Identification of bacteria in drinking water with Raman spectroscopy
Jack
van de Vossenberg
*a,
Heli
Tervahauta
c,
Kees
Maquelin
b,
Carola H. W.
Blokker-Koopmans
a,
Marijan
Uytewaal-Aarts
a,
Dick
van der Kooij
a,
Annemarie P.
van Wezel
a and
Bram
van der Gaag
a
aKWR Watercycle Research Institute, Groningenhaven 7, 3433 PE Nieuwegein, The Netherlands. E-mail: Jack.vandeVossenberg@gmail.com; Fax: +31-30-6061165; Tel: +31-30-6069511
bCenter for Optical Diagnostics & Therapy, Department of Dermatology, Erasmus MC, 's Gravendijk-wal 230, 3015 CE Rotterdam, The Netherlands
cDepartment of Medical Microbiology & Infectious Diseases, Erasmus MC, 's Gravendijkwal 230, 3015 CE Rotterdam, The Netherlands
First published on 3rd April 2013
Abstract
Raman spectroscopy was used to discriminate between Legionella strains and between E. coli and coliform strains. The relationship between triplicate Raman spectra derived from Legionella bacteria was compared with that derived from a blind set of samples and amplified fragment length polymorphism (AFLP) data from the same strains. Triplicate Raman spectra of E. coli and coliform bacteria were compared with their 16S phylogeny. In all cases Raman spectra were reproducible and could be distinguished from spectra of other organisms down to the strain level. All samples in a blind fourth set were identified correctly. Raman spectra of organisms of the same coliform species clustered according to 16S rRNA gene phylogeny, except for Enterobacter spp. At higher taxonomic levels the relationship between species was less comparable. For Legionella strains the Raman spectra grouped according to AFLP groups, based on the dataset used in this study. Raman spectroscopy could correctly distinguish E. coli from other coliform bacteria and L. pneumophila from non-pneumophila strains. Incubation of Legionella strains in different types of drinking water at different temperatures over a period of one week introduced so little variation in the Raman spectra that only very closely related L. pneumophila strains could not be distinguished from each other. Temperature, ageing and water type did not influence the identification potency of Raman spectroscopy in all cases. Given the accuracy, speed and simplicity of the Raman spectroscopy technique this method seems a welcome addition to the current tools for identification of waterborne bacteria.
Introduction
Drinking water suppliers aim to provide safe drinking water to consumers. Safety is primarily achieved by source protection, water treatment and a reliable distribution system. Over the last two decades molecular methods have been developed to replace ‘classical’ phenotype based identification methods for bacteria.1 Nevertheless, methods like selective plating and enzyme activity tests still are the most extensively studied, most used and most often validated identification tests in drinking water testing. These methods are embedded in legislation on drinking water quality, but require a long time between sampling and result.A phenotype based method that can be used for identification of bacteria is Raman spectroscopy. The method is rapid and straightforward and does not require invasive experimental procedures nor extra dyes or other reagents. A Raman spectrum from microorganisms is a result of the combination of the spectra of all molecules that form the bacterial cell (carbohydrates, proteins, fatty acids, nucleic acids, small molecules).2 Together, these spectra show similar discriminatory abilities of various phenotypical characterizations to other phenotypic tests like lipid analysis, whole-cell protein electrophoresis, metabolic tests.3 Raman spectroscopy can leave a unique fingerprint even at the strain level.4–6 The method is not yet extensively explored for drinking water analysis. Raman spectroscopy has been successfully described as a tool to discriminate between waterborne species of bioterror agents, pathogens and indicators as Yersinia, Bacillus and Escherichia coli.7,8 Next to its discriminatory properties and the short experimental procedure, the method does not require highly skilled labor. These characteristics make Raman spectroscopy a promising tool for the assessment of water quality.
Even though Raman spectroscopy can be used for identification of bacteria, the standard for bacterial species identification is 16S rRNA gene sequence information.9 The information content of the 1.6 kbase long 16S rRNA gene sequence is limited and therefore the 16S rRNA gene cannot be used to distinguish between strains of one species. The amplified fragment length polymorphism (AFLP) technique,10 which is based on the reproducible amplification of restriction enzyme-digested genomic DNA, is more sensitive than 16S rDNA sequencing and can therefore be used to distinguish between strains. As with 16S rRNA, the relationships between AFLP patterns reflect phylogenetic relationships, i.e. patterns with a higher similarity come from more closely related strains or species than AFLP patterns that show more variation.
