 Open Access Article
Open Access ArticleEffect of hydrogen peroxide oxidation systems on human urinary steroid profiles
Unnikrishnan
Kuzhiumparambil
and
Shanlin
Fu
*
Centre for Forensic Science, School of Chemistry and Forensic Science, University of Technology, Sydney (UTS), Ultimo, NSW 2007, Australia. E-mail: Shanlin.Fu@uts.edu.au; Fax: +61 2 9514 1460; Tel: +61 2 9514 8207
First published on 30th May 2013
Abstract
In sports drug testing the steroid profile is the most versatile and informative screening tool for the detection of steroid abuse. Despite the introduction of observed urine collection procedures by the World Anti-Doping Agency (WADA), chemical manipulation of urine specimens by athletes to conceal drug use still occurs and poses an ongoing challenge for doping control laboratories worldwide. In vitro urine adulteration using highly oxidative chemicals have been reported several times in the past. In this study we report the effect of two oxidising agents, Fenton's reagent and peroxidase–peroxide system on the human urinary steroid profile. Varying concentrations of these oxidants were reacted with urine and the reactions monitored by gas chromatography-mass spectrometry. A significant decrease in the absolute concentrations of androsterone, etiocholanolone, 5α-androstane-3α,17β-diol, 5β-androstane-3α,17β-diol and epitestosterone was observed with consequent alteration of the steroid profile ratios. Adulteration of urine sample with these oxidants can thus mask the abnormality in a steroidal profile following steroid abuse. Drug testing authorities should take into account the effects of these oxidizing adulterants while interpreting the steroid profile data for doping control purposes.
1 Introduction
The primary task of doping control laboratories is to detect prohibited substances in athlete specimens and chemical and physical manipulation of samples during the collection process.1,2 Even though doping control laboratories follow numerous measures to ensure the integrity of urine specimens, several cases of sample manipulation have been reported.3 This includes urine substitution, urine dilution, and urine adulteration with highly oxidative chemicals. Twelve incidences of physical or chemical manipulation of doping control samples, mostly by urine substitution or tampering, have been reported between 2006 and 2009.3 Some of these urine manipulations were done by corrupted doping control officials. Powerful oxidants such as nitrite, hypochlorite, peroxides, permanganate and iodate are commonly used chemicals for urine adulteration purposes.4–6 Many of these chemicals have been successfully used to produce false negative urine test results by altering the physicochemical properties of the samples.7 Many commercial products containing these oxidants are readily available over the internet. Examples include Stealth (containing peroxidase and peroxide), Klear (potassium nitrite) or Whizzies (sodium nitrite), Urine Luck (pyridinium chlorochromate), and UrinAid (glutaraldehyde).8 These adulterants act by either interfering with immunoassay procedures or by converting the target drugs to other compounds.9 These drug-masking chemicals are believed to be used by athletes across a range of sports for the same purposes. Finding an effective way of combating such adulteration activities has drawn considerable interest among scientists worldwide working in the field of drug testing.In sports drug testing the steroid profile is the most putative marker for the detection of steroid abuse.10 A urinary steroid profile is made up of the concentrations and ratios of several endogenous steroidal hormones, their metabolites and precursors present in urine during normal metabolic processes.2 These steroids include testosterone (T), epitestosterone (E), dihydrotestosterone (DHT), androsterone (A), etiocholanolone (Etio), 5α-androstane-3α,17β-diol (Adiol) and 5β-androstane-3α,17β-diol (Bdiol) as well as other steroidal species (Fig. 1).11,12 The Steroid profile is a naturally well balanced system that does not show much intra-individual variations especially with regard to the ratios utilized for doping control purposes such as T/E, A/Etio, A/T and Adiol/Bdiol.13 However it is highly sensitive to applications of endogenous as well as synthetic anabolic steroids. According to the World Anti-Doping Agency (WADA) guidelines, when an abnormal steroid profile is observed, further analysis is then performed on the sample to determine the origin of the altered steroidal analytes.14
