Evaluation of a drop-on-demand micro-dispensing system for development of artificial fingerprints
Jessica L.
Staymates
*,
Matthew E.
Staymates
and
Greg
Gillen
National Institute of Standards and Technology, Surface and Microanalysis Science Division, Gaithersburg, MD 20899, USA. E-mail: jessica.staymates@nist.gov
First published on 2nd November 2012
Abstract
Precision micro-dispensing is an evolving technique that has many applications in the scientific and additive manufacturing communities. Here we describe a method for dispensing viscous materials, including the oily substance found in human fingerprints, known as sebum. In this work, a dispense jet system was used to deposit known amounts of sebum onto surfaces to represent an artificial human fingerprint. Ultraviolet-visible spectrophotometry (UV-Vis) and microgravimetry were used to verify the sebum mass loadings of the samples. The dispense jet was capable of printing a viscous sebum mixture as well as a less viscous solution of sebum dissolved in heptane. This method was shown to be repeatable, and UV-Vis was found to be a simple and useful technique for verifying the mass of sebum deposited. This method could be used to prepare artificial fingerprint samples for a variety of applications including the preparation of test materials for emerging trace detection technologies.
Introduction
Materials deposition has become a common and useful technique for a variety of applications including bioprinting,1–3 fabrication of metal microstructures,4 development of components of optical systems,5,6 and medicine formulation.7,8 There are a variety of other dispensing technologies available, including some that have the capability to deposit viscous materials onto a surface for applications such as printing solder, conformal coatings, and adhesives. More recently, it has also been used to deposit explosives and narcotics solutions onto surfaces, for the production of test materials to evaluate trace contraband detectors such as ion mobility spectrometers (IMS).9 This method of test material preparation utilizes piezo-electric based inkjet technology, which allows for the deposition of extremely small (picoliters) and precise volumes of low viscosity (1 cP to 20 cP10) solutions with known concentrations onto various surfaces,9,11,12 such as IMS collection swabs.A potential novel extension of this approach is for the preparation of artificial fingerprints, since several forensic and trace chemical detection techniques investigate fingerprint residues left behind by a subject. Many times these latent fingerprints are collected not only for biometric information, but also for analysis of any number of compounds (such as drugs or explosives) and other trace evidence.13–15 However, in order to use these trace chemical screening techniques such as IMS, there is a need for standard test materials to validate their performance. Currently, such test materials are typically prepared using pure compounds.9 However, there is a need to develop more realistic test materials that represent a field sample which contains not only the chemical analyte of interest, but also background interferents and environmental species that are inherent to the sample. Of particular interest is the development of reference materials that include the oily substance that is present in latent fingerprints, known as sebum. However such viscous materials and particle suspensions cannot easily be printed with the inkjet technology that is currently utilized for the dispensing of standard analyte solutions. This paper describes an evaluation of a specialized micro-dispensing system for depositing a known mass of high viscosity sebaceous material on to substrates in a repeatable manner. This system allows for printing of solutions with a large range of viscosities (1 cP to 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cP) and can print solutions and particle suspensions with volumes as low as one nanoliter (nL) per drop. The ability to print a suspension (as opposed to a dissolved analyte solution) would also help prepare realistic samples with the particle size distributions that mimic actual explosive or narcotic mixtures. Constituents of sebum in human fingerprints can vary based on physiological differences such as gender and age.16–18 However for this feasibility study, a simplified artificial sebaceous material was used for testing the deposition method. The ultimate goal is to develop a technique to accurately and reproducibly generate an artificial fingerprint test material that contains the chemical constituents representative of real human fingerprints.
000 cP) and can print solutions and particle suspensions with volumes as low as one nanoliter (nL) per drop. The ability to print a suspension (as opposed to a dissolved analyte solution) would also help prepare realistic samples with the particle size distributions that mimic actual explosive or narcotic mixtures. Constituents of sebum in human fingerprints can vary based on physiological differences such as gender and age.16–18 However for this feasibility study, a simplified artificial sebaceous material was used for testing the deposition method. The ultimate goal is to develop a technique to accurately and reproducibly generate an artificial fingerprint test material that contains the chemical constituents representative of real human fingerprints.