Because Raman spectroscopy is a chemotaxonomic method, reproducibility of the spectra is affected by the change in molecular composition of the cell due to variations in growth conditions. Hence aging of cells in the drinking water and different water types may influence the Raman spectra of the organisms. Tripathi et al.8 studied the aging of lyophilized samples in distilled water and tap water over one week. They used spores of Bacillus vegetative cells and compared with spores of B. atrophaeus and B. thuringiensis. They could still distinguish between the spores and vegetative cells, and they could not observe significant changes in Raman signatures after one week, indicating that Raman spectroscopy is not very sensitive to age-related changes.
The aim of this study was to see whether drinking water relevant bacterial species and strains could be correctly identified by Raman spectroscopy. We were also interested in finding how the Raman spectrum for bacteria surviving in drinking water would change over time. Two groups of bacteria in drinking water were chosen, Legionella and E. coli plus fecal coliform bacteria. E. coli bacteria are strong indicators of fecal contamination, while coliform bacteria mostly do not have fecal origin.11 For E. coli bacteria and other coliform bacteria 16S rRNA gene species information was used for comparison. For even more closely related Legionella bacteria, which sometimes grow in drinking water and may cause legionellosis, we used AFLP as the method for comparison. Survival in drinking water over a one-week period was tested with three different strains of Legionella pneumophila species and one closely related Legionella anisa species. We also tested whether the Raman spectra of these Legionella bacteria were influenced by the drinking water matrix.
Materials and methods
For the reproducibility and identification tests in this study, bacteria were grown on culture plates under reproducible conditions, and collected. For other tests, bacteria were incubated in drinking water, after which cells were collected by centrifugation. Raman spectra were taken from the collected cells.16S and AFLP analyses
For 16S rRNA gene sequencing, DNA was isolated from the bacteria followed by PCR with Taq DNA polymerase and primers against conserved domains.12 Nearly full 16S rRNA gene sequences were analyzed using two terminal primers (8F and 1392R). The 16S rRNA gene sequences were assembled with the DNAstar software package (DNAStar Inc., WI, USA). The sequences were aligned with sequences in the ARB SSU rDNA database tree.13 The identity of isolates was deduced from the phylogenetic position of their sequences using maximum likelihood and maximum parsimony tree building methods.The amplified fragment length polymorphism (AFLP) method was adapted from an analysis method for Campylobacter strains.14 The method was used as a DNA fingerprinting method, using restriction enzymes HhaI and HindIII. The final products were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 together with internal size standard Genescan-500 (PE Applied Biosystems) and analyzed on a short capillary/POP-4 polymer using a model ABI 310 automated DNA sequencer. The obtained chromatograms were normalized with the internal size standard and compared using Bionumerics software version 3.5. Dendrograms were based on similarity values obtained with the UPGMA method.15
1 together with internal size standard Genescan-500 (PE Applied Biosystems) and analyzed on a short capillary/POP-4 polymer using a model ABI 310 automated DNA sequencer. The obtained chromatograms were normalized with the internal size standard and compared using Bionumerics software version 3.5. Dendrograms were based on similarity values obtained with the UPGMA method.15
Selection of strains
Unless specified otherwise the bacteria used in this study were taken from the collection of isolates from water samples held at KWR Watercycle Research Institute (KWR). For all bacteria, a pre-culture was made and stored in aliquots at −80 °C. Inocula were made by plating these frozen cultures on Lab Lemco Agar (LLA, Oxoid, Cambridge, UK) plates.Coliform and E. coli bacteria were selected from the strain collection at KWR and from the ECOR collection of reference strains.16 The coliform bacteria used in this study were Salmonella enterica (SP5, WG49), Enterobacter cloacae (WR3), Enterobacter hormaechei (M979284, M970287), Citrobacter freundii (M951100, M970282, 295, 021127-4554), Enterobacter amnigenus (M970501, M979274, 290), Buttiauxella noackiae (M970273), Enterobacter aerogenes (EPA_202), Raoulterra terrigena (M970279), Klebsiela pneumoniae (M970305, M970304, M981147) and Serratia fonticola (M970289, M970292). E. coli WR1 and E. coli M970443 were taken from the KWR collection, we used ECOR strains: ECOR-8, ECOR-10, ECOR-11, ECOR-12, ECOR-26 and ECOR-41.16E. coli and coliform bacteria were inoculated in triplicate from separate −80 °C stocks and grown on lauryl sulfate agar plates (LSA, Oxoid, Cambridge, UK) without phenol red at 35 or 37 °C, i.e. depending on the location of the incubators. The E. coli/coliform plates were incubated overnight (for 18–20 hours) at 35 °C, and prepared for Raman spectroscopy measurements the following day.