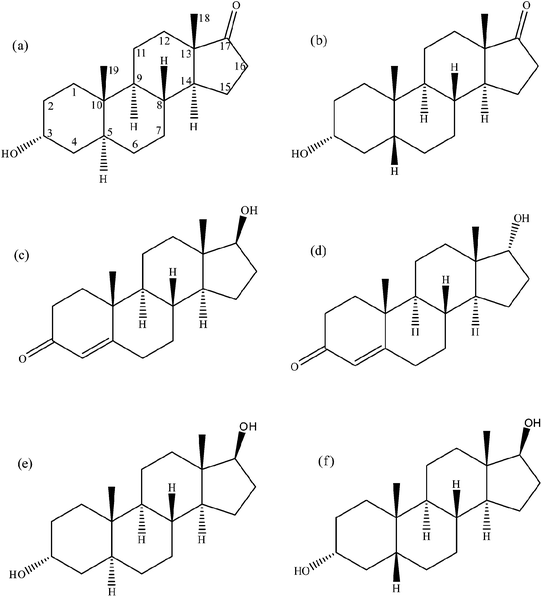 | ||
| Fig. 1 Steroid structures. (a) Androsterone, (b) etiocholanolone, (c) testosterone, (d) epitestosterone, (e) 5α-androstane-3α,17β-diol and (f) 5β-androstane-3α,17β-diol. | ||
Due to the vital importance of the steroid profile in the fight against doping in sports, several factors need to be considered while interpreting the steroid profile data for doping control purposes.2 We have recently reported that adulteration of urine samples with oxidising agents such as nitrite, permanganate, hypochlorite and chromate can lead to significant changes in endogenous steroid profile parameters and can thus mask drug abuse.15 In this study we report the effect of another two popular oxidising adulterants containing hydrogen peroxide, i.e., the peroxidase–hydrogen peroxide system and the Fenton's system on the steroid profiles of human urine.
Peroxidase–hydrogen peroxide system and Fenton's system are strong oxidising systems in which oxidation reaction is mediated through the highly reactive hydroxyl free radical.16,17 A combination of peroxidase and hydrogen peroxide is reported to provide strong oxidation potential to urine sample and thus can be an effective urine adulterant to mask drug abuse. The oxidant is readily available over the internet marketed under names such as Stealth.17,18 Stealth is reported to have an effect to mask several drugs of abuse including the opiates, morphine and codeine.18,19 The Fenton's system, consisting of hydrogen peroxide and ferrous sulphate is another frequently used oxidation system that is capable of oxidising and degrading organic substances owing to its high oxidation power and simplicity.20
2 Experimental
2.1 Reagents, chemicals and standards
Horse radish peroxidase (Type-V1 A), hydrogen peroxide (30% w/w), mercaptoethanol, ammonium iodide and β-glucuronidase from Helix pomatia (Type H-3) were purchased from Sigma Aldrich (Sydney, NSW, Australia). T, E, A, Etio, Adiol, Bdiol and the corresponding deuterated analogues (16,16,17-d3)-testosterone (d3-T), (16,16,17β-d3)-epitestosterone (d3-E), (2,2,4,4-d4)-androsterone (d4-A), (16,16,17-d3)-5α-androstane-3α,17β-diol (d3-Adiol), and (2,2,3β,4,4-d5)-5β-androstane-3α,17β-diol (d5-Bdiol) were purchased from the National Measurement Institute (North Ryde, NSW, Australia). N-Methyl-N-trimethylsilyltrifluoroacetamide (MSTFA) was obtained from UCT (Bristol, PA, USA). Surine (synthetic urine devoid of human urinary steroids) was purchased from Ceriliant (Austin, TX, USA). Ferrous sulphate, potassium bicarbonate, potassium carbonate, sodium acetate, and glacial acetic acid were obtained from Univar (Ingleburn, NSW, Australia). All other reagents were of analytical grade.2.2 Collection of urine
All volunteers were asked to provide informed consent, in compliance with the requirements of the National Health and Medical Research Council (NHMRC). Ethics approval for this study was obtained from the UTS Human Research Ethics Committee (ethics approval no. UTS HREC 2010-268A). Urine samples from twenty healthy subjects (10 males and 10 females) of Asian and Caucasian origin in age group of 20–40 years were collected into sterile plastic containers. Two pools (1 from male urines and 1 from female urines) of urines were then obtained by combining them. Each pool was then divided to 30 mL aliquots which were stored at −20 °C before being used for the study.2.3 Extraction of urine
A standard urinary steroid screening procedure was adapted to determine the concentration of selected endogenous steroids and ratios.21,22 To 1 mL of urine were added 1 mL of acetate buffer (0.2 M, pH 5.2), 50 μL of β-glucuronidase and internal standards mixture and these samples were incubated for 3 h at 58 °C. After hydrolysis, the hydrolysate was mixed with 1 mL of carbonate buffer (pH 9) and a liquid–liquid extraction was carried out with diethyl ether (5 mL) for ten minutes on a Ratek roller mixer (Boronia, VIC, Australia). The diethyl ether layer containing free steroids was then transferred into a screw cap glass tube and evaporated to dryness under nitrogen at 35 °C. The residue was then derivatised at 72 °C for 30 min, using 50 μL of MSTFA–NH4I–2-mercaptoethanol (1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/w/v) and injected directly into the GC-MS.
