Most researchers aiming to successfully culture neural cells in vitro currently use laminin-coated substrates due to its critical role in axonal development.43 The results of this study demonstrate that biological conditioning can give rise to enhanced neuronal cell attachment and directional outgrowth. Further investigation into radial glia secretary factors present on the surface may aid the development of protocols and materials for superior tissue engineering of the nervous system44 and advanced in vitro models of the nervous system.
Conclusions
Challenges in achieving regeneration of mammalian nervous tissue surround the ability of a diverse population of neuronal cells to adhere, migrate, align and communicate to provide the required support for such a network. In the present study, we have used a dual gradient platform as a high-throughput means to establish the effect of material surface characteristics on cell responses of both neurons and glia independently of each other and during the sequential culture of neurons on pre-adhered glia. The results presented show a dependence of both primary radial glia and neuron responses on surface chemistry and micro-groove width. Surfaces pre-conditioned by radial glial cells give rise to enhanced initial attachment and prolonged alignment of subsequently cultured neurons. Further work is required to better understand the process of surface conditioning by radial glia, although it is clear that neuron attachment and alignment can be significantly enhanced by careful consideration of the surface onto which they reside. Future surface engineering of neural scaffolds may lead to advances in the regeneration of neural tissue.Acknowledgements
This work was funded by the Medical Research Council (MRC). The authors would like to thank Sue Willington, Mary Robertson and Tim Self for their help in cell culture, fabrication of the topographically-modified substrates and fluorescence microscopy, respectively.Notes and references
- K. Y. Tsang, M. C. H. Cheung, D. Chan and K. S. E. Cheah, Cell Tissue Res., 2010, 339, 93–110 CrossRef CAS.
- T. Rozario and D. W. DeSimone, Dev. Biol., 2010, 341, 126–140 CrossRef CAS.
- T. Dvir, B. P. Timko, D. S. Kohane and R. Langer, Nat. Nanotechnol., 2011, 6, 13–22 CrossRef CAS.
- H. Kaji, G. Camci-Unal, R. Langer and A. Khademhosseini, Biochim. Biophys. Acta, Gen. Subj., 2011, 1810, 239–250 CrossRef CAS.
- F. Gelain, S. Panseri, S. Antonini, C. Cunha, M. Donega, J. Lowery, F. Taraballi, G. Cerri, M. Montagna, F. Baldissera and A. Vescovi, ACS Nano, 2011, 5, 227–236 CrossRef CAS.
- J.-S. Cho, H.-W. Park, S.-K. Park, S. Roh, S.-K. Kang, K.-S. Paik and M.-S. Chang, Neurosci. Lett., 2009, 454, 43–48 CrossRef CAS.
- C. Murray-Dunning, S. L. McArthur, T. Sun, R. McKean, A. J. Ryan and J. W. Haycock, 3D Cell Culture: Methods and Protocols, 2010, pp. 155–166 Search PubMed.
- M. F. B. Daud, K. C. Pawar, F. Claeyssens, A. J. Ryan and J. W. Haycock, Biomaterials, 2012, 33, 5901–5913 CrossRef CAS.
- M. Siemionow, M. Bozkurt and F. Zor, Microsurgery, 2010, 30, 574–588 CrossRef.
- P. Roach, T. Parker, N. Gadegaard and M. R. Alexander, Surf. Sci. Rep., 2010, 65, 145–173 CrossRef CAS.
- M. J. Dalby, N. Gadegaard, R. Tare, A. Andar, M. O. Riehle, P. Herzyk, C. D. W. Wilkinson and R. O. C. Oreffo, Nat. Mater., 2007, 6, 997–1003 CrossRef CAS.
- H. V. Unadkat, M. Hulsman, K. Cornelissen, B. Papenburg, R. K. Truckenmüller, G. F. Post, M. Uetz, M. J. T. Reinders, D. Stamatialis, C. A. van Blitterswijk and J. de Boer, Proc. Natl. Acad. Sci. USA, 2011, 108(40), 16565–16570 CrossRef CAS.
- A. Sorensen, T. Alekseeva, K. Katechia, M. Robertson, M. O. Riehle and S. C. Barnett, Biomaterials, 2007, 28, 5498–5508 CrossRef CAS.
- D. Hoffman-Kim, J. A. Mitchel and R. V. Bellamkonda, Annual Review of Biomedical Engineering, 2010, vol 12, pp. 203–231 Search PubMed.
- G. N. Li and D. Hoffman-Kim, Tissue Eng., Part B: Rev., 2008, 14, 33–51 CrossRef CAS.
- A. Sudarov and A. L. Joyner, Neural Development, 2007, 2, 26 CrossRef.
- K. W. McDermott, D. S. Barry and S. S. McMahon, J. Anat., 2005, 207, 241–250 CrossRef.
