Sensitive detection of prion protein through long range resonance energy transfer between graphene oxide and molecular aptamer beacon†
Hong Lin
Zhuang
a,
Shu Jun
Zhen
a,
Jian
Wang
b and
Cheng Zhi
Huang
*ab
aEducation Ministry Key Laboratory on Luminescence and Real-Time Analysis, College of Chemistry and Chemical Engineering, Southwest University, Chongqing, PR China
bCollege of Pharmaceutical Sciences, Southwest University, Chongqing, PR China. E-mail: chengzhi@swu.edu.cn; Fax: +86-23-68254659; Tel: +86-23-68367257
First published on 6th November 2012
Abstract
The traditional molecular aptamer beacon (MAB) is designed by combining an aptamer to a molecular beacon, and its two terminals are labelled with a fluorescent moiety (donor) and quenching moiety (acceptor), respectively. However, it usually has a high background because of the low energy transfer efficiency between the donor and the acceptor. In order to overcome these drawbacks, we have developed a novel MAB with just one fluorescently labelled end, which acts as the donor, and graphene oxide (GO) introduced as the acceptor for target detection by employing long range resonance energy transfer (LrRET) as the signal-transduction mechanism from GO to MAB. To test the validity of the designed MAB system, cellular prion protein (PrPC) has been used as the model target. It was found that the fluorescence of the designed MAB is completely quenched by GO, supplying a very low background. Conversely, the quenched fluorescence is recovered significantly with the addition of PrPC, so that PrPC can be detected over a wide range of 10.2–78.8 μg mL−1 with a detection limit as low as 0.309 μg mL−1 and with high selectivity. This GO-based MAB approach is a successful application of LrRET for the detection of PrPC, with advantages such as low costs, high quenching efficiency and good specificity, and it opens up new opportunities for the sensitive detection of biorecognition events.
Introduction
Molecular aptamer beacons (MABs) are specifically designed oligonucleotides with hairpin structures that have the advantages of both the generality of aptamers and the good signal transduction capability of molecular beacons (MBs).1,2 Applications of traditional MABs range from genetic screening, biosensor development, biochip construction, and the detection of target biomolecules3,4 such as thrombin,5 platelet-derived growth factor,6 and so on. These methods, however, suffer from several limitations such as the high-cost of synthesis as the opposite two termini of the MAB are respectively labelled with the fluorophore (donor) and the quencher (acceptor),7 high background signal because of the low fluorescence resonance energy transfer (FRET) efficiency between the conventional fluorophore and quencher, easy digestion by endogenous nuclease,8 and so on. To overcome these shortcomings, our group has designed a new MAB for ATP detection based on the long range resonance energy transfer (LrRET) between dye and multi-walled carbon nanotubes (MWCNTs), which is cost-effective and has reduced the background signal.7 Compared with carbon nanotubes (CNTs), graphene with a single-atom-thick and two-dimensional structure9 can quench the fluorescence of dye more effectively because the rate of energy transfer has R−5 dependence from dye to CNTs, but R−4 to graphene, where R is the distance between the donor and the acceptor.10,11 Moreover, graphene can interact with the soft loop of the single-stranded DNA (ssDNA) portion of MAB nonconvalently by means of π–π stacking interactions between nucleotide bases and graphene but hardly interacts with the rigid double-stranded DNA (dsDNA).8,12–14 Therefore, graphene not only acts as an excellent “nanoquencher” to quench the fluorescence of organic dye but also has the superquenching ability to minimize background fluorescence.15 In addition, it can protect DNA against nuclease digestion and enhances the self-delivery capability and the intracellular stability of DNA compared with free DNA probes.16 Thus, several lines of work have reported the fluorescent detection of target molecules such as ATP,8 Mucin 1 (MUC1),17 insulin,18 and thrombin19 by using aptamer–graphene oxide (GO)-based fluorescence probes. However, up to now, there has been no relevant literature on the detection of cellular prion protein (PrPC) using GO-based MAB probes.Inspired by these reports, herein we have developed a new strategy to design a MAB with only one end labelled by a fluorophore as the energy donor and GO as the energy acceptor, by taking PrPC, an important glycosylphosphatidylinositol (GPI)-anchored plasma membrane protein,20,21 as a target molecule.