6, v/w/v) and injected directly into the GC-MS.
2.4 GC-MS
Quantitative analysis was performed following a previously reported and validated method on an Agilent 7890 series GC coupled to an Agilent quadrupole MS (5975N) (with a representative total ion chromatogram shown in Fig. 2).15 Three characteristic ions were monitored for each analyte. Ions monitored were as follows: A, 419, 434, 330; Etio, 419, 434, 330; Adiol, 241, 256, 336; Bdiol, 256, 241, 336; E, 432, 417, 327; T, 432, 417, 327; d4-A, 423, 438, 334; d3-Adiol, 244, 259, 339; d5-Bdiol, 261, 246, 341; d3-E, 435, 420, 330; d3-T, 435, 421, 330. The first ion was used as the target ion for quantification and the rest two as the qualifier ions. The dwell time was 30 ms for each monitored ion.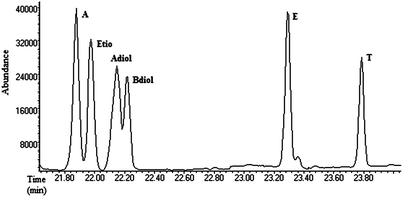 | ||
| Fig. 2 Total ion chromatogram of the six endogenous steroids; androsterone (A), etiocholanolone (Etio), 5α-androstane-3α,17β-diol (Adiol), 5β-androstane-3α,17β-diol (Bdiol). | ||
2.5 Reaction with oxidative adulterants
A range of concentrations of hydrogen peroxide in the presence of horse radish peroxidase and ferrous sulphate was reacted with 1 mL male and female urine at physiological pH for a time span of 2 h at room temperature. The oxidation reactions with Fenton's system was also performed at acidic pH based on previous reports that efficiency of Fenton's reagent is high at acidic pH.23 Acidic pH was achieved by addition of 25 μL of 1 M hydrochloric acid. The optimal ferrous ion concentration for the tested reaction conditions was determined as 0.025 mM.24 A series of experiments were performed with varying ferrous ion concentration (0.01 mM to 0.05 mM) and highest level of activity was observed with 0.025 mM (data not shown). In peroxidase–hydrogen peroxide system, excess peroxidase (5 units, 20 μL from stock solution 1 mg mL−1 in water) was added to reaction mixture. After the reaction with oxidants, the urinary steroid screening procedures as described in Sections 2.3 and 2.4 were followed to determine the recovery of steroids of interest. Urine samples without addition of oxidative adulterants were included in each experiment as controls.2.6 Statistical analysis
Statistical analysis was performed using one-way ANOVA. The significance level was set to 0.05 for all tests.3 Results
3.1 Effect of peroxidase–hydrogen peroxide oxidation system
Varying volumes (10, 20, 50 and 100 μL) of hydrogen peroxide (30% w/w) in the presence of peroxidase (5 units) was reacted with 1 mL urine at physiological pH (6.5 to 6.1). Urine colour slightly faded on addition of hydrogen peroxide, but not to an extent that would raise suspicion of adulteration. Considerable depletion of all endogenous steroids except T was observed on addition of hydrogen peroxide. The recovery of A, Etio, Adiol, Bdiol and E was reduced significantly in a concentration dependant manner (Fig. 3). In male urine, depletion of A, Etio and Bdiol were more profound than other steroids. With 100 μL hydrogen peroxide the recovery of A, Etio and Bdiol were reduced to less that 15%, whereas the recovery of Adiol and E were 35 and 55% respectively. T was found to be less reactive towards hydrogen peroxide with 79% recovery at highest concentration of hydrogen peroxide tested. The oxidant in female urine exhibited similar reactivity as observed in male urine.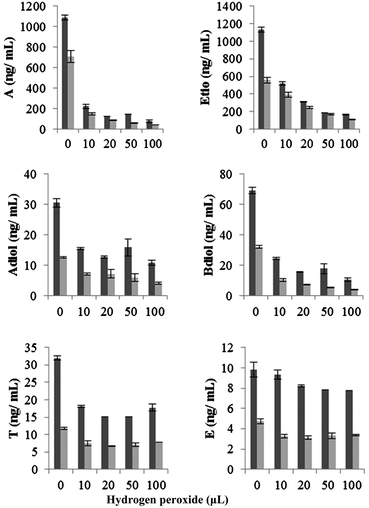 | ||
