The road to medical vibrational spectroscopy – a history†
Henry H. Mantsch*
NRC (National Research Council) Researcher Emeritus, Ottawa, Canada. E-mail: henry.mantsch@rogers.com
Abstract
The present Editorial chronicles the journey from classical infrared and Raman spectroscopy to medical vibrational spectroscopy, as experienced by a contemporary witness of the times. During the second half of the last century vibrational biospectroscopy became a topic of increasing global interest and has spawned a number of international conferences of which the most recent, SPEC 2012 – Shedding New Light on Disease, constitutes the basis of the present themed issue.
1 Introduction
Why should one be interested in the history of vibrational biospectroscopy, why indeed be concerned with the history of any branch of science? A fitting answer would be that since science is undertaken by and for humans, the historical perspective introduces a human element into the otherwise austere science. Journal publications, important as they may be, reveal little about the individuals who carried out the work. Oftentimes precious nuggets of information may only emerge from oral conference presentations or from chance personal encounters with the individuals involved. In my opening allocution I shall not “shed new light on disease”, the title of this conference, but I will try to shed light on how medicine has discovered infrared and Raman spectroscopy, or perhaps the other way round, whilst addressing the arrival of vibrational biospectroscopy as a new addition to the medical toolbox.I intend to recount the history of vibrational biospectroscopy from my own perspective, based on personal recollections as a witness of the times. My own journey along this path began in the 1950s with a PhD dissertation on infrared spectroscopic studies of natural products. At that time the term “biomolecule” had not yet been coined, in any case it was not commonly used. Thus I may be regarded as a dinosaur of vibrational spectroscopy, a fact further confirmed by my first “toy”, shown in Fig. 1.
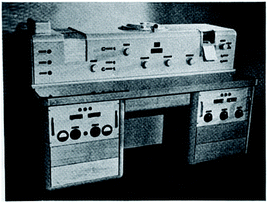 | ||
| Fig. 1 Front view of the infrared spectrophotometer UR-10 produced by Carl Zeiss Jena in the 1950s (personal photograph). | ||
This was an early dispersive double beam spectrophotometer with a water-cooled globar source and a PbS detector. The dispersive element was a monochromator in the Littrow arrangement fitted with three prisms, KBr (400–700 cm−1), NaCl (700–1800 cm−1) and LiF (1800–5000 cm−1) which upon scanning were automatically and successively rotated into and out of the beam. Percentage transmission was measured by using an optical-wedge attenuator and the spectrum was recorded with a hard pen that left a trace on the wax-coated red paper. These delicate paper spectra had to be handled carefully as they were easily scratched. The Zeiss UR-10 spectrophotometer was a contemporary with the wider known Perkin-Elmer Model 21 instrument.
2 History
I took the liberty of dividing the history of vibrational biospectroscopy into three time frames or epochs:2.1 The dark ages. This era stands for the distant past and encompasses the time period prior to World War II.
2.2 The modern era. This time period stands for the second half of the 20th century and can be further subdivided into two parts, the early past (1945–1980) and the recent past (1980–2000).