Because of the high energy transfer between GO and dye on MAB, the background fluorescence is highly reduced and the signal-to-background ratio is improved greatly. In addition, our designed GO-based one-end-labelled MAB is simple and cost-effective, and it can be applied in the detection of other targets.
Experimental
Apparatus
The fluorescence spectra and fluorescence anisotropy were measured with a Hitachi F-2500 fluorescence spectrophotometer (Hitachi, Tokyo, Japan). A vortex mixer, QL-901 (Haimen, China), was employed to mix the solution, and high-speed TGL-16M centrifuge (Hunan, China) was used for the centrifugation process. A CyberScan pH510 desktop pH meter (EUTECH, Singapore) was used to detect the pH values.Materials and reagents
Graphene oxide was purchased from Sinopharm Chemical Reagent Co. Ltd. (China). Human immunoglobulin (H-IgG) was purchased from Sigma-Aldrich (Missouri, USA). Transferrin, ferritin, pepsin, and bovine serum albumin (BSA) were purchased from Shanghai Biotech. (Shanghai, China). 2-(N-morpholino) ethanesulfonic acid (MES) was purchased from Sino-American Biotech. (USA). 20 mM MES buffer was prepared by dissolving 0.3905 g MES in 100 mL ultra-pure water and the pH was adjusted to 6.42 with NaOH. Ultrapure water obtained from a Millipore water purification system (18 MΩ, Milli-Q, Millipore, Billerica, MA) was used throughout. All other reagents were of analytical grade.The sequence of the designed MAB was 5′-tetramethyl-6-carboxyrhodamine (TAMRA)-CC![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif)
![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) AT TTC TCG GG-3′, in which the underlined portion was the core region of anti -PrPC aptamer, and it was synthesized by Beijing SBS Genetech Co., Ltd. (Beijing, China).
AT TTC TCG GG-3′, in which the underlined portion was the core region of anti -PrPC aptamer, and it was synthesized by Beijing SBS Genetech Co., Ltd. (Beijing, China).
Preparation of recombinant human prion protein
The PrPC was purified according to the method developed by Xiao's group.22 The plasmid of recombinant human prion protein (23–231) was constructed and expressed in Escherichia coli BL21 (DE3). Briefly, a fresh overnight culture was used for inoculation by 50 μg mL−1 isopropyl-β-D-thiogalactopyranoside. After 4 h, bacteria were harvested by centrifugation and sonicated in buffer A (50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole, pH 8.0).The resulting solution was then denatured in 6 M guanidine hydrochloride (Gdn-HCl) overnight and purified by nickel–nitrilotriacetic acid agarose resin. The concentration of purified PrPC was quantified by ultraviolet-visible (UV-vis) absorption spectrophotometry.
General procedures
A certain concentration of PrPC was added to the solution of 30 μg mL−1 GO, at pH 6.42. The mixture was kept at room temperature for 30 min. Then, the solution was transferred for fluorescence measurements, at an excitation wavelength of 525 nm, using the F-2500 fluorescence spectrophotometer.Results and discussion
Design strategy
The whole procedure for the fabrication of the GO-based MAB detection platform and its application in PrPC detection are illustrated in Scheme 1. The novel MAB was designed by integrating a single-labelled hairpin-shaped aptamer and GO. The hairpin-shaped aptamer was constructed with anti-PrPC aptamer in the loop and another two nucleotides added to the 5′-end of the aptamer which are complementary to nucleotides at the 3′-end of the aptamer to form a hairpin-shaped probe.23 Only one end of the designed MAB was labelled with a fluorophore-TAMRA, and GO was used as a nanoquencher. In the absence of PrPC, MAB can be adsorbed on the surface of GO through π–π interaction between GO and the loop structure of MAB. Thus, the background fluorescence signal of MAB is quenched efficiently through the LrRET from TAMRA to GO. In the presence of PrPC, the MAB forms a ligand-binding structure, leaving the surface of GO, and thus the fluorescence is restored. | ||
| Scheme 1 Illustration of the fabrication of GO based MAB and the procedure of fluorescent detection of PrPC. | ||
This GO-based MAB strategy is simple and cost-effective as the designed MAB only needs to be labelled on one end. More importantly, this strategy might have a very low fluorescence background because of the high LrRET efficiency between GO and the fluorophore.