Fig. 3 Change in absolute concentration of endogenous steroids on addition of hydrogen peroxide in presence of peroxidase (5 units) at physiological pH. Data were mean values from 3 repeated experiments (n = 3) with standard deviation represented by the error bars.  male urine; male urine;  female urine. female urine. | ||
The effect of oxidant concentration on the steroid ratios is depicted in Fig. 4. Adiol/Bdiol ratio showed a gradual increase while the A/Etio and A/T ratios showed a gradual decrease over the oxidant concentration range (10 μL to 100 μL) tested. In male urine, the T/E ratio was increased marginally from 0.26 to 0.40 and the Adiol/Bdiol ratio was increased by 2-fold to approximately 1.25 from the basal level of around 0.52. Due to high reactivity of hydrogen peroxide against A, a sharp decrease in A/Etio and A/T ratios was observed. In female urine, the A/T ratio was decreased steeply from 87 (basal level) to 6 and A/Etio ratio decreased from 0.85 to 0.37. A marginal increase in T/E ratio was observed from 0.41 to 0.47 (P ≤ 0.05).
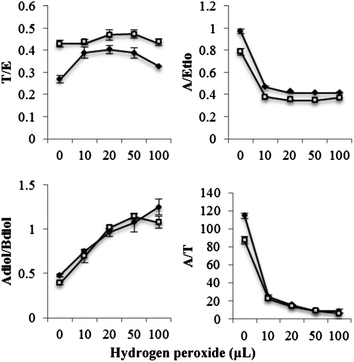 | ||
Fig. 4 Change in steroidal ratios on addition of hydrogen peroxide in presence of peroxidase (5 units) at physiological pH. Data were mean values from 3 repeated experiments (n = 3) with standard deviation represented by the error bars.  male urine; male urine;  female urine. female urine. | ||
3.2 Effect of Fenton's reagent
Fenton's system comprising of ferrous sulphate (0.025 mM) and hydrogen peroxide (25, 50, 75 and 100 μL) was reacted with urine at acidic pH (3.5–4.3) and physiological pH (6.3–7.1). No marked variation in the appearance of urine was observed with peroxide concentration studied; however addition of hydrogen peroxide above 100 μL (tested at 125 and 150 μL) resulted in the formation of a precipitate. The pH dependence of the effect of Fenton's reagent on steroids was minimal with steroids exhibiting similar reactivity at normal and acidic pH.In male urine, Fenton's reagent was found to be very reactive against A, Etio, Adiol, Bdiol and E under both test conditions. A significant loss of these steroids was found at the lowest hydrogen peroxide concentration (25 μL) tested. The recovery of A, Etio, Adiol, Bdiol and E were found to be 10, 20, 26, 12 and 41% respectively at physiological pH. No significant variation in the activity was observed at higher hydrogen peroxide concentrations. For example the recovery values for these steroids at an increased hydrogen peroxide concentration of 100 μL were 10, 15, 23, 11 and 38% respectively (Fig. 5). T exhibited moderate reactivity towards Fenton's reagent. The recovery of T was found to be 57% and 83% at physiological and acidic pH value at 25 μL hydrogen peroxide concentration. An evident decrease in the A/Etio and A/T ratio was observed, whereas Adiol/Bdiol ratio exhibited a steep increase. T/E ratio was found to increase marginally with an increase in oxidant concentration (Fig. 6).