2.3 The current era. This is the contemporary time period and stands for the 21st century.
2.1 The dark ages
This time span encompasses the period from the proof of infrared radiation by Herschel in 1801 and the experimental demonstration in 1928 of what is now referred to as the Raman effect, up to and including the turbulent times of World War II. During this time a number of advancements occurred in the area of vibrational spectroscopy but in terms of biospectroscopy there was little of significance. I shall not dwell further on this stage for two reasons: firstly, I was not around to witness this early era of emerging vibrational spectroscopy and secondly, there already exist excellent historical reviews written by Andreas Barth and Parvez Haris on infrared spectroscopy1 and by Derek Long on Raman spectroscopy.22.2 The modern era
After the enmity and the devastation of World War II, in a gesture of reconciliation between former enemies Sir Harold Thompson from London, Jean Lecomte from Paris and Reinhard Mecke from Freiburg, lay the past to rest and agreed to start anew. These far-sighted individuals took on the task of bringing together spectroscopists from across Europe and beyond. Since much of the work during WW II, and specifically research in infrared spectroscopy, was carried out under secrecy and war time restrictions, the contributions of the American, British, French, German and other schools could be made public only much later. A first concrete step was to establish what became known as the European Congress on Molecular Spectroscopy, in short the EUCMOS conferences. The first meeting was organized by Ernst Miescher in 1951 on neutral Swiss territory in Basel, followed by regular biennial meetings in different European cities (the most recent EUCMOS conference was held in 2012 in Cluj-Napoca, Romania). While this conference continued under its European name, it soon became a truly international event dedicated to infrared and Raman spectroscopy. Indeed, the single most significant event in my early professional career was my good fortune to attend the 1963 EUCMOS meeting in Budapest, Hungary, precisely 50 years ago. The three wise men from Europe, Thompson, Lecomte, and Mecke, were there, as were other prominent practitioners of vibrational spectroscopy from all over the world: Herzberg, Jones, Bernstein, Lord, Miller, Lippincott, Shimanouchi, Tsuboi, Stammreich, Longuett-Higgins, Mangini, Josien, Bellanato, Hadzi, Sheppard, Orville-Thomas, Long, Clark, Hester, Connes, Zerbi and many more – the list reads like a Who’s Who in Spectroscopy. These were all pioneers in an emerging field of their time, yet it was not so much their science as it was their fascinating personalities that inspired and motivated young participants like myself to pursue a career in vibrational spectroscopy.
As is often the case, there were early dreamers, or Jules Verne type visionaries, who were first in using vibrational spectroscopy to investigate medical specimens. There are two publications which are always quoted in reviews and introductions to this topic, namely the paper by Elkan Blout and Robert Mellors on infrared spectra of tissues, published in Science in 1949,4 and the paper by Donald Woerner on infrared absorption curves for normal and neoplastic tissues, published in Cancer Research in 1952.5 I have to mention though that the introduction of the group frequency concept in the early 1950s by Lionel Bellamy,6 Werner Bruegel7 and Norman Jones8 greatly benefitted vibrational spectroscopic studies of biomolecules.
Norman Jones (born 1913–died 2001) was undeniably one such early pioneer who succeeded in turning dreams into reality. In 1937, after obtaining a PhD on the structure of vitamin D from the University of Manchester, he took up a post-doctoral position at Harvard where he developed spectroscopic techniques to monitor biochemical reactions. In 1946 he came to the National Research Council in Ottawa, an institution that was rapidly expanding after the war. As consultant to the Sloan Kettering Institute for Cancer Research in New York, he was instrumental in developing a steroid hormone metabolism program based on infrared spectroscopy. The upshot was a remarkable, though not well known, atlas of infrared absorption spectra of 760 steroids.9 I quote from the preface to volume 1, published in 1953:
| “Advance in the control of disease depends upon progress in our ability to recognize, to define, and to control basic etiologic abnormalities…as medicine has progressed it has used and expanded fundamental information from the fields of chemistry, physics, and mathematics…the work presented in this volume is in the realm of research for the acquisition of basic knowledge but with the aim of using the results in the solution of urgent medical problems”. |
When Jones left Europe he was determined to make his mark in the New World. Interestingly, his claim to fame came in an odd area of spectroscopy, in his own words he “married spectroscopy to the computer”. Throughout the 1960s, Norman Jones, working at the NRC in Ottawa and Abe Savitzky working for Perkin-Elmer in Norwalk, Connecticut, together pioneered the electronic revolution in infrared spectroscopy. They took numerical data recording from paper spectra to punched paper strips, then to magnetic tapes and later to magnetic disks. This was a first necessary step, which then allowed them to perform digital data reduction, initially on macro-computers later on dedicated mini-computers. When I joined Jones's spectrochemistry lab in Ottawa in the late 1960s, he was already the proud owner of a Perkin-Elmer 521 spectrophotometer that had provision for digital data readout on punched paper tape (see Fig. 2).
 | ||
| Fig. 2 Norman Jones turning knobs on his new toy, the Perkin-Elmer 521 spectrometer, ca. 1967 (personal photograph). | ||
Let me illustrate how the infrared spectra recorded on this spectrophotometer were digitally encoded on paper tapes. On the punched paper strip shown in Fig. 3 the first five digits represent the wavenumber and the next three digits the transmittance, followed by an end-of-word character. Thus the top eight-digit number on this strip, 14000946, indicates a transmittance of 0.946 at 1400.0 cm−1.