Investigation of the designed GO-based MAB for PrPC detection
In order to confirm our design strategy, the fluorescence spectra of GO-based MAB before and after the addition of PrPC were measured, and the results are shown in Fig. 1. It was found that the free MAB showed strong fluorescence emission in MES (Fig. 1a) which was quenched by more than 96.8% in MES upon addition of 30 μg mL−1 GO (Fig. 1b), indicating that GO could quench the fluorescence of TAMRA due to the highly efficient LrRET from dye to GO. When the MAB were bonded with target PrPC, the fluorescence was obviously enhanced (Fig. 1c). These results clearly indicate that the introduction of GO significantly improves the sensitivity as it greatly minimizes the fluorescence background and thus significantly improves the signal-to-background ratio. | ||
| Fig. 1 Fluorescence emission spectra of MAB (50 nM) before (a) and after (b) adding GO (30 μg mL−1) and then incubating with PrPC (20.5 μg mL−1) (c) in MES buffer. | ||
To further verify the mechanism of this method, the fluorescence anisotropy of the MAB–GO–PrPC system was measured (Fig. 2). The fluorescence anisotropy of a fluorophore reflects the ability of a molecule to rotate in its microenvironment, and is commonly used to investigate molecular interaction.24 The fluorescence anisotropy of free MAB in MES was 0.204, however, it increased 3.17-fold after addition of GO, indicating that the MAB was adsorbed on the GO surface. Nevertheless, the fluorescence anisotropy decreased by 1.89-fold after further addition of the PrPC into the mixture of GO and MAB. It illustrates that in the presence of PrPC, the MAB and PrPC forms the MAB–PrPC complex, resulting in the delivery of MAB from the GO surface, making the dyes far away from the GO surface. These results further demonstrate that the strong interaction between PrPC and the MAB achieves the purpose of detection of PrPC.
 | ||
| Fig. 2 Change of fluorescence anisotropy of MAB (50 nM) under different conditions: (a) MAB in MES buffer; (b) MAB + GO; (c) MAB + 20.5 μg mL−1 PrPC + GO. Excitation was monitored at 525 nm, and emission was monitored at 580 nm. | ||
GO-based MAB as a low-fluorescence background signal platform
We found that the concentrations of GO strongly influenced the detection of PrPC. With the increasing concentration of GO, the fluorescence intensity of the MAB decreased gradually and trended to a minimum value at 30 μg mL−1 (Fig. 3). Further experimental results showed that when GO was 30 μg mL−1, the ratio of F/F0 (where F0 and F are the fluorescence intensities of TAMRA at 580 nm in the absence and presence of PrPC, respectively) reached the highest value, 11.9 (Fig S1†). As a result, 30 μg mL−1 was used as the optimized concentration for GO. | ||
| Fig. 3 Fluorescence emission spectra of MAB (50 nM) at GO concentrations of 0, 5, 10, 15, 20, 25, 30, 35 and 40 μg mL−1 (a–i). The inset is the fluorescent intensity ratio of MAB upon addition of GO at different concentrations. | ||
Highly sensitive and selective detection of PrPC
After the addition of a series of PrPC solutions with final concentrations from 10.2 to 78.8 μg mL−1, the fluorescence emission intensity increased gradually (Fig. 4A). It was found that the restored fluorescence intensity follows a linear relationship, which could be expressed as the linear equation F/F0 = 20.6978logc − 14.409, where c is the concentration of PrPC (regression coefficient R2 = 0.993) (Fig. 4B). The detection limit (3σ/k) was estimated to be 0.309 μg mL−1, which was comparable to that of the other reported PrPC detection methods. In recent years, methods including capillary electrophoresis-based noncompetitive immunoassay,25 nanomechanical resonator assays26 have been employed in the detection of PrPC. However, most of these methods are based on the recognition of the antibody of prion protein, and need some complicated functionalization steps. On the other hand, the affinity of the clearly identified antibodies of prion protein in the native form is too low to be applied in practical conditions.27 Therefore, our designed GO-based MAB strategy is obviously more suitable for PrPC detection due to its cost-effective, simple, and sensitive properties.