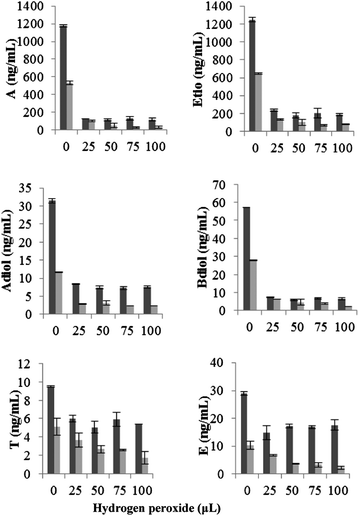 | ||
Fig. 5 Change in absolute concentration of endogenous steroids on addition of hydrogen peroxide in presence of ferrous sulphate (0.025 mM) at physiological pH. Data were mean values from 3 repeated experiments (n = 3) with standard deviation represented by the error bars.  male urine; male urine;  female urine. female urine. | ||
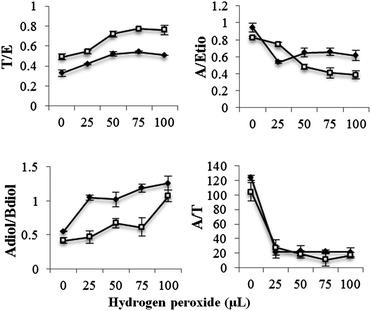 | ||
Fig. 6 Change in steroidal ratios on addition of hydrogen peroxide in presence of ferrous sulphate (0.025 mM) at physiological pH. Data were mean values from 3 repeated experiments (n = 3) with standard deviation represented by the error bars.  male urine; male urine;  female urine. female urine. | ||
Fenton's reagent in female urine exhibited significant reactivity against A, Etio, Adiol and Bdiol with an 80% decrease in recovery value at 25 μL hydrogen peroxide concentration. A similar trend of changes in steroid ratios was observed in female urine to that observed in the male urine.
3.3 Effect of oxidants on internal standards
Examination of the peak areas of the internal standards showed a decrease in the concentration of d4-A, d3-E and d3-T, indicating possible oxidation of these compounds by hydrogen peroxide. In male urine, 10–15% decrease in internal standard peak area (P ≤ 0.05) of d4-A was observed on addition of 20 to 100 μL hydrogen peroxide in presence of peroxidase. The effect on d3-E and d3-T were more intense with 15 to 30% decrease in peak area on addition of 10 to 100 μL (Fig. 7). Reaction of female urine with hydrogen peroxide also resulted in significant decrease in the concentration of d3-E and d3-T. With 50 to 100 μL concentration of hydrogen peroxide, 8 to 21% (P ≤ 0.05) and 23 to 35% (P ≤ 0.05) decrease in the peak areas of d3-E and d3-T was observed. Concentration of d4-A was also found to decrease by 13% with 100 μL of hydrogen peroxide. With Fenton's reagent in male urine 31 to 40% and 39 to 48% decrease in peak area of d3-E and d3-T respectively was observed at the range of hydrogen peroxide concentration tested (Fig. 8).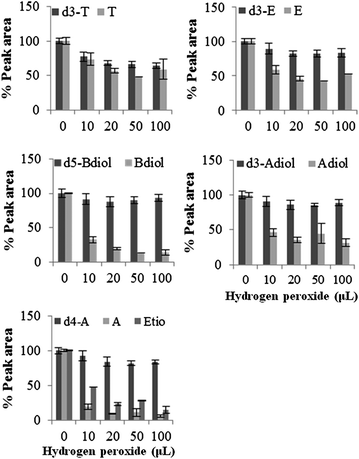 | ||
| Fig. 7 Percentage recovery of internal standard on addition of hydrogen peroxide in presence of peroxidase at physiological pH. Data were mean values of peak area of total ions (n = 3) with standard deviation represented by the error bars. | ||
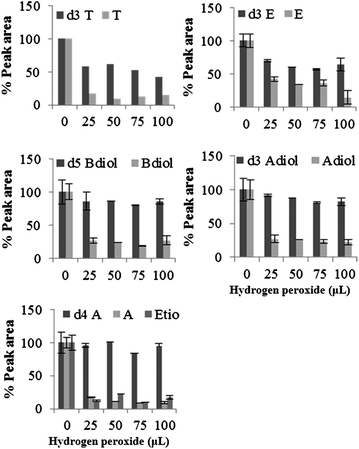 | ||
| Fig. 8 Percentage recovery of internal standard on addition of hydrogen peroxide in presence of ferrous sulphate (0.025 mM) at physiological pH. Data were mean values of peak area of total ions (n = 3) with standard deviation represented by the error bars. | ||
4 Discussion
Doping control authorities pay particular attention to atypical analytical results and conspicuous urinary steroid profile data using population based statistics.3 The absolute concentrations of endogenous steroids in human urine and steroidal ratios such as T/E, A/Etio, A/T and Adiol/Bdiol are the indicators utilized by doping control laboratories to screen steroid misuse. WADA has defined threshold values for absolute concentrations of endogenous steroids and specific steroidal ratios.25 A statistical outlier value and/or a disrupted steroid profile support a doping violation.This study demonstrates that two common oxidising systems containing hydrogen peroxide, Fenton's reagent and HRP-HP system, can reduce the absolute concentrations of endogenous steroids and consequently led to the alteration of steroidal ratios. Thus any possible higher excretion levels of endogenous steroids after doping can be masked by adulterating the urine sample by these oxidising agents which can lead to false negative results.