Nine strains of L. pneumophila were compared with one L. anisa and two L. bozemanii strains. To obtain the best information from a limited amount of strains, we selected both closely and distantly related bacteria, based on their AFLP phylogeny, with emphasis on L. pneumophila. Nine Legionella pneumophila strains and three non-pneumophila Legionella strains were selected according to their similarity based on AFLP patterns. The Legionella strains were: Legionella pneumophila (M990333 = ATCC 22284, M994475, M001829, M995113, M010675A, M004233, M004237, M052261, M020434), Legionella bozemanii (M001387 = ATCC 35545, M001388 = ATCC 33217), Legionella anisa (M030276 = ATCC 35292). All strains, except the ATCC strains were taken from the KWR strain collection. Legionella bacteria were grown on Buffered Charcoal Yeast Extract (BCYE, Oxoid) plus 80 IE per ml polymyxin B sulfate and 8.8 μg ml−1 Na-cefazoline (BCYE + AB) agar plates at 35 or 37 °C. The Legionella plates were incubated at 35 °C for seven days and subsequently prepared for Raman spectroscopy measurements.
For Raman spectroscopy, 4 individual inocula were prepared for each isolate and stored at −80 °C. Each −80 °C stock of each isolate was inoculated on a culture plate, BCYE + AB for Legionella, LSA without phenol red for E. coli/coliforms. The identity of the bacteria was determined with the first three sets of plates. The fourth set of replicates was numbered in a random order, and analyzed without knowing the identity during analysis.
Survival in drinking water
For the water matrix we selected three drinking water types that cover the range of drinking water types found in The Netherlands. The water was collected from three clear water pumping stations, labeled PS1, PS2 and PS3. PS1 was ground water and contained the lowest quantities of most of the dissolved components (Table 1). PS2 was ground water with higher concentrations of some components. PS3 water originated from surface water and contained higher concentrations of other components. The water was collected in a 20 liter bottle and stored at 4 °C until use.| Parameter | Pumping station | ||
|---|---|---|---|
| PS1 | PS2 | PS3 | |
| Temperature (°C) | 10–10.5 | 10–12 | 2.1–22 |
| pH | 7.94 | 7.76 | 8.1 |
| EGV (mS m−1) | 23.7 | 47.7 | 49.3 |
| Sulphate (mg l−1) | 18 | <2 | 70.1 |
| Nitrate (mg l−1) | 4.4 | 12 | 2.6 |
| Colour (mg Pt/Co/l) | <3 | 16 | <3 |
| TOC (mg l−1) | <0.5 | 7.6 | 3.46 |
Strains were inoculated on BCYE + AB plates and grown for 1 week at 37 °C. The colony material was taken from the agar plate and inoculated into 1 liter of drinking water at a final density of 107 cells per ml. The cell suspensions were incubated at 10 °C or 37 °C.
Survival of the bacteria was assessed by plating bacteria on BCYE + AB plates every time when samples were taken for Raman spectroscopy measurements. In parallel tests, 50 μl of samples were taken from steps in the integrity loss assay for Legionella cells. Integrity loss in the different water types was followed with SybrGreen and propidium iodide (PI). The parallel samples were stained with 2 μl of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 100× diluted SybrGreen (10
1 mixture of 100× diluted SybrGreen (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 concentrate in DMSO, Invitrogen) and 50 μg ml−1 PI (Becton-Dickinson), and incubated for 15 min. The ratio of living and dead cells was assessed with a Leica DM-RXA epifluorescence microscope.17
000 concentrate in DMSO, Invitrogen) and 50 μg ml−1 PI (Becton-Dickinson), and incubated for 15 min. The ratio of living and dead cells was assessed with a Leica DM-RXA epifluorescence microscope.17
During the initial tests with the survival of Legionella samples in drinking water, we noticed that over time it became more and more difficult to collect cells by centrifugation. To confirm this we tested centrifugation of a one week old sample in 50 ml Greiner tubes according to the standard lab procedure at 3775 × g for 10 min in a swing-out rotor, without brake while spinning down. 100 μl of the initial suspension and 100 μl of supernatant were plated out on Legionella specific agar plates. The supernatant was removed by vacuum suction, leaving 1 ml in the tube. 100 μl was taken from that fraction that should include the (invisible) pellet. We found out that with this procedure, cells remained in the supernatant. The number of cells in the supernatant had only decreased from 4.4 × 105 ml−1 to 1.4 × 105 ml−1. The number of cells in the pellet fraction had increased to 3.5 × 106 ml−1, which is insufficient to produce a visible pellet. Therefore, we changed to Vivaspin 20 tubes. These centrifuge tubes contain two tilted 0.2 μm pore size PES membrane filters that concentrate cells into a small volume of medium. Concentration of bacteria with these tubes did yield visible pellets that could be harvested for Raman spectroscopy.
