 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A simple analytical method involving the use of a monolithic silica disk-packed spin column and HPLC-ECD for determination of L-DOPA in plasma of patients with Parkinson's disease
Makoto Tsunoda*a,
Masaaki Hirayamab,
Kinji Ohnoc and
Takao Tsudad
aGraduate School of Pharmaceutical Sciences, University of Tokyo, Tokyo, 1130033, Japan. E-mail: makotot@mol.f.u-tokyo.ac.jp; Fax: +81-3-5841-4761; Tel: +81-3-5802-3339
bDepartment of Pathophysiological Laboratory Sciences, Nagoya University Graduate School of Medicine, Nagoya, Japan. E-mail: hirasan@met.nagoya-u.ac.jp
cDivision of Neurogenetics, Center for Neurological Diseases and Cancer, Nagoya University Graduate School of Medicine, Nagoya, Japan. E-mail: ohnok@med.nagoya-u.ac.jp
dPico-device. Co. Ltd., Nagoya, Japan. E-mail: tsuda@pico-device.co.jp
First published on 15th August 2013
Abstract
L-DOPA (L-3,4-dihydroxyphenylalanine) is commonly used in the treatment of Parkinson's disease. Monitoring the concentration of L-DOPA in human plasma will enable dose optimization, but is rarely performed because current quantification methods are tedious and time-consuming. In this study, a simple method for the determination of L-DOPA in the plasma of patients with Parkinson's disease was developed. A monolithic silica disk-packed spin column with a phenylboronate moiety, which forms stable anionic complexes with the cis-hydroxyl groups of L-DOPA, was used to extract L-DOPA from plasma with extraction recoveries exceeding 90%. The extracted L-DOPA was then separated on a reversed-phase column and detected electrochemically with a boron-doped diamond electrode. The method, which has a limit of detection of 10 fmol, was then successfully applied for the determination of L-DOPA in the plasma of healthy volunteers and patients with Parkinson's disease.
Introduction
Parkinson's disease (PD) is a common neurodegenerative disease that affects 0.3% of the population over the age of 50. PD is caused by the loss of dopaminergic neurons in the substantia nigra pars compacta1 and is characterized by resting tremors, bradykinesia, rigidity, and postural imbalance.The drug L-DOPA (L-3,4-dihydroxyphenylalanine) is currently the most effective treatment option for PD, but continuous administration of L-DOPA generally leads to narrowing of the therapeutic window.2 Also, when the concentration of L-DOPA in plasma exceeds an upper threshold, patients may develop choreoathetosis, which is characterized by uncomfortable, involuntary movement of the limbs, trunk, and orolingual muscles.3 Alternatively, if the concentration of L-DOPA falls below a certain threshold, PD symptoms can intensify to where the patients cannot move, which is known as wearing-off or the on–off phenomenon.
Orally administered L-DOPA is taken up in the proximal upper gastrointestinal tract (duodenum and jejunum) via a facilitated, saturable uptake process. In advanced PD, dysfunction of gastrointestinal mobility can occur which often culminates in the delay of the medication effect, which is known as the delayed-on phenomenon.4 Hence, the measurement of L-DOPA in the plasma of patients with PD will help to prevent overdosing and can be used to optimize L-DOPA dosing for advanced PD.
High-performance liquid chromatography instruments coupled to electrochemical detectors (HPLC-ECD) are commonly used for the determination of L-DOPA in plasma.5–10 However, current HPLC-ECD methods require complicated, time-consuming pretreatment steps involving alumina and solid-phase extraction. For clinical applications, pretreatment needs to be simplified.
Recently, monolithic silica disk-packed spin columns have been developed.11–13 Monolithic silica differs from classical silica in that it consists of high surface area silica rods, which allow for analyte adsorption, instead of particles. Procedures such as sample loading, washing, and elution of target analytes can be accomplished by simple centrifugation of the spin column, and multiple samples can be processed simultaneously in the same centrifuge. Because of ease of use and the low volume of solvent required for extractions, spin columns are well-suited for sample preparation in clinical settings.