Depending on the drug abused, doping can leave specific imprints on potential doping markers. Administration of adrenocorticotropic hormone (ACTH), human chorionic gonadotropin (hCG), androstenedione and i.v. infusion of E is known to increase urinary E concentration and thereby decrease the T/E ratio.2,26 Administration of 1-androsterone, and dehydroepiandrosterone (DHEA) can significantly increase A/Etio ratio approximately twice their basal levels.12,26,27 Dihydrotestosterone (DHT), often being misused in sports for its anabolic and psychotropic benefits, can increase the urinary concentration of A and subsequently the A/Etio ratio.15,26 Our research has revealed that these alterations may be reverted back by the act of urine adulteration with hydrogen peroxide in the presence of peroxidase or ferrous sulphate, thus preventing detection of doping of these steroids.
It was also observed that addition of hydrogen peroxide to urine can degrade internal standards such as d4-A, d3-E and d3-T which can further underestimate the absolute concentration loss of steroids and thus alter the correct evaluation of the urinary steroid profile in doping control analysis. Doping control laboratories should take this decrease in internal standard peak area as a warning sign to investigate potential involvement of urine manipulation by oxidizing agents such as hydrogen peroxide.
In this study, the variation inflicted by oxidizing adulterants on steroid profile has not exceeded the WADA action limits based on population reference ranges. Therefore population based nonspecific thresholds on absolute concentrations of steroids and their respective ratios may not be fit for purpose to evidence steroid abuse and subsequent manipulation of urine sample.10 An individualisation of the reference ranges based on a longitudinal analysis of an individual steroid profile in an athelete's endocrinological passport can be an increasingly efficient approach to detect the biological fingerprint that doping leaves in athlete's body. This study supports the importance of establishing the Athlete Steroidal Passport (ASP), the steroidal module of Athlete Biological Passport presently being finalised by WADA for future implementation.25
5 Conclusion
The use of the steroid profile as an index of misuse of anabolic steroids is necessary since its parameters are useful indicators for further analysis to confirm the suspicion. Several factors need to be considered before interpreting a steroid profile data for doping control purposes. This study reports the effect of adulteration of urine samples with peroxidase–hydrogen peroxide system and Fenton's reagent. Both these oxidising agents can alter endogenous steroidal profile parameters and thus mask the abnormality in steroidal profile following steroid abuse. The study intends to improve the analytical capability of anti-doping laboratories to detect steroid abuse and/or chemical manipulation in sports.Acknowledgements
The study was funded by the Anti Doping Research Program (ADRP) of the Australian Government Department of Regional Australia, Local Government, Arts and Sport with a project ID 20-UTS-2011-12.References
- E. Castellanos-Rizaldos, P. Liu, C. A. Milbury, M. Guha, A. Brisci, L. Cremonesi, M. Ferrari, H. Mamon and G. M. Makrigiorgos, Clin. Chem., 2012, 58, 1130–1138 CAS.
- B. Hauser, T. Deschner and C. Boesch, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2008, 862, 100–112 CrossRef CAS.