In preparation for Raman spectroscopy 175 ml samples were taken from the water bottles and centrifuged in sequential runs of 20 ml per run in Vivaspin 20 tubes (Sartorius, Göttingen, Germany) for 5 min at 3000 × g. The concentrate was transferred to an Eppendorf cup and centrifuged for 15 min at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g in an Eppendorf centrifuge. The pellet was resuspended in 100 μl of MilliQ water and transported on ice to the Raman spectrometer facility at River Diagnostics (Rotterdam, The Netherlands).
000 × g in an Eppendorf centrifuge. The pellet was resuspended in 100 μl of MilliQ water and transported on ice to the Raman spectrometer facility at River Diagnostics (Rotterdam, The Netherlands).
Raman spectroscopy
Sample preparation of the isolates on the plate was carried out according to the River Diagnostics's (Rotterdam, The Netherlands) standard operating procedure. A calibrated 1 μl loop was filled with biomass and suspended in 10 μl of de-mineralized water. From this step onwards, plate samples and samples from water incubation were treated the same. After a centrifugation step for 3 minutes at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, the pellet was re-suspended and 7–10 μl of this suspension was transferred onto a test slide.
000 × g, the pellet was re-suspended and 7–10 μl of this suspension was transferred onto a test slide.
The test slide consisted of a fused silica slide (Hellma Benelux BV, Aartselaar, Belgium) and a removable silicone isolator with 24 wells (Sigma-Aldrich, Zwijndrecht, The Netherlands). The suspensions were allowed to dry for 30–40 minutes at 35 °C.
The Raman spectroscopy measurements were performed on an advanced prototype of a near-infrared (NIR) Raman SpectraCell RA™ system (River Diagnostics, Rotterdam, The Netherlands) as described before.6 Briefly, this system excites the samples using approx. 220 mW near-infrared laser light (785 nm). The light is focused on a measurement volume of 5 × 2 micrometers (height × width).
Data analysis
Software scripts for spectrum pretreatment and data analysis were written in MATLAB version 7.1 (The MathWorks, Natick, MA, USA).5,6To analyze spectral relationships between different isolates, a cluster analysis of sets of spectra was done using the pairwise similarities (squared Pearson correlation coefficient; R2) as a distance matrix in combination with Ward's cluster algorithm. For each isolate the R2 was calculated over the Raman spectra obtained for the three replicates. The 95% confidence interval was calculated from these R2 values and the lowest level was used as the similarity cut-off level in the dendrograms resulting from hierarchical cluster analyses of data. In total, 29 values were obtained for E. coli/coliform isolates. The lowest value (for K. pneumoniae, (M970305)) was used as the similarity cut-off level in the dendrograms resulting from hierarchical cluster analyses of data. Above this cut-off level the isolates were considered to be clonally related (i.e. belonging to the same strain).
Results and discussion
Distinction between E. coli and coliform bacteria, comparison with 16S
The 16S rRNA gene sequence is commonly used for molecular identification of bacteria down to the species level. In earlier studies, with endospore forming in Bacillus and Brevibacillus bacteria, good discrimination between species was shown with UV resonance Raman spectroscopy and the data correlated with 16S rRNA gene sequence data.18 The same type of comparison was done for eight Mycobacterium species, and for 95% (60/63) the Raman spectra corresponded to the 16S rRNA gene data.19 Kirschner et al.20 found that Fourier-transform IR (FTIR) and Raman data of Enterococci correlated better with 16S rRNA gene data than phenotype based methods (API, Microscan). In the same study FTIR data were compared with Raman spectroscopy. These spectroscopic methods correlated well, which implies that for Enterococci Raman spectroscopic data reflect 16S rRNA gene data better than biochemical tests.The ECOR strains for this study were primarily selected because they clustered in different groups in a standard reference strain study by Ochman and Selander.16 The cluster of ECOR strains 8, 10 and 11 in that study was different from the cluster of ECOR-26 and 41, and in their turn all were different from ECOR-12. The ECOR collection has shown its usefulness as test collection for rapid whole-organism fingerprinting with Surface Enhanced Raman Spectroscopy, which is different from the method we used in this study and is not directly comparable.4
Twenty-nine E. coli and coliform bacteria were grown on agar plates. Plates without phenol red were used instead of standard E. coli selective plates, because the dye in the standard plates interfered with the bacterial Raman signal. Using Raman spectroscopy 28 clusters were found in the dataset. One of the clusters contained the data of 2 isolates, Enterobacter amnigenus strains 290 and M979274, suggesting that these isolates are in fact the same strain. The Raman spectra from all other isolates clustered with their own triplicates only (Fig. 1b). In a group similarity comparison, averaged spectra of E. coli bacteria against spectra of other coliform bacteria, the E. coli bacteria could be distinguished from the other coliform bacteria and vice versa in all cases. This indicated that repeatability was excellent and that the sensitivity of the method is well able to distinguish between species.