In this study, a simple LC method was developed for the analysis of L-DOPA in plasma, where a spin column was used for extraction, an ODS HPLC column was used for separation, and electrochemical detection was achieved with a boron-doped diamond electrode. Phenylboronate-modified monolithic silica was used because phenylboronate forms a stable, anionic complex with the cis-hydroxyl groups of catechol compounds. The developed method was applicable to the determination of L-DOPA in the plasma of patients with PD.
Experimental
Materials
L-DOPA, norepinephrine, epinephrine, and dopamine were obtained from Sigma (Tokyo, Japan). Acetonitrile (HPLC grade) was purchased from Wako Pure Chemical (Osaka, Japan). Purified water was prepared using a Millipore ultra-pure water system (Milford, USA).HPLC
The HPLC system consisted of a pump (PU1580, Jasco, Tokyo, Japan), a manual sample injector (Rheodyne 7725), and an electrochemical detector (ED703, GL Sciences, Tokyo, Japan). The cell of the electrochemical detector was set to 1 μA with a response time of 3 s. Separations were performed on a C18 reversed-phase analytical column (Inertsil ODS-4 column, 150 × 3.0 mm I.D., 5 μm, GL Sciences) at 35 °C. The mobile phase was composed of 20 mmol L−1 sodium acetate–citrate buffer/acetonitrile (100/5, v/v) containing 1 g L−1 sodium 1-octanesulfonate. Isocratic elution was performed at a flow-rate of 0.5 mL min−1. The chromatograms were analyzed using the software Chromato-Pro (Run Time Corporation, Kanagawa, Japan).Standard solutions
Stock solutions were prepared with 0.1% trifluoroacetic acid (TFA) in water. Working solutions were made by diluting stock solutions with water. All solutions were stored at 4 °C.Human plasma samples
All studies involving patients and volunteers were approved by the ethical review committees of Nagoya University Graduate School of Medicine and the Graduate School of Pharmaceutical Sciences of University of Tokyo. After the appropriate consent forms were signed, blood (5 mL) was drawn from 3 volunteers and 5 patients with Parkinson's disease taking L-DOPA. Each patient was given 300 mg L-DOPA orally 3 h prior to blood drawing. All plasma samples were kept at −80 °C prior to analysis.Sample preparation
The phenylboronate monolithic spin column (GL Sciences) was conditioned with acetic acid and phosphate buffer. First, 0.2 mL of 1% acetic acid was added to the column, and the column was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. Then, 0.2 mL of 100 mmol L−1 phosphate buffer (pH 8.0) was added to the column, and the column was centrifuged at 10
000 rpm for 1 min. Then, 0.2 mL of 100 mmol L−1 phosphate buffer (pH 8.0) was added to the column, and the column was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. The analyte sample was then applied to the conditioned monolithic spin column, and the column was centrifuged at 10
000 rpm for 1 min. The analyte sample was then applied to the conditioned monolithic spin column, and the column was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. The column was then rinsed with 200 μL of 100 mmol L−1 phosphate buffer (pH 8.0), and centrifuged. Analytes were eluted from the column with 1% acetic acid (200 μL), and 20 μL eluate samples were used for HPLC analysis.
000 rpm for 1 min. The column was then rinsed with 200 μL of 100 mmol L−1 phosphate buffer (pH 8.0), and centrifuged. Analytes were eluted from the column with 1% acetic acid (200 μL), and 20 μL eluate samples were used for HPLC analysis.