Materials and methods
Sebum deposition was conducted with an Asymtek DJ9000 dispense jet system from Nordson† (Carlsbad, CA) which is a dispensing system commonly used to dispense viscous materials for applications such as soldering, adhesives, and conformal coating for circuit boards and semi-conductors. This drop-on-demand dispensing system cannot print the small volumes that piezoelectric inkjet printing systems can, but its capabilities for reproducible printing of highly viscous materials and particles in a suspension are promising and may play a key role for emerging forensic science dispensing applications.This dispense jet system features a pneumatically controlled piston with a ball-shaped tip on the end that rapidly contacts a seat within the nozzle assembly resulting in the ejection of the sample fluid through a narrow orifice at the nozzle tip. The geometry of the orifice as well as the throw of the piston and the rate at which the piston strikes the seat can be changed to adjust the droplet size (typically 100 μm to 300 μm diameter). Because the viscosity of many materials may change with temperature, the print-head can be heated at the tip to ensure repeatable operation. The system can dispense over an area of 350 mm × 350 mm (14 inches × 14 inches) onto samples that vary by 7.5 cm in height. A schematic diagram of the dispense jet head is shown in Fig. 1 and illustrates how this dispensing system operates.
 | ||
| Fig. 1 Schematic diagram of DJ 9000 jetting mechanism. As the piston contacts the nozzle seat, hydraulic pressure within the fluid forces a droplet to be ejected. Each stroke of the piston creates a single ejection event (image design based on Asymtek www.asymtek.com). | ||
A unique feature of this system is the use of an integrated Satorius WZ64S microbalance calibrated by Asymtek using weights traceable to NIST for mass-based gravimetric printing. In this mode of operation, the system translates the print-head to the balance and ejects a known number of microdrops. The drops are weighed and the resulting mass/drop is used to calibrate the total deposited mass of subsequent depositions. This is done automatically within the specialized Fluidmove software (Nordson, Carlsbad, CA) control program for the instrument.
Initial experiments involved the printing of artificial sebum material (components listed in Table 1), which is a soft wax-like mixture that is solid at room temperature. Although the print-head could be heated to melt (52 °C) the sebum material to a liquid for dispensing, the solid sebum in the unheated reservoir and transfer line made it difficult to print accurately and repeatably. Therefore, a removable heater was made in-house using a solid PTFE tube and heating tape to keep the sebum material in liquid form throughout the duration of dispensing. The temperature of the heater can be controlled to prevent excess heating of the material and avoid over-pressurization of the dispensing reservoir. This heater can be easily removed when the printing solution does not need to be heated. A photograph of the heater is shown in Fig. 2.
 | ||
| Fig. 2 Photo of the heating unit (A) and the controls (B) made in-house to heat the reservoir and keep solid materials in liquid form during printing. White arrows in (A) indicate the heating cartridges. The blue arrow in (A) shows the location of the nozzle orifice. | ||
To begin printing, 5 mL to 10 mL of artificial sebum was heated to form a liquid and added to the printer reservoir. The print-head was heated to 65 °C and the reservoir and fluid transfer lines were heated to approximately 60 °C in order to keep the sebum in a liquid state. The reservoir fluid pressure was set to 45.5 kPa, and the valve pressure was set to 620.5 kPa with a valve on time of 2 ms and off time of 3 ms. The integrated microbalance was used to measure the approximate mass of deposited sebum solution before and after each print sequence. The readability of this balance is 0.1 mg, and it was calibrated using a 50 g standard weight (Troemner, Thorofare, NJ) and verified using 1 mg to 20 g standard weights. All printing functions are controlled by the Fluidmove software.
For one series of experiments, a diluted solution containing 1 g sebum dissolved in 20 mL heptane was printed so the solvent would evaporate after dispensing, leaving behind a thin layer of sebum in each drop. The heater was not needed here since the solution was a liquid at room temperature. The fluid reservoir pressure was set to 19.3 kPa and the valve on and off times were changed to determine how this affects the mass per drop. A series of ten dots was printed for each valve time parameter. Once a consistent method was determined, an array of dots was created to represent the shape of a fingerprint, and the sebum mixture was printed.