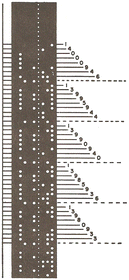 | ||
| Fig. 3 Illustration of the punched paper tape containing data encoded on the Perkin-Elmer Model 521 Spectrophotometer (from Computerized infrared spectroscopy: Beginnings and some stops by the wayside10). | ||
However, digital encoding was only the first step. Next the spectroscopic data had to be transferred to the main-frame computer, initially a venerable SDS 920, later an IBM 360. This was done by manually punching holes in IBM cards using the data points from the paper tapes. It was a slow process and prone to typing errors but we were very popular because the debris from punching holes in the colored IMB cards was in great demand for festive occasions as a substitute for confetti. Of course, data mining in those days was performed using programs written in FORTRAN. During the period between 1968 and 1986 the spectroscopy group at NRC in Ottawa produced over 50 computer programs for infrared spectroscopy, all written in FORTRAN. Shown in Fig. 4 is the cover page of Bulletin no. 18, the last of these NRC internal publications which were only produced in a limited number and thus not widely distributed. Nonetheless, many if not all programs were used by various instrument manufacturers and freely incorporated into their commercial data reduction programs (at that time NRC was not interested in commercialization).
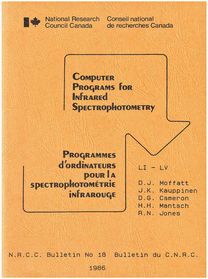 | ||
| Fig. 4 Cover page of NRC Bulletin no. 18 with the FORTRAN source codes for a number of modular computer programs, freely distributed by the Molecular Spectroscopy Group in 1986 (private copy). | ||
Progress was realized in the 1970s with the advent of teletype technology, which allowed us now to process data directly from our laboratories, reducing the time it took to communicate with the main-frame computer, located in the computation centre at the other end of town. The acquisition in 1977 of a Digilab FTS-14 Fourier transform infrared spectrometer, equipped with a NOVA Data General minicomputer (16 kbytes core memory), permitted further detachment from the computation centre and the main-frame computer. Nevertheless, space in the spectroscopy lab continued to be taken up by reels of bulky magnetic tapes and disks, used to store the raw FT-IR spectra (see Fig. 5). By learning about these historical developments in spectroscopic data processing, today's researchers should fully appreciate the dedicated personal computers now comfortably available at their own workspace.