 | ||
| Fig. 1 Specificity and reproducibility of Raman spectroscopy for identification of bacterial strains. (a) Legionella. La = Legionella anisa, Lb = L. bozemanii, other strains are L. pneumophila. (b) E. coli and coliforms. Isolation, growth and analysis were replicated four times. Blue branches depict significantly different Raman spectra. Spectra that are linked with red branches are not significantly different from each other, showing that Raman spectra are highly reproducible. | ||
The maximum parsimony phylogenetic tree of E. coli and coliform bacteria 16S rRNA gene sequences was compared with the dendrogram for the Raman spectra from the same organisms (Fig. 3). The dendrograms were matched by swapping branches while the topology was left intact. Most spectra, but not all, clustered into one cluster for each species. K. pneumoniae, C. freundii and S. fonticola strains clustered according to 16S based speciation in both Raman and 16S rRNA dendrograms. Most of the E. coli data clustered accordingly, but E. coli WR1, and to a lesser extent its most related neighbor ECOR-41, fell out of the major E. coli cluster in the Raman data. 16S sequence data for the two Raman E. amnigenus spectra mentioned above were identical, which confirmed that they are the same species. Both spectra grouped with C. freundii spectra while the spectra of the other E. amnigenus and closely related B. noackiae clustered with single strains of Salmonella, E. coli and E. cloacae. The Raman spectroscopy data for Salmonella and E. hormaechei did not cluster as one species. Both with 16S gene and Raman spectroscopy comparisons Enterobacter species do not cluster unequivocally, indicating that the used ‘Enterobacter’ group is more diverse than can be deduced from the genus name. At phylogenetic levels above species 16S rRNA gene phylogeny and Raman data did not match.
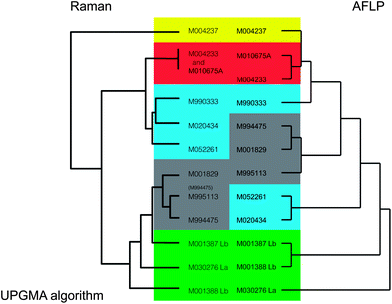 | ||
| Fig. 2 Comparison of topologies of dendrograms for Legionella calculated with Raman spectroscopy versus AFLP. Colored areas mark clusters of related L. pneumophila strains in the Raman spectra and non-pneumophila Legionella species. Most strains clustered in a corresponding fashion for Raman and AFLP data. | ||
 | ||
| Fig. 3 Comparison of topologies of dendrograms for E. coli/coliforms calculated with Raman spectroscopy versus 16S rRNA gene sequencing. Clusters in the dendrograms have the same color. | ||
With the obtained spectra, we also tested whether a reliable judgment could be made for a given spectrum if it is E. coli or coliform. Every obtained spectrum was compared to average spectra for E. coli or non-E. coli. In all cases the spectra could correctly be identified as either E. coli or non-E. coli coliform.
Distinction between Legionella bacteria, comparison with AFLP
Bacillus strains that were nearly identical in 16S rRNA gene sequence could be well distinguished from each other with Raman spectroscopy,3 indicating that Raman spectroscopy allows a higher resolution than can be obtained with 16S rRNA gene sequencing. The AFLP method also has higher resolution than 16S rRNA gene sequencing, and can detect the minute differences that exist between strains. Dendrograms derived from AFLP and Raman spectroscopy were also compared in earlier studies. Maquelin et al.21 obtained highly similar groupings for Acinetobacter species with both methods. Listeria monocytogenes strains could be correspondingly divided in two groups with AFLP and Raman spectroscopy.22 The subdivision correlated with the susceptibility to a compound that is coupled to variations in the cell wall, which could well explain the variations in Raman spectra. Serology and Raman spectra also coincide, because serology of L. monocytogenes is coupled to carbohydrate containing proteins on the cell surface, which will likely influence the whole-cell Raman spectra as well.In 4 replicate experiments 12 Legionella isolates were grown on agar plates and biomass was analysed using Raman spectroscopy. The fourth replicate was used as a blind test to determine whether the bacteria could be correctly identified based on the information gathered with the first three replicates. The Raman spectra for all 12 Legionella isolates clustered separately (i.e. in strain specific clusters), except for two pairs of L. pneumophila isolates. M004233/M010675A were probably the same strain, both with AFLP and Raman spectroscopy. M995113/M994475, which were closely related, but different according to their AFLP data, appeared not to have significantly different Raman spectra (Fig. 1a). When regarding these two pairs of L. pneumophila strains as one single strain, all blind test samples were identified correctly.