To improve our ability to measure the mass of sebum deposited per drop, a stand-alone Sartorius model SE2-F microbalance (Elk Grove, IL) with a readability of 0.1 μg was used to weigh tin capsules before and after printing the sebum into them. The mass of standard weights ranging from 2 mg to 2 g were measured daily to determine the variability of the microbalance.
A Shimadzu 1800 Ultraviolet-Visible Spectrometer (UV-Vis) was used in a second set of experiments to verify the mass of sebum printed on a surface. Early experiments showed that cholesterol and jojoba oil had UV absorption peaks at around 195 nm. Since it is assumed that the sebum mixture is homogeneous, the absorbance of these compounds in sebum should provide the information needed to determine the total sebum concentration in a sample. Calibration curves were made by first pipetting various concentrations of a sebum–heptane solution into quartz cuvettes. Samples were analyzed by printing into small 2 mL capacity glass vials and allowing the solvent to evaporate. Then, 1 mL of heptane was added to each vial and the samples were sonicated for five minutes. The solutions were then transferred to clean quartz cuvettes for analysis with the UV-Vis.
Results and discussion
There are a variety of printing methods available in the software for dispensing, including single drops, multiple drops per spot, lines, circles, underfill applications, etc. A weighted line can also be jetted, where the print-head dispenses a predetermined mass of material over a line marked with fiducials. In this work, an array was created to print a square containing ten rows and ten columns of single dots of sebum. Heated sebum arrays were printed in 2 cm by 2 cm squares on a painted metal surface to simulate fingerprint secretions on painted automobile surfaces. Fig. 3 shows the 16 arrays, each containing sub-arrays of 10 × 10 individual drops. Fig. 4 is a micrograph of the individual sebum droplets. The low-sensitivity integrated mass balance determination measured these dots to be approximately 185 μg of sebum per drop. Each spot in an array contains a single drop of sebum.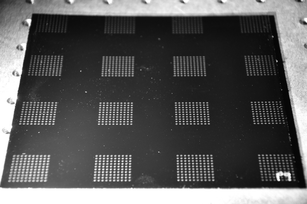 | ||
| Fig. 3 Photo of painted metal surface with 16 arrays of sebum dots dispensed with the Asymtek printer system. | ||
 | ||
| Fig. 4 Reflected light micrograph of sebum dots on painted metal surface. | ||
Deposition of the viscous artificial sebum mixture with the dispense jet was successful, and the arrays of drops were uniform in size and shape, as seen in Fig. 3 and 4. A software program called ImagePro (Media Cybernetics Inc.) was used for post-processing to determine the area of each full dot in Fig. 4 based on histogram intensity levels. The relative standard deviation (RSD) for the average area of the six dots was 2.49%, indicating that the dot size ejected from the printer is extremely repeatable. The software was used to also measure the average mean diameter of the six dots based on pixels, and that measurement had a lower RSD at 1.27%. This was a useful measurement tool for determining the repeatability of the dispensed drop size.
To simplify the printing process and to eliminate the need for the modified reservoir heater, a different approach was also evaluated. In this case, sebum was dissolved in heptane so that it remained liquid at room temperature and, once printed, the solvent would evaporate and leave behind a thin residue of sebum.
Experiments for measurement purposes were completed using this solution (1 g sebum dissolved in 20 mL heptane). Fig. 5 shows a micrograph of a single drop of the sebum–heptane solution dispensed with the Asymtek system. This droplet has a larger diameter (approximately 2 mm) than with the previous method, but the mass of sebum is much less because the majority of the dispensed material (heptane) has evaporated. The result is a large circular deposit that contains a thin layer of sebum that has dried to form many small droplets over the surface and closely resembles the global features of a real fingerprint.