 | ||
| Fig. 5 Snapshot of the spectroscopy lab at NRC in Ottawa, ca. 1978 (personal photograph). Note the filing cabinets in the background with racks of magnetic disks and tapes. Left to right: D. Moffatt (technician), H. Casal (post-doc), R. Jones (retired by then), S. Capes (summer student), H. Mantsch (author) and W. Surewicz (research associate). | ||
Another visionary from the early past was Harold Bernstein (born 1914–died 1984). In 1938, after obtaining a PhD from the University of Toronto, Harold moved to Copenhagen to study Raman spectroscopy at the institute of Niels Bohr. There he was picked up by the Nazis when they invaded Denmark in 1940 and spent the next five years under house arrest as a civilian prisoner of war. Bernstein resigned himself to these events rationally, without bitterness, and after establishing his own molecular spectroscopy lab at the National Research Council in Ottawa, he accepted a number of post-doctoral fellows from Germany, notably Wolfgang Kiefer who developed his rotating Raman cell during the time he spent at NRC in Ottawa (1970–1972). Bernstein grew up with the Toronto Arc as the source for Raman spectra (which was developed by Boris Stoicheff at NRC), but he turned to the laser as soon as this became available. Always alert to new developments, in the mid 1960s his lab broke new ground for the young field of resonance Raman spectroscopy. Bernstein recognized the growing role of Raman spectroscopy and organized the first International Conference on Raman Spectroscopy in Ottawa in 1969. Later on, in 1980 the 7th International Conference on Raman Spectroscopy (ICORS VII) was held again in Ottawa to honor his contributions to Raman spectroscopy. Bernstein also was co-founder of the Journal of Raman Spectroscopy and served as its co-editor until his retirement in 1978. Having established well known spectroscopy laboratories at the National Research Council in Ottawa, Harold Bernstein and Norman Jones both attracted many young post-docs and visiting scientists from all over the world. Several of these post-docs further stimulated Harold's interest for vibrational biospectroscopy, notably Paul Carey and Richard Mendelsohn, who after spending a few years in his lab, later continued to develop new biomedical applications in their own labs.
During the second half of the last century Gerhard Herzberg (born 1904–died 1999) reigned as the grandmaster of spectroscopy at the National Research Council of Canada. Herzberg transformed the NRC in Ottawa into a Mecca for molecular spectroscopists. Harry Kroto, a post-doctoral fellow from 1964 to 1966, who shared the 1996 Nobel Prize with Robert Curl, a visiting scientist during that time, explicitly stated that the stimulating atmosphere at NRC had contributed to the discovery of the buckminsterfullerenes. Many other Nobel Laureates and distinguished spectroscopists from all over the world visited Ottawa at one time or another during their career.
Every day at three o'clock Gerhard Herzberg, Norman Jones, Harold Bernstein and the rest of us gathered for afternoon tea to discuss spectroscopy as well as other issues. While some considered this to be a waste of time, it proved to be a free exchange of ideas. At one of these afternoon teas Herzberg shared with us how he first met Raman at the 1929 Faraday Society Meeting on Molecular Spectra and Structure in Bristol. Herzberg was dismayed that he could not understand the first speaker, O. W. Richardson, who apparently mumbled, but he understood every single word of the second speaker, C. V. Raman, who spoke beautifully clear English. From then on Herzberg and Raman became good friends. Once after he returned from visiting Raman in Bangalore Herzberg shared with us a tell-tale story (which he rarely did). Raman, certain that he would receive the Nobel Prize, apparently told Herzberg that he should have received it already in 1929 but that Rutherford wanted Richardson to have it first, so he (Raman) had to wait until 1930. Herzberg had many interesting stories to tell as he had worked and rubbed shoulders with many famous people like Max Born, James Frank, Edmund Teller, Niels Bohr, Werner Heisenberg, Peter Debye, Walter Heitler, Victor Weisskopf, Enrico Fermi, Max Delbrueck and many more. Although he never worked on biological molecules himself, Herzberg showed great interest and was amazed to see how far we had taken his classical spectroscopy. Later on he even sponsored our biospectroscopic work for publication in the Proceedings of the National Academy of Sciences of the USA, whereof he was a member. The great thing we all learnt from Herzberg was that spectroscopy is not only a product of the brain and the hands, but also of the heart and soul. Herzberg generously opened up his home and his cottage in the Gatineau Hills to colleagues and guests from Canada and from abroad (see Fig. 6).
 | ||
| Fig. 6 Discussing spectroscopy at Herzberg's home, ca. 1985. Left to right: Andy Cole (visiting from Perth, Down Under), Norman Jones, Gerhard Herzberg and Henry Mantsch (personal photograph). | ||
Yet another dreamer from the early past was Nils Kaiser (born 1921) who in 1958 filed a first patent for the non-invasive determination of blood glucose levels.11 At that time he was the official medical doctor for the German Max Planck Society and also a practising scientist at the Max Planck Institute for Plasma Physics in Munich. When I recently met Nils in the summer of 2012, he expressed great interest in SPEC 2012 and was wondering how far the spectroscopic determination of blood analytes had advanced by today. At age 92 he is still flying his private plane, so it appears that practising vibrational spectroscopy may be good for one's longevity. Even Herzberg proved this point, working well into his 90s and mingling happily with 12 aging Nobel Laureates at the event that we organized to celebrate his 90th birthday (see Fig. 7).