Comparison of the Raman dendrogram with the AFLP dendrogram of Legionella showed that in general the spectra of closely related strains matched well with AFLP data (Fig. 2). There were two noticeable exceptions. The first one was L. pneumophila M004237, of which the Raman spectrum grouped outside all other spectra, while its AFLP profile was an average L. pneumophila profile and most closely related to strains M004233 and M010675A. The second exception was that AFLP patterns of both L. bozemanii strains in this study were closely related, but their Raman spectra were not.
Another observation was that some clusters of L. pneumophila strains (M994475, M001829 and M995113) clustered among L. bozemanii and L. anisa for Raman spectroscopy, while more distinct strains M052261 and M020434 on the basis of AFLP clustered better with other L. pneumophila strains for Raman spectroscopy. This phenomenon was also observed in previous studies on Candida and Enterococcus bacteria, which also showed that variation of Raman spectra within a species can be as large as between species.20,23 Therefore, in our datasets we can only reliably state that isolates in the same cluster are related, but the more distant relationships between clusters only have limited taxonomic significance.
Raman spectra for Legionella and AFLP data in our study matched reasonably. The spectra for L. pneumophila were spread over multiple clusters. The variance between these clusters was equal to variance between species. The most distinct discrepancy was found with L. pneumophila strain M004237. The reason for the different spectrum could be that this organism has a unique biochemical feature. On the plate and on inspection with a binocular magnifier the colonies for M004237, M004233 and M010675A had the same phenotype. These strains are closely related according to AFLP, but according to the Raman spectra, M004237 is more distinct from the other two strains than any of the other Legionella strains. Possibly this strain produces more poly-β-hydroxybutyric acid (PHB) than other L. pneumophila strains (Fig. 4),24,25 which influences the spectrum so much that the strain is located in a completely different position in the dendrogram. Although the difference cannot completely be explained by PHB, it is a strong indication that isolate M004237 already stored PHB granules while the other isolates did not. Such a phenotype may not be visible in the AFLP patterns.
 | ||
| Fig. 4 Difference in Raman spectra of closely related L. pneumophila strains. Likely the difference in strain M004237 is caused by higher levels of storage compound poly-β-hydroxybutyric acid. The Raman band at 1726 cm−1, indicated with the vertical line, is most indicative of this difference. | ||
When the (nine triplicate) L. pneumophila and the (two triplicate) L. anisa spectra were averaged according species, all spectra in the fourth “blind” dataset could be assigned to the correct species (data not shown). This indicates that Raman spectroscopy could correctly distinguish L. pneumophila from the other Legionella species.
From the data it can be concluded that information obtained with Raman spectroscopy can correctly cluster closely related strains. However, the topology for less related strains is different from AFLP analysis and with this small dataset Raman spectroscopy alone does not seem to be able to determine the species of Legionella. The method would perform better when a larger reference database would be made.
We can conclude that Raman spectroscopy is specific, but above the species level Raman spectroscopy is not a good indicator for phylogeny. Unlike with nucleic acid based methods, variations in Raman spectra are caused by biochemical differences that reflect differences of phenotype. As mentioned above, genotype versus phenotype comparisons will always reveal differences.
Variation in the environment/change in the spectrum after incubation/survival in drinking water
Drinking water in The Netherlands originates from ground water and surface water. The water is distributed without disinfectant residual. Three drinking water types were chosen that cover the range of water types as found in The Netherlands. The water composition differed in organic and inorganic composition (TOC, nitrate, phosphate, etc.).Two temperatures were chosen. 10 °C is comparable with ground water temperature in The Netherlands and the modal drinking water temperature in the distribution network. The temperature of drinking water derived from surface water may fluctuate from little over 0 °C in rare cold winter periods to more than 25 °C in rare hot summer periods. Depending on factors such as residence time, drinking water can be influenced by soil temperatures of the distribution system, resulting in temperatures from 4 to 25 °C. We expected Legionella bacteria to survive for many weeks, and therefore we chose 37 °C for the higher temperature incubations, enabling us to study an accelerated transition from stationary to dead bacteria.
Incubation in one of the drinking water samples (PS3 water) resulted in a brown precipitate, possibly iron oxide, which interfered with the Raman spectroscopy measurements, making further experiments impossible. Therefore, only samples that were incubated in water from the other locations (PS2 and PS1) were used for Raman spectroscopy.