 | ||
| Fig. 5 Droplet of sebum when the heptane has evaporated after the sebum–heptane mixture was dispensed on the surface. | ||
Initial tests were completed with a nozzle that has a 100 μm orifice, 3 ms valve-on and valve-off times, and a pressure of 13.8 kPa. The deposits were designed in the shape of fingerprints with either 10 or 16 drops per array. A negligible amount of fluorescent dye was also added to the sebum solution as a visual aid for locating the deposit on the sample. Since the starting solution concentration is known (1 g sebum in 20 mL heptane) and the mass per drop can be measured, this information can be used to calculate an estimate of the mass of sebum left on the deposit once the heptane has evaporated. The ultimate goal is to deposit a mass of sebum that is similar to that of an actual latent fingerprint, which has been found to be between 0.5 μg and 15 μg of sebum.18,23
One unique application of the printer system is the ability to print a suspension of particles, such as those found in military explosives including C-4 plastic explosive. Pure cyclotrimethylenetrinitramine (RDX) crystals were homogenized by water-milling to reduce the particle diameter size to 20 μm or less and were added to the heptane–sebum solution. In order to print particles with this dispensing system, they need to remain in a suspension without settling to the bottom of the reservoir too quickly. This was not possible with the sebum–heptane solution because it was not viscous enough. Since printing the viscous sebum mixture without solvent requires heating, it would be unlikely for RDX particles to remain stable at elevated temperatures. Therefore, adding polyisobutylene (PIB) to the sebum–heptane–RDX mixture helped keep the RDX particles in suspension, and because this polymer is commonly used as a binder for the plastic explosive C-4, this solution would create a more realistic explosive fingerprint on the test surfaces. Fig. 6 shows two micrograph images of dispensed droplets of this solution printed in an array that is shaped like a fingerprint. The image on the left is a reflected white light image showing the drop size and formation. On the right is a polarized light image showing the birefringent RDX particles and their location within each droplet.
 | ||
| Fig. 6 White light (left) and polarized light (right) micrographs of sebum–heptane–RDX–polyisobutylene mixture printed in a fingerprint-like array. | ||
After determining the feasibility of printing the sebum–heptane mixture with PIB and RDX particles in suspension, the simplified sebum–heptane solution was used for measurement purposes in the next experiments. The sebum arrays were printed on several glass microscope slides for visual inspection and to develop an extraction procedure to confirm the mass of sebum for each deposit. A UV-visible spectrophotometer was used in spectrum mode to determine whether the printer was depositing the expected mass of sebum. First a calibration curve was created by pipetting the original printing solution into cuvettes and diluting with heptane to get concentrations ranging from 40 μg mL−1 to 120 μg mL−1 sebum. It was found that extracting the printed glass slides in small volumes necessary for UV-Vis was difficult; therefore drops of sebum solution were printed directly into small glass vials.
Fig. 7 shows the resulting calibration curve for the pipetted solutions, as well as the calculated mass printed in each of the glass vials. The error bars are one standard deviation of the average of the absorbance measurements. A linear fit to the calibration curve has an R2 value of 0.9674, and the variability in the curve is likely due to pipetting errors. The mass of the printed solutions was calculated using the equation of this line (shown in the graph legend). The lower masses of printed sebum have larger error bars because the UV-Vis is not sensitive enough to reliably detect these sebum concentrations.
 | ||
| Fig. 7 UV-Vis absorption as a function of sebum concentration for a pipetted calibration curve. The mass of printed sebum samples are calculated using the absorbance (averaged for each drop #) in the linear equation of the calibration curve. Error bars are one standard deviation of two replicates. | ||
Microgravimetry
One additional method that can be used to verify the mass of sebum that is deposited with the high viscosity printer is microgravimetry. The integrated mass balance in the printer system can be used to roughly calculate the mass of the sebum solution deposited before and after each printing sequence. This calculation can vary depending on the nozzle size, pressure, valve-on and valve-off times, and viscosity of the solution. One pitfall to this method is that it appears that the software collects the mass measurement from the balance immediately without allowing the measurement to stabilize and reach a steady-state value. Since the drops are forcefully ejected onto the balance plate, this could result in a higher mass than what is actually deposited. This integrated mass measurement system was precise and was useful for initial tuning, but was found to be inaccurate in terms of actual mass of material deposited. Alternatively, a stand-alone microbalance was used for mass measurements. In this approach, the masses of ten small tin weighing capsules were measured before and after depositing 10 to 50 drops of sebum into each tin. The pre-weigh, print, and post-weigh were all completed within a six hour period and the difference of the measurements before and after printing was calculated. In Fig. 8, the measured mass of sebum deposited in the tins is compared to the calculated mass of sebum dispensed in the glass vials from UV-Vis measurements. | ||
| Fig. 8 Printed mass of sebum as a function of number of drops dispensed. Error bars are one standard deviation. | ||
For these measurements, the lower numbers of drops push the limits of the measurement techniques for both UV-Vis and gravimetry, resulting in larger error bars (larger standard deviations). Each of these lines are relatively linear (R2 values of 0.9959 and 0.9968 for UV-Vis and gravimetry, respectively), showing the repeatability of the dispense jet. However, there is a difference in the slopes for each line. The reason for this is still to be determined, but it is likely due in part to the measurement sensitivity of the lower sebum mass levels.