 | ||
| Fig. 7 Herzberg turns 90, a celebration of minds in Toronto, December 1994. From left to right: (front row) Herzberg, Porter, Watson, Prigogine; (back row) Perutz, Smith, Herschbach, Brockhouse, Polanyi, de Duve, Townes, Kendal (from Molecular spectroscopy with Gerhard Herzberg12). | ||
While this author was most familiar with the early vibrational spectroscopy scene in Europe and Canada (primarily at the NRC in Ottawa), I also met other individuals and witnessed exciting developments in the US, Japan and in other parts of the world. A prominent figure from that time in the US was Richard (Dick) Lord (born 1910–died 1989) who was director of the George Harrison Spectroscopy Lab at MIT after the war. There he became highly interested in biomolecules, spurred on by PhD students like Rich Mendelsohn, George Thomas and others. In the period between the advent of laser Raman spectroscopy in the early 1960s and the introduction of Fourier Transform infrared spectroscopy in the late 1970s, the former was the preferred vibrational spectroscopic technique for the study of biological molecules. Consequently the studies of the laser Raman spectra of nucleic acids and proteins carried out in Lord's laboratory opened up novel fields of research in vibrational biospectroscopy. Lord's seminal papers, on DNA hydration with Mike Falk (later at NRC in Halifax) or with George Thomas on the first infrared spectra of viruses, are still quoted to this day. As educator Lord also recognized the need to capture the interest of young spectroscopists and established a first post-graduate training course in infrared spectroscopy, held initially at MIT and later at Bowdoin College in Maine.
Once the dust of WW II had settled, Japan re-joined the international spectroscopic community. A prominent representative from Japan during that time period was Hiko Shimanouchi (born 1915–died 1980). Wherever he showed up, Shimanouchi always stood out, certainly not due to his height as can be seen from the photograph in Fig. 8, but thanks to his gregarious character (he could drink everybody under the table). While Shimanouchi himself was less interested in biospectroscopy, several of his students, noticeably Mitsuo Tasumi and Hiro-o Hamaguchi, became prominent Japanese envoys for vibrational biospectroscopy.
 | ||
| Fig. 8 Group photograph taken at the 1967 EUCMOS Congress in Madrid, September, 1967 (personal conference photograph). Hiko Shimanouchi is 5th from the left in the front row; also in the front row is Norman Jones, 7th from the right, next to Magda his wife (they always travelled together). | ||
With more and more vibrational spectroscopists turning their attention to the world of biomolecules the need became apparent to create a venue, a forum where the practitioners of this art could meet, discuss their results, share their experience and also vent their frustration. A first opportunity presented itself on the occasion of the NATO Advanced Study Institute on the Spectroscopy of Biological Molecules, a meeting organized in 1983 by two naturalized Canadians, Theo Theophanides (born in Greece) and Camille Sandorfy (born in Hungary), both from the University of Montreal. Held in Acquafredda di Maratea, in the relaxed atmosphere of Southern Italy, the desire was expressed to have follow-up meetings on this topic. As NATO did not have unlimited funds for such specialized meetings, the prominent rector of the University of Reims who was in the audience, offered to host the next meeting at his university. This was the naissance of the 1st European Conference on the Spectroscopy of Biological Molecules (ECSBM), held two years later in 1985 in Reims, France. Since this successful first meeting, ECSBM conferences were held every other year: 1987 in Freiburg/Germany, 1989 in Rimini/Italy, 1991 in York/UK, 1993 in Loutraki/Greece, 1995 in Lille/France, 1997 in Escorial/Spain, 1999 in Enschede/Netherlands, 2001 in Prague/Czech Republic, 2003 in Szeged/Hungary, 2005 in Aschaffenburg/Germany, 2007 in Paris/France, 2009 in Palermo/Italy and last 2011 in Coimbra/Portugal. The published proceedings of these meetings bear witness to the progress made over the last 25 years in vibrational biospectroscopy. Throughout the years a certain camaraderie and also a friendly competition developed amongst the individuals who usually attended these meetings. A number of the present day vibrational biospectroscopists should be able to recognize themselves in the photograph in Fig. 9.