Legionella cells that were kept on a lab bench at room temperature (20–22 °C) and not exposed to centrifugal stress did not show decrease of integrity over a period of 3 weeks, i.e. 2% of the cells were stained by propidium iodide (PI) over the whole period. In the period of 1 week that was used for the Raman experiments, the number of cells that was stained with PI increased from 2 to around 40% at 37 °C (data not shown). These cells were not dead, because plating assays of the same samples did not show significant reduction of cell numbers over that period. For Legionella, SybrGreen and PI staining do not predict the number of viable cells in a sample. It can be concluded that for the survival of Legionella in drinking water PI/SybrGreen data should be interpreted with caution. The survival of Legionella bacteria, as determined with plate assays, differed for the different water types. In all water types and at both 10 and 37 °C, survival was between 75 and 100% over the first week. Raman spectra were taken over this period.
Raman data showed clustering of spectra according to the Legionella strain. Neither the composition of drinking water nor incubation temperature nor incubation for one week did influence clustering: spectra could still be assigned to the correct strain, albeit that the closely related strains M020434 and M052261 could not be distinguished from each other after incubation (Fig. 5). The changes in environmental conditions may have reduced the resolution of the Raman spectroscopy method, but the more complex procedure to collect the cells may also have been a major cause for the drop in resolution.
 | ||
| Fig. 5 Comparison of Legionella Raman spectra upon incubation for one week, for different strains, in different types of drinking water, and at 10 and 37 °C, respectively. S = strain, W = water type, T = temperature. | ||
The Raman spectra clustered correctly according to Legionella species. Raman spectra did not change so much over time, and the different Legionella strains could be correctly assigned at all times. Temperature and water type did not seem to significantly influence the Raman spectra. Likewise, the water type was of little influence on the Raman spectra for PS1 or PS2 water. Raman spectra of the bacteria used in this study are stable under varying conditions, and therefore the method of identification is reasonably robust. Tripathi et al.,8 who compared vegetative E. coli cells with more distantly related Bacillus spores, also found minimal spectral variability with respect to the age of the suspension and of the water matrix. And heat-inactivated bacteria showed minimal differences compared to the spectra of viable bacteria, enabling identification of Mycobacteria with less stringent biosafety precautions.19 The reproducibility found with different water temperatures, water types and starvation times makes clear that Raman spectroscopy is better for identification than for measurement of subtle changes in water quality.
Conclusion
Raman spectroscopy is a promising and easy method for identification of waterborne bacteria. The method can reproducibly assess the biochemical signature at the strain level both for E. coli/coliform strains and for Legionella strains. The method is not very sensitive for variation in drinking water composition or incubation time. Larger datasets to define the range of spectra for one species will improve the accuracy of the method.These results enable us to make a next step in a larger project (TTI-W photonic crystals) to develop a method to quickly identify waterborne bacteria that are trapped in optical tweezers.
Because of the confirmed high specificity and reproducibility of the Raman spectroscopy method the method may be used in drinking water research for colony confirmation. Such a method would be equally fast as nucleic acid amplification methods that are currently in use, and faster and more economical than AFLP analysis. The method is likely to be more promising for Legionella colony confirmation than for E. coli/coliform distinction.
The method would also be suitable for source tracking of Legionella strains. Strains that have caused legionellosis could then quickly be compared with strains isolated from possible contamination sites.
Abbreviations
| AFLP | Amplified fragment length polymorphism; |
| TOC | Total organic carbon; |
| PI | Propidium iodide. |
Acknowledgements
This work was performed in the TTIW-cooperation framework of Wetsus, Centre of Excellence for Sustainable Water Technology (http://www.wetsus.nl) and by BTO, the joint research program of Dutch water utilities. Wetsus is funded by the Dutch Ministry of Economic Affairs. The authors like to thank the participants of the TTIW research theme “Sensoring” for the fruitful discussions and their financial support. The authors thank Bart Wullings, Leo Heijnen and Gertjan Medema (KWR) and Gerwin Puppels (River Diagnostics BV) for the helpful discussions. The authors thank Mitchell Laurens and Diana Willemse (Erasmus MC) Meindert de Graaf, Lonneke Hensen, Ronald Italiaander, Anita van der Veen, Gaby van Doorn-Abelman, Ton Braat and Anke Brouwer-Hanzens (KWR) for technical assistance.References
- C. L. Meays, K. Broersma, R. Nordin and A. Mazumder, J. Environ. Manage., 2004, 73, 71–79 CrossRef.
- K. Maquelin, C. Kirschner, L. P. Choo-Smith, N. van den Braak, H. P. Endtz, D. Naumann and G. J. Puppels, J. Microbiol. Methods, 2002, 51, 255–271 CrossRef CAS.
- D. Hutsebaut, J. Vandroemme, J. Heyrman, P. Dawyndt, P. Vandenabeele, L. Moens and P. de Vos, Syst. Appl. Microbiol., 2006, 29, 650–660 CrossRef.