- E. Basco, F. Hajos and Z. Fulop, Anat. Embryol., 1977, 151, 219–222 CrossRef CAS.
- L. A. B. Elias, D. D. Wang and A. R. Kriegstein, Nature, 2007, 448, 901–U903 CrossRef CAS.
- S. C. Noctor, A. C. Flint, T. A. Weissman, R. S. Dammerman and A. R. Kriegstein, Nature, 2001, 409, 714–720 CrossRef CAS.
- P. Malatesta, I. Appolloni and F. Calzolari, Cell Tissue Res., 2008, 331, 165–178 CrossRef.
- T. E. Anthony, C. Klein, G. Fishell and N. Heintz, Neuron, 2004, 41, 881–890 CrossRef CAS.
- A. K. Soe, S. Nahavandi and K. Khoshmanesh, Biosens. Bioelectron., 2012, 35, 1–13 CrossRef CAS.
- J. M. Peyrin, B. Deleglise, L. Saias, M. Vignes, P. Gougis, S. Magnifico, S. Betuing, M. Pietri, J. Caboche, P. Vanhoutte, J. L. Viovy and B. Brugg, Lab Chip, 2011, 11, 3663–3673 RSC.
- J. Yang, F. R. A. J. Rose, N. Gadegaard and M. R. Alexander, Adv. Mater., 2009, 21, 300–304 CrossRef CAS.
- M. Taylor, A. J. Urquhart, M. Zelzer, M. C. Davies and M. R. Alexander, Langmuir, 2007, 23, 6875–6878 CrossRef CAS.
- D. Barry and K. McDermott, Glia, 2005, 50, 187–197 CrossRef.
- M. Zelzer, R. Majani, J. W. Bradley, F. R. A. J. Rose, M. C. Davies and M. R. Alexander, Biomaterials, 2008, 29, 172–184 CrossRef CAS.
- P. Malatesta, E. Hartfuss and M. Gotz, Development, 2000, 127, 5253–5263 CAS.
- T. Ueda-Yukoshi and T. Matsuda, Langmuir, 1995, 11, 4135–4140 CrossRef CAS.
- P. Clark, S. Britland and P. Connolly, J. Cell Sci., 1993, 105, 203–212 CAS.
- K. R. Milner and C. A. Siedlecki, Int. J. Nanomedicine, 2007, 2, 201–211 CAS.
- B. Wojciak-Stothard, A. S. G. Curtis, W. Monaghan, M. McGrath, I. Sommer and C. D. W. Wilkinson, Cell Motil. Cytoskeleton, 1995, 31, 147–158 CrossRef CAS.
- M. Zelzer, D. Albutt, M. R. Alexander and N. A. Russell, Plasma Processes Polym., 2012, 9, 149–156 CrossRef CAS.
- A. Harsch, J. Calderon, R. B. Timmons and G. W. Gross, J. Neurosci. Methods, 2000, 98, 135–144 CrossRef CAS.
- P. Roach, D. Farrar and C. C. Perry, J. Am. Chem. Soc., 2006, 128, 3939–3945 CrossRef CAS.
- M. Zelzer, M. R. Alexander and N. A. Russell, Acta Biomater., 2011, 7, 4120–4130 CrossRef CAS.
- L. J. Millet, M. E. Stewart, R. G. Nuzzo and M. U. Gillette, Lab Chip, 2010, 10, 1525–1535 RSC.
- R. D. Fields and B. Stevens-Graham, Science, 2002, 298, 556–562 CrossRef CAS.
- J. B. Recknor, D. S. Sakaguchi and S. K. Mallapragada, Biomaterials, 2006, 27, 4098–4108 CrossRef CAS.
- K. Hasegawa, Y. W. Chang, H. D. Li, Y. Berlin, O. Ikeda, N. Kane-Goldsmith and M. Grumet, Exp. Neurol., 2005, 193, 394–410 CrossRef CAS.
- R. Murugan, P. Molnar, K. P. Rao and J. J. Hickman, International Journal of Biomedical Engineering and Technology, 2009, 2, 104–134 CrossRef CAS.
- B. Grimpe, S. C. Dong, C. Doller, K. Temple, A. T. Malouf and J. Silver, J. Neurosci., 2002, 22, 3144–3160 CAS.
- J. H. A. Bell and J. W. Haycock, Tissue Eng., Part B: Rev., 2012, 18, 116–128 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2bm00060a |
| ‡ Current address: Institute for Science and Technology in Medicine, Guy Hilton Research Centre, Thornburrow Drive, Keele University, Stoke-on-Trent, Staffordshire, ST4 7QB, United Kingdom. |
| This journal is © The Royal Society of Chemistry 2013 |