It is also important to characterize individual nozzles used in these types of micro-dispensing technologies. This particular Asymtek system has over 20 different nozzle configurations with different orifice sizes and piston seat designs. Fig. 9 shows the calculated sebum mass from UV-Vis absorbance data as a function of the number of drops dispensed for two different nozzles. Each curve is the average of two separate datasets, which were collected over a 16 month period. Each of these measurements was collected using different sebum–heptane solutions with slight variations in concentration, so a separate calibration curve is associated with each dataset for the mass calculations. Each dataset was also printed with varying flow rates, which were normalized to the largest measured flow rate for simplified comparison. Although the nozzles used here have the same orifice size of 100 μm, this graph shows a difference in mass deposited between the two nozzles. Because of the mass difference between the two nozzles, we decided to characterize each nozzle independently before printing to facilitate a better estimate of the mass of material that each nozzle deposits, along with an idea of the repeatability of each nozzle design.
 | ||
| Fig. 9 Comparison of the mass deposited using two different 100 μm-orifice nozzles. Sebum mass is calculated based on UV-Vis calibration curves for each dataset. Error bars are one standard deviation. | ||
In a separate part of the experiment, increments of 50 drops of sebum were deposited in ten tin capsules and ten glass vials. Fig. 10 shows the sebum mass per drop deposited as determined by microgravimetry and UV-Vis analysis. It is important to note that these measurements were obtained from separate sample sets. The sebum solution, flow rate and nozzle type were consistent in both sets. There is an offset in the two measurement methods which at this moment cannot be explained. The difference between the results of the two measurement methods is approximately 20%. There appears to be a systematic bias; the reasons for this effect are currently being investigated. The relative standard deviation (RSD) is 4.66% for the average UV-Vis measurements and 9.04% for gravimetry measurements. Based on the lower RSD, UV-Vis may be the more reliable and less variable technique to use for measuring the mass deposited using this printer system. It is also a simple and less time consuming process. In contrast, microgravimetry suffers from several environmental factors such as humidity changes, electrostatic effects, and lack of sufficient vibration isolation, all of which can lead to erroneous mass readings.
 | ||
| Fig. 10 Total mass of sebum per drop for ten 50-drop samples as determined by microgravimetry and UV-Vis. Error bars are one standard deviation. | ||
Conclusions
The high viscosity dispensing method system has been shown to be a useful technique for micro-deposition applications and has high-throughput capabilities (an estimated 2000 samples per h). This printer system has certain advantages compared to piezo-electric based inkjet printing systems, the most practical being the capability to dispense viscous materials and particles in suspension in a repeatable manner. The integrated mass balance is beneficial for initial tuning and estimating the mass of material that is being deposited onto the surfaces; however other techniques are necessary to verify the amount of material that was truly dispensed. Some applications may not need a more accurate measurement than the integrated mass balance provides. UV-Vis was shown to be a reliable method of determining the mass of analyte that is deposited on a surface with the dispense jet. This technique can be used to estimate the ideal number of drops for each application and desired mass of analyte, and can be used to confirm the mass that is dispensed. While UV-Vis is a fast and reliable method, there are many other methods that may be amenable to measuring the composition of sebum including GC/MS, LC/MS, FTIR, ambient ionization and secondary ion mass spectrometry. Future efforts will explore techniques that can verify the amount of material that is present in each printed sample, preferably without destroying the sample.Acknowledgements
The U.S. Department of Homeland Security sponsored the production of this material under an Interagency Agreement with the National Institute of Standards and Technology.References
- V. Mironov, G. Prestwich and G. Forgacs, J. Mater. Chem., 2007, 17, 2054–2060 RSC.