Method validation
Linear regression analysis was performed to calculate the slope, intercept, and correlation coefficient of the calibration curve. Precision was evaluated by analyzing five replicates of four plasma samples containing L-DOPA (0, 1, 2, and 4 μM) within the same day (intra-day precision) and over five different days (inter-day precision). The differences between peak areas before and after spiking were interpolated on the respective calibration curves, and compared to the theoretical concentrations to obtain analyte recoveries.Results and discussion
Monolithic spin column extraction
Monolithic silica spin columns were used to prepare the human plasma samples for HPLC analysis. Advantages of spin columns over traditional solid phase extraction include: (1) small sample volumes can be analyzed; (2) samples can be easily separated via centrifugation; (3) multiple samples can be processed simultaneously. For this work, a phenylboronate moiety was bonded to the monolithic silica surface, as catechol compounds are known to form complexes with boronate, and selective extraction should be possible with the derivatized spin columns. With modified conditions for the extraction of catecholamines, the extraction recovery of L-DOPA from the human plasma sample reached 90%.Chromatographic conditions
Based on previous work concerning the analysis of catecholamines,14–16 a variety of mobile phases were evaluated for the HPLC separation. L-DOPA was poorly retained with a mobile phase composed of 20 mmol L−1 sodium acetate–citrate buffer/acetonitrile (100/16, v/v) containing 1 g L−1 sodium 1-octanesulfonate, so the concentration of the ion-pair reagent and acetonitrile content were further optimized. The acetonitrile content affected the separation to a greater extent than the ion-pair reagent, and L-DOPA was retained sufficiently with a decrease in acetonitrile content. Accordingly, optimal separation was obtained with a mobile phase consisting of 20 mmol L−1 sodium acetate–citrate buffer/acetonitrile (100/5, v/v) containing 1 g L−1 sodium 1-octanesulfonate.Fig. 1(a) shows a typical chromatogram of an L-DOPA standard solution, which exhibits a retention time of 5 min. Catechol compounds other than L-DOPA were also extracted by the spin column, but the retention times of the catecholamines, 3,4-dihydroxymandelic acid, and 3,4-dihydroxyphenylacetic acid were different from that of L-DOPA.
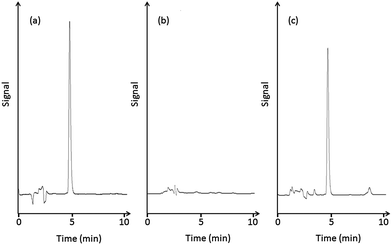 | ||
| Fig. 1 HPLC chromatograms of (a) a standard solution of L-DOPA, (b) healthy human plasma, and (c) human plasma sample from a patient with PD. | ||
To determine the optimal detection potential for electrochemical detection, a hydrodynamic voltammogram measurement was taken. As shown in Fig. 2, the peak response gradually increased up to 1400 mV (vs. Ag/AgCl). Although the highest voltage is generally desirable for detection, 1000 mV was chosen because of the increase of background noise and the rise of endogenous peaks at high voltage.
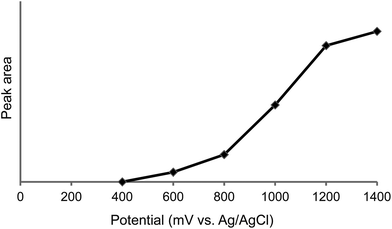 | ||
| Fig. 2 Hydrodynamic voltammogram of L-DOPA. | ||
Method validation
The calibration curve of L-DOPA had a linear working range from 100 fmol to 100 pmol with a correlation coefficient of 0.999. The limits of detection and quantitation were 10 and 35 fmol, respectively. These values were lower than those reported in previous studies. Precision, expressed as the percent coefficient of variation, was less than 3.9% and accuracy was high (Table 1).| Added (μM (n = 5)) | Concentration (Mean ± SD, μM) | Precision (RSD, %) | Recovery (%) |
|---|---|---|---|
| Intra-day | |||
| 0 | 1.76 ± 0.94 | 3.7 | |
| 1 | 2.73 ± 0.92 | 2.8 | 97 |
| 2 | 3.69 ± 0.91 | 1.1 | 96 |
| 4 | 5.66 ± 0.89 | 2.1 | 97 |
| Inter-day | |||
| 0 | 1.74 ± 0.90 | 3.9 | |
| 1 | 2.76 ± 0.92 | 3.3 | 102 |
| 2 | 3.74 ± 0.89 | 2.6 | 99 |
| 4 | 5.71 ± 0.87 | 2.5 | 99 |
Analysis of human plasma samples
Plasma samples collected from patients with PD and healthy volunteers were assayed with the developed spin column-HPLC-ECD method. A typical chromatogram of the plasma from a healthy human is shown in Fig. 1(b). L-DOPA is clearly absent from the chromatogram. A typical chromatogram of the plasma sample from a patient with PD is shown in Fig. 1(c). From the analysis, the L-DOPA concentration in 5 PD patients was 1.76 ± 0.94 μM (mean ± SD, n = 5).Although this method measures only the L-DOPA concentration, and not other metabolites such as 3-O-methyl DOPA, the method works for small sample volumes (100 μL). In addition, it has sufficient sensitivity, as a linear working range between 1.5 and 8 μM gives the most consistent clinical response.