- M. Thevis, H. Geyer, G. Sigmund and W. Schänzer, J. Pharm. Biomed. Anal., 2012, 57, 26–32 CrossRef.
- B. D. Paul, J. Anal. Toxicol., 2004, 28, 599–608 CrossRef CAS.
- A. Pham, S. Fu and M. Dawson, TIAFT Bulletin, 2011, 41, 49–51 Search PubMed.
- S. Luong, R. Shimmon, J. Hook and S. Fu, Anal. Bioanal. Chem., 2012, 403, 2057–2063 CrossRef CAS.
- M. Thevis, M. Kohler and W. Schänzer, Drug Discovery Today, 2008, 13, 59–66 CrossRef CAS.
- W. B. Jaffee, E. Trucco, S. Levy and R. D. Weiss, J. Subst. Abuse Treat., 2007, 33, 33–42 CrossRef.
- A. H. B. Wu, B. Bristol, K. Sexton, G. Cassella-McLane, V. Holtman and D. W. Hill, Clin. Chem., 1999, 45, 1051–1057 CAS.
- E. Strahm, P.-E. Sottas, C. Schweizer, M. Saugy, J. Dvorak and C. Saudan, Br. J. Sports Med., 2009, 43, 1126–1130 CrossRef CAS.
- C. Ayotte, in Doping in Sports: Biochemical Principles, Effects and Analysis, ed. D. Thieme and P. Hemmersbach, Springer, Berlin, Heidelberg, 2010, vol. 195, ch. 4, pp. 77–98 Search PubMed.
- P. Van Renterghem, P. Van Eenoo, H. Geyer, W. Schänzer and F. T. Delbeke, Steroids, 2010, 75, 154–163 CrossRef CAS.
- M. K. Parr and W. Schänzer, J. Steroid Biochem. Mol. Biol., 2010, 121, 528–537 CrossRef CAS.
- M. Thevis, W. Schänzer, H. Geyer, D. Thieme, J. Grosse, C. Rautenberg, U. Flenker, S. Beuck, A. Thomas, R. Holland and J. Dvorak, Br. J. Sports Med., 2013, 47, 109–114 CrossRef.
- U. Kuzhiumparambil and S. Fu, Steroids, 2013, 78, 288–296 CrossRef CAS.
- B. Petri, R. Watts, A. Teel, S. Huling and R. Brown, in In Situ Chemical Oxidation for Groundwater Remediation, ed. R. L. Siegrist, M. Crimi and T. J. Simpkin, Springer, New York, 2011, vol. 3, ch. 2, pp. 33–88 Search PubMed.
- J. T. Cody and S. Valtier, J. Anal. Toxicol., 2001, 25, 466–470 CrossRef CAS.
- A. Dasgupta, Am. J. Clin. Pathol., 2007, 128, 491–503 CrossRef CAS.
- J. T. Cody, S. Valtier and J. Kuhlman, J. Anal. Toxicol., 2001, 25, 572–575 CrossRef CAS.
- M. Ghaly, S. Hawash, A. Mahdy, S. Marsafy and E. Ahmed, J. Eng. Appl. Sci., 2005, 52, 1245–1264 Search PubMed.
- R. Ahmadkhaniha, A. Shafiee, N. Rastkari, M. R. Khoshayand and F. Kobarfard, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2010, 878, 845–852 CrossRef CAS.
- P. Van Renterghem, P. Van Eenoo, W. Van Thuyne, H. Geyer, W. Schänzer and F. T. Delbeke, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2008, 876, 225–235 CrossRef CAS.
- K. Barbusinski and K. Filipek, Pol. J. Environ. Stud., 2001, 10, 207–212 CAS.
- C. Bouasla, F. Ismail and M. E. Samad, Int. J. Ind. Chem., 2012, 3, 1–30 CrossRef.
- P.-E. Sottas, N. Robinson, O. Rabin and M. Saugy, Clin. Chem., 2011, 57, 969–976 CAS.
- M. Rodica, L. Mia and R. Gabriel, Rom. Biotechnol. Lett., 2011, 16, 6008–6016 Search PubMed.
- M. K. Parr, G. Opfermann, H. Geyer, F. Westphal, F. D. Sönnichsen, J. Zapp, D. Kwiatkowska and W. Schänzer, Steroids, 2011, 76, 540–547 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