 | ||
| Fig. 9 Group picture of the invited speakers from the 1991 ECSBM meeting in York (personal photograph). | ||
It is historically well documented that rapid scientific progress often ensues from novel instrumental developments, as was the case following the introduction of vibrational micro-spectroscopy. The coupling of both infrared and Raman spectrometers to microscopes led to a surge in vibrational biospectroscopic papers, especially in the field of cytology and pathology. Assisted by the laser optics and the CCD detectors used in Raman spectroscopy, the development of Raman micro-spectroscopy antedated that of infrared micro-spectroscopy. The Raman microscope MOLE, developed by Michel Delhaye and Paul Dhamelincourt at the Technical University in Lille, was first announced at the 1974 Raman conference at Bowdoin College, Maine. FT-IR microscopes were developed much later and only became readily available in the 1990s. Moreover, in their early days, the FT-IR microscopes used for spectral imaging had to be content with focal arrays obtained from US military rejects with defect pixels.
Obviously there were other spectroscopy meetings where only special sessions and a limited number of presentations were dedicated to vibrational biospectroscopy. These included the International Conferences on Raman Spectroscopy, ICORS, which started 1969 in Ottawa, Canada and the International Conferences on FT-IR Spectroscopy, ICOFTS, which started 1977 in Columbus, Ohio and after 2001 turned into the International Conference on Advanced Vibrational Spectroscopy, ICAVS. It was at one of these conferences, the 1985 ICOFTS meeting in Ottawa, where Dieter Naumann from the Robert Koch Institute in Berlin first reported on FT-IR applications in microbiology.13 Initially this was thought to be a rather esoteric field but it has since led to a burgeoning interest with its own meetings dedicated exclusively to the vibrational spectroscopy of micro-organisms.
During the roaring 1990s, vibrational biospectroscopy gathered momentum drastically. The idea of using infrared or Raman spectroscopy to explore the properties of living organisms, and specifically humans, was as outlandish as it was compelling. The attraction, however, proved irresistible to many and led to a host of new biomedical applications. Not surprisingly along the way it also raised a few problems when it was realized that the typical cancer surgeon's ignorance of vibrational spectroscopy was probably only matched by the typical physicist's ignorance of tumour biology. This meant that for sound biomedical experiments the spectroscopists had to work closely with their medical counterparts and so complement each other. Clearly, vibrational spectroscopic publications are more trustworthy if the list of authors includes medical collaborators and vice versa for papers published in medical journals.
Throughout their existence, infrared and Raman spectroscopy competed for the attention of scientists interested in molecular structures. However, biomedical spectroscopy has united the two rivalling techniques and nowadays most biospectroscopists use both modalities. Biomedical vibrational spectroscopic studies fall in one of two categories: (i) ex vivo applications, i.e. the analysis or imaging of extracted biofluids or excised tissues and (ii) in vivo applications, i.e. the in situ analysis or imaging of body parts, affording a live window into metabolism. The major impact areas for medical practitioners can be summarized under:
“Vibrational clinical chemistry”, the reagent-free spectral analysis of infrared or Raman spectra. These spectra contain the “fingerprints” of all analytes present, and after applying appropriate data mining routines the analytes can be determined both qualitatively and quantitatively.