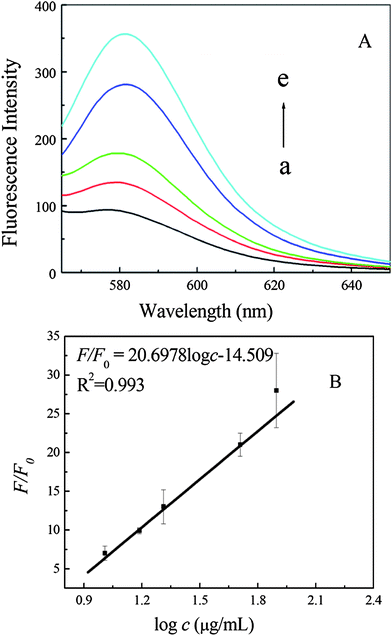 | ||
| Fig. 4 Fluorescence emission spectra of MAB (50 nM) after incubation with 10.2, 15.4, 17.9, 20.5, 51.2 and 78.8 μg mL−1 PrPC (a–e) (A), and then addition of GO (30 μg mL−1). (B) The plot of fluorescence intensity ratio F/F0vs. logarithm of PrPC concentration. | ||
The specificity of the designed GO-based MAB fluorescence assay was also examined using other proteins. Under the optimized detection conditions, IgG, transferrin, ferritin, BSA and pepsin were detected individually with the GO-based MAB fluorescence assay, and the experimental results are shown in Fig. 5. The fluorescence signal has a slight change after the addition of these proteins (F/F0 = 4.87 for transferrin, 5.04 for BSA, 0.97 for IgG, 1.25 for ferritin and 1.22 for pepsin) because the active site of the GO was occupied by BSA and transferrin through the electrostatic and π–π interaction between them, leading to the separation of MAB from the surface of GO, but it does show significant enhancement after the introduction of 20.5 μg mL−1 PrPC. Therefore, the GO-based MAB can be applied in the highly selective detection of PrPC.
 | ||
| Fig. 5 Fluorescence intensity changes (F/F0) of GO-based MAB (50 nM) after incubation with transferrin (20.5 μg mL−1), bovine serum albumin (BSA) (20.5 μg mL−1), IgG (20.5 μg mL−1), pepsin (20.5 μg mL−1) and ferritin (20.5 μg mL−1), respectively, where F and F0 are the fluorescence intensities of MAB after and before hybridization in the presence of GO at 580 nm. | ||
Conclusions
In summary, we designed a simple, selective and sensitive fluorescent GO-based MAB for PrPC detection by using GO as a quenching reagent. This strategy was based on the change in binding affinity of GO to MAB upon recognition of the target PrPC. As a result, the TAMRA-labelled MAB moves away from the surface of GO, and the fluorescence of MAB is recovered. Through the signal-transduction mechanism of LrRET, the background fluorescence signal is minimized and signal-to-background ratio is improved greatly. Compared with the conventional MAB, our present strategy possesses two advantages. Firstly, only one end of the MAB needs to be labelled, which is simple and cost-effective. Secondly, the background signal is significantly reduced owing to the high energy transfer efficiency between the GO and the fluorophore. Thus, the platform of GO-based MAB has great potential in biochemical studies and clinical diagnosis.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no: 21035005), National Basic Research Program of China (973 Program, no. 2011CB933600) and the Special Fund of Chongqing Key Laboratory (CSTC).Notes and references
- Z. Chen, G. Li, L. Zhang, J. F. Jiang, Z. Li, Z. H. Peng and L. Deng, Anal. Bioanal.Chem, 2008, 392, 1185–1188 CrossRef.
- J. W. Liu, Z. H. Cao and Y. Lu, Chem. Rev, 2009, 109, 1948–1998 CrossRef CAS.
- W. H. Tan, K. M. Wang and T. J. Drake, Curr. Opin. Chem. Biol., 2004, 8, 547–553 CrossRef CAS.
- K. M. Wang, Z. W. Tang, C. Y. J. Yang, Y. M. Kim, X. H. Fang, W. Li, Y. R. Wu, C. D. Medley, Z. H. Cao, J. Li, P. Colon, H. Lin and W. H. Tan, Angew. Chem., Int. Ed, 2008, 47, 2–17 CrossRef.