Conclusion
A method for the determination of L-DOPA in human plasma, involving extraction with monolithic spin columns combined with high-performance liquid chromatography-electrochemical detection, was developed. This validated, relatively simple method provides a way to optimize the dosage of L-DOPA for patients with Parkinson's disease and also to perform pharmacokinetic studies of orally or intravenously administered L-DOPA.Acknowledgements
We are grateful to Fumiko Ozawa, Yui Okada, Sae Goto, Satoru Hasegawa, and Saki Maeda for their technical assistance. We also would like to thank PD patients for participating in this study. This study was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, the Ministry of Health, Labor, and Welfare of Japan, and the Aichi Science and Technology Foundation.References
- S. K. Van Den Eeden, C. M. Tanner, A. L. Bernstein, R. D. Fross, A. Leimpeter, D. A. Bloch and L. M. Nelson, Am. J. Epidemiol., 2003, 157, 1015–1022 CrossRef.
- O. Rascol, C. Goetz, W. Koller, W. Poewe and C. Sampaio, Lancet, 2002, 359, 1589–1598 CrossRef.
- J. E. Ahlskog and M. D. Muenter, Mov. Disord., 2001, 16, 448–458 CrossRef CAS.
- R. Djaldetti, J. Baron, I. Ziv and E. Melamed, Neurology, 1996, 46, 1051–1054 CrossRef CAS.
- M. Tsunoda, Anal. Bioanal. Chem., 2006, 386, 506–514 CrossRef CAS.
- F. Bugamelli, C. Marcheselli, E. Barba and M. A. Raggi, J. Pharm. Biomed. Anal., 2011, 54, 562–567 CrossRef CAS.
- A. Di Stefano, M. Carafa, P. Sozio, F. Pinnen, D. Braghiroli, G. Orlando, G. Cannazza, M. Ricciutelli, C. Marianecci and E. Santucci, J. Controlled Release, 2004, 99, 293–300 CrossRef CAS.
- C. Lucarelli, P. Betto, G. Ricciarello, M. Giambenedetti, C. Corradini, F. Stocchi and F. Belliardo, J. Chromatogr., 1990, 511, 167–176 CrossRef CAS.
- A. Tolokán, I. Klebovich, K. Balogh-Nemes and G. Horvai, J. Chromatogr., B: Biomed. Sci. Appl., 1997, 698, 201–207 CrossRef.
- S. Letellier, J. P. Garnier, J. Spy and B. Bousquet, J. Chromatogr., B: Biomed. Sci. Appl., 1997, 696, 9–17 CrossRef CAS.
- A. Namera, A. Takeuchi, T. Saito, S. Miyazaki, H. Oikawa, T. Saruwatari and M. Nagao, J. Sep. Sci., 2012, 35, 2506–2513 CrossRef CAS.
- S. Reichelt, C. Elsner, A. Prager, S. Naumov, J. Kuballa and M. R. Buchmeiser, Analyst, 2012, 137, 2600–2607 RSC.
- M. Tsunoda, C. Aoyama, S. Ota, T. Tamura and T. Funatsu, Anal. Methods, 2011, 3, 582–585 RSC.
- M. Tsunoda, K. Takezawa, T. Santa and K. Imai, Anal. Biochem., 1999, 269, 386–392 CrossRef CAS.
- K. Takezawa, M. Tsunoda, K. Murayama, T. Santa and K. Imai, Analyst, 2000, 125, 293–296 RSC.
- M. Tsunoda, M. Yamagishi, K. Imai and T. Yanagisawa, Anal. Bioanal. Chem., 2009, 394, 947–952 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