- A. Skardal, J. Zhang, L. McCoard, X. Xu, S. Oottamasathien and G. D. Prestwich, Tissue Eng., Part A, 2010, 16(8), 2675–2685 Search PubMed.
- S.-J. Song, J. Choi, Y.-D. Park, S. Hong, J. J. Lee, C. B. Ahn, H. Choi and K. Sun, Artif. Organs, 2011, 35(11), 1132–1136 Search PubMed.
- A. Tropmann, N. Lass, N. Paust, T. Metz, C. Ziegler, R. Zengerle and P. Koltay, Microfluid. Nanofluid., 2012, 12, 75–84 CrossRef CAS.
- R. Danzebrink and M. A. Aegerter, Thin Solid Films, 1999, 351, 115–118 CrossRef CAS.
- R. Danzebrink and M. A. Aegerter, Thin Solid Films, 2001, 392, 223–225 Search PubMed.
- N. Scoutaris, M. R. Alexander, P. R. Gellert and C. J. Roberts, J. Controlled Release, 2011, 156, 179–185 Search PubMed.
- P. A. Melendez, K. M. Kane, C. S. Ashvar, M. Albrecht and P. A. Smith, J. Pharm. Sci., 2007, 97(7), 2619–2636 Search PubMed.
- E. Windsor, M. Najarro, A. Bloom, B. Benner, R. Fletcher, R. Lareau and G. Gillen, Anal. Chem., 2010, 82, 8519–8524 Search PubMed.
- W. R. Cox, C. Guan and D. J. Hayes, Proc. SPIE, 2000, 3952, 400–407 Search PubMed.
- R. M. Verkouteren and J. R. Verkouteren, Anal. Chem., 2009, 81, 8577–8584 CrossRef CAS.
- R. M. Verkouteren, G. Gillen and D. W. Taylor, Rev. Sci. Instrum., 2006, 77, 085104 CrossRef.
- C. M. Wynn, S. Palmacci, R. R. Kunz and M. Aernecke, Opt. Express, 2011, 19(19), 18671–18677 Search PubMed.
- P. H. R. Ng, S. Walker, M. Tahtouh and B. Reedy, Anal. Bioanal. Chem., 2009, 394, 2039–2048 CrossRef CAS.
- J. S. Day, H. G. M. Edwards, S. A. Dobrowski and A. M. Voice, Spectrochim. Acta, Part A, 2004, 60, 563–568 CrossRef.
- K. M. Antoine, S. Mortazavi, A. D. Miller and L. M. Miller, J. Forensic Sci., 2010, 55(2), 513–518 CrossRef CAS.
- K. G. Asano, C. K. Bayne, K. M. Horsman and M. V. Buchanan, J. Forensic Sci., 2002, 47(4), 805–807 CAS.
- G. M. Mong, C. E. Petersen and T. R. W. Clauss, Advanced Fingerprint Analysis Project: Fingerprint Constituents, Pacific Northwest National Laboratory, Richland (WA), Report no. PNNL-13019, September 1999 Search PubMed.
- ASTM Standard guide for evaluating cleaning performance of ceramic tile cleaners, ASTM standard # D5343–97.
- S. Valivetu, J. Wesley and G. W. Lu, Int. J. Pharm., 2008, 346, 10–16 Search PubMed.
- P. Airey, J. Verran and A. McMahon, Food Bioprod. Process., 2006, 84(C4), 359–365 Search PubMed.
- W. Musial and A. Kubis, Eur. J. Pharm. Biopharm., 2003, 55, 237–240 Search PubMed.
- N. E. Archer, Y. Charles, J. A. Elliott and S. Jickells, Forensic Sci. Int., 2005, 154, 224–239 CrossRef CAS.
Footnote |
| † Certain commercial equipment, instruments, or materials are identified in this document. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the products identified are necessarily the best available for the purpose. |
| This journal is © The Royal Society of Chemistry 2013 |