- N. Hamaguchi, A. Ellington and M. Stanton, Anal. Biochem, 2001, 294, 126–131 CrossRef CAS.
- M. C. Vicens, A. Sen, A. Vanderlaan, T. J. Drake and W. H. Tan, ChemBioChem, 2005, 6, 900–907 CrossRef CAS.
- S. J. Zhen, L. Q. Chen, S. J. Xiao, Y. F. Li, P. P. Hu, L. Zhan, L. Peng, E. Q. Song and C. Z. Huang, Anal. Chem., 2010, 82, 8432–8437 CrossRef CAS.
- Y. He, Z.-G. Wang, H.-W. Tang and D.-W. Pang, Biosens. Bioelectron., 2011, 29, 76–81 CrossRef CAS.
- H. F. Dong, W. C. Gao, F. Yan, H. X. Ji and H. X. Ju, Anal. Chem., 2010, 82, 5511–5517 CrossRef CAS.
- R. S. Swathi and K. L. Sebastian, J. Chem. Sci., 2009, 121, 777–787 CrossRef CAS.
- R. S. Swathi and K. L. Sebastian, J. Chem. Phys., 2008, 129, 054703 CrossRef CAS.
- C. H. Lu, C. L. Zhu, J. Li, J. J. Liu, X. Chen and H. H. Yang, Chem. Commun., 2010, 46, 3116–3118 RSC.
- H. X. Chang, L. H. Tang, Y. Wang, J. H. Jiang and J. H. Li, Anal. Chem., 2010, 82, 2341–2346 CrossRef CAS.
- Y. Wang, Z. H. Li, D. H. Hu, C. T. Lin, J. H. Li and Y. H. Lin, J. Am. Chem. Soc., 2010, 132, 9274–9279 CrossRef CAS.
- S. J. He, B. Song, D. Li, C. F. Zhu, W. P. Qi, Y. Q. Wen, L. H. Wang, S. P. Song, H. P. Fang and C. H. Fan, Adv. Funct. Mater., 2010, 20, 453–459 CrossRef.
- C.-H. Lu, C.-L. Zhu, J. Li, J.-J. Liu, X. Chen and H.-H. Yang, Chem. Commun., 2010, 46, 3116–3118 RSC.
- Y. He, Y. Lin, H. W. Tang and D. W. Pang, Nanoscale, 2012, 4, 2054–2059 RSC.
- Y. Pu, Z. Zhu, D. Han, H. X. Liu, J. Liu, J. Liao, K. J. Zhang and W. H. Tan, Analyst, 2011, 136, 4138–4140 RSC.
- Y. P. Wang, Y. H. Xiao, X. L. Ma, N. Lia and X. D. Yang, Chem. Commun., 2012, 48, 738–740 RSC.
- N. Stahl, D. Borchelt, K. Hsiao and S. Prusiner, Cell, 1987, 51, 229–240 CAS.
- M. Vey, S. Pilkuhn and H. Wille, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 14945–14949 CrossRef CAS.
- S.-L. Yu, J. Lei, M.-S. SY, F.-H. Mei, S.-L. Kang, G.-H. Sun, P. Tien, F.-S. Wang and G.-F. Xiao, Eur. J. Hum. Genet., 2004, 12, 867–870 Search PubMed.
- S. J. Xiao, P. P. Hu, Y. F. Li, C. Z. Huang, T. Huang and G. F. Xiao, Talanta, 2009, 79, 1283–1286 CrossRef CAS.
- J. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed.
- W.-C. Yang, M. J. Schmerr, R. Jackman, W. Bodemer and E. S. Yeung, Anal. Chem., 2005, 77, 4489–4494 CrossRef CAS.
- M. Varshney, P. S. Waggoner, C. P. Tan, K. L. Aubin, R. A. Montagna and H. G. Craighead, Anal. Chem., 2008, 80, 2141–2148 CrossRef CAS.
- J. Grassi, S. Maillet, S. Simon and N. Morel, Vet. Res., 2008, 39, 33–45 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ay26156a |
| This journal is © The Royal Society of Chemistry 2013 |