- R. M. Jarvis and R. Goodacre, Anal. Chem., 2003, 76, 40–47 CrossRef.
- H. F. M. Willemse-Erix, J.-W. Jachtenberg, H. Barutçi, G. J. Puppels, A. van Belkum, M. C. Vos and K. Maquelin, J. Clin. Microbiol., 2010, 48, 736–740 CrossRef CAS.
- D. F. M. Willemse-Erix, M. J. Scholtes-Timmerman, J.-W. Jachtenberg, W. B. van Leeuwen, D. Horst-Kreft, T. C. Bakker Schut, R. H. Deurenberg, G. J. Puppels, A. van Belkum, M. C. Vos and K. Maquelin, J. Clin. Microbiol., 2009, 47, 652–659 CrossRef.
- K. S. Kalasinsky, T. Hadfield, A. A. Shea, V. F. Kalasinsky, M. P. Nelson, J. Neiss, A. J. Drauch, G. S. Vanni and P. J. Treado, Anal. Chem., 2007, 79, 2658–2673 CrossRef CAS.
- A. Tripathi, R. E. Jabbour, P. J. Treado, J. H. Neiss, M. P. Nelson, J. L. Jensen and A. P. Snyder, Appl. Spectrosc., 2008, 62, 1–9 CrossRef CAS.
- W. Ludwig and K. H. Schleifer, FEMS Microbiol. Rev., 1994, 15, 155–173 CrossRef CAS.
- P. Vos, R. Hogers, M. Bleeker, M. Reijans, T. van de Lee, M. Hornes, A. Frijters, J. Pot, J. Peleman and M. Kuiper, Nucleic Acids Res., 1995, 23, 4407–4414 CrossRef CAS.
- S. C. Edberg, E. W. Rice, R. J. Karlin and M. J. Allen, Symp. Ser. - Soc. Appl. Microbiol., 2000, 29, 106S–116S Search PubMed.
- R. Devereux and S. G. Willis, in Molecular microbial ecology manual, ed. A. D. L. Akkermans, J. D. Van Elsas and F. J. De Bruijn, Kluwer Academic Publishers, Dordrecht, The Netherlands, 1995, pp. 1–11 Search PubMed.
- W. Ludwig, O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Förster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. König, T. Liss, R. Lüssmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode and K. H. Schleifer, Nucleic Acids Res., 2004, 32, 1363–1371 CrossRef CAS.
- B. Duim, T. M. Wassenaar, A. Rigter and J. Wagenaar, Appl. Environ. Microbiol., 1999, 65, 2369–2375 CAS.
- C. D. Michener and R. R. Sokal, Evolution, 1957, 11, 130–162 CrossRef.
- H. Ochman and R. K. Selander, J. Bacteriol., 1984, 157, 690–693 CAS.
- S. Barbesti, S. Citterio, M. Labra, M. D. Baroni, M. G. Neri and S. Sgorbati, Cytometry, 2000, 40, 214–218 CrossRef CAS.
- E. C. Lopez-Diez and R. Goodacre, Anal. Chem., 2004, 76, 585–591 CrossRef CAS.
- P. C. A. M. Buijtels, H. F. M. Willemse-Erix, P. L. C. Petit, H. P. Endtz, G. J. Puppels, H. A. Verbrugh, A. van Belkum, D. van Soolingen and K. Maquelin, J. Clin. Microbiol., 2008, 46, 961–965 CrossRef CAS.
- C. Kirschner, K. Maquelin, P. Pina, N. A. Ngo Thi, L. P. Choo-Smith, G. D. Sockalingum, C. Sandt, D. Ami, F. Orsini, S. M. Doglia, P. Allouch, M. Mainfait, G. J. Puppels and D. Naumann, J. Clin. Microbiol., 2001, 39, 1763–1770 CrossRef CAS.
- K. Maquelin, L. Dijkshoorn, T. J. K. van der Reijden and G. J. Puppels, J. Microbiol. Methods, 2006, 64, 126–131 CrossRef CAS.
- A. Oust, T. Møretrø, K. Naterstad, G. D. Sockalingum, I. Adt, M. Manfait and A. Kohler, Appl. Environ. Microbiol., 2006, 72, 228–232 CrossRef CAS.
- K. Maquelin, L. P. Choo-Smith, H. P. Endtz, H. A. Bruining and G. J. Puppels, J. Clin. Microbiol., 2002, 40, 594–600 CrossRef CAS.
- D. Helm and D. Naumann, FEMS Microbiol. Lett., 1995, 126, 75–79 CAS.
- A. Hermelink, M. Stammler and D. Naumann, Analyst, 2011, 136, 1129–1133 RSC.
| This journal is © The Royal Society of Chemistry 2013 |