“Vibrational pathology”, centered around spectral cytopathology and spectral histopathology. This makes up the bulk of all biomedical applications today. The “molecular dance”, an expression often used for the motions studied by vibrational spectroscopy, of diseased tissue is slightly different from that of healthy tissue, making vibrational spectroscopy a tool well suited for staging the progression of disease, as sketched-out in the cartoon of Fig. 10.
 | ||
| Fig. 10 Cartoon depicting the progression of disease as a stepwise transformation from benign (angelic) to malignant (diabolic). | ||
As summarized in a recent review by Max Diem et al.14 good progress has been achieved when using spectral cyto/histo-pathology as opposed to classical cyto/histo-pathological stains. And yes, caution needs to be exercised when announcing “early and certain detection of cancer” as such claims raise expectations prematurely and may harm the cause of vibrational biospectroscopy.
“Vibrational radiology”, based on novel biomedical imaging modalities. Here the source of radiation is not X-rays or microwaves but comes from the vibrational energy in the electromagnetic spectrum. These spectral images are customarily expressed as false color images (a picture is worth a thousand words) and are becoming increasingly popular in biomedical publications.
“Biomedical uses of vibrational spectroscopy beyond direct disease control”. This is a fast growing area which encompasses a wide and diverse range of biomedical applications such as monitoring wound healing by tracking surgical margins or tissue viability. Other applications include the monitoring of skin hydration, oxidative stress, effect of ionizing radiation, interactions with drugs, the study of bone or kidney stone composition and more.
2.3 The current era
The contemporary history of vibrational biospectroscopy parallels that of the 21st century, both are still quite young. By the turn of the last century a large number of biospectroscopists had graduated from the study of biomolecules and were applying their infrared and Raman spectroscopic tools to the study of human cells and tissues. As this topic became of increasing global interest we decided to organize an international meeting in 2000 in Winnipeg, dedicated exclusively to this theme, for which we chose the catchy title Shedding New Light on Disease: Optical Diagnostics for the New Millennium. The success of this first conference, also known under the abbreviated name of SPEC 2000, led to a series of follow-up meetings, SPEC 2002 in Reims/France, SPEC 2004 in Newark/USA, SPEC 2006 in Heidelberg/Germany, SPEC 2008 in São José/Brazil, SPEC 2010 in Manchester/UK and of course the SPEC 2012 conference in Chiang Mai/Thailand. The proceedings from SPEC 2002 and SPEC 2004 were published in special issues of the journal Vibrational Spectroscopy, those from SPEC 2006 in a special issue of Analytical and Bioanalytical Chemistry and those from SPEC 2008 and SPEC 2010 appeared as themed issues in Analyst.It is undeniable that during the first 12 years of this century many of the present-day vibrational spectroscopists have made significant contributions to the field of biomedical vibrational spectroscopy which collectively have advanced the field more so than everything achieved throughout its entire previous history. It is therefore not surprising that even at meetings such as this one “history is being made”. The latest developments are amply documented in a number of recent reviews written by experts in the specific area.15–21 Still, if I had to identify a single most important development in biomedical vibrational spectroscopy during this contemporary era, it would be the emergence of bioinformatics as an equal partner to the disciplines of vibrational spectroscopy and medicine. Today biomedical vibrational spectroscopy relies heavily on mathematical methods for data mining, so following the electronic revolution of the last century we need a new software revolution to aid spectroscopists scrambling to decode the mountains of experimental data generated by biomedical vibrational spectroscopy and help translate these findings into clinically relevant information, i.e. data (records) that can be used by the medical practitioners.
By joining the diplomatic service shortly after the SPEC 2002 meeting in Reims, sadly for me, I had to step back from witnessing first hand the latest developments in biomedical vibrational spectroscopy. Hence I am hesitant to comment here on the most recent advancements and so, for the following SPEC meeting I would like to call on someone currently active in the field to appraise the progress made with the hindsight of history, and review the latest developments in this exciting multidisciplinary area.
3 Epilogue
Although historians are by nature looking backwards, into the past, I cannot end this history without a look into the crystal ball. What I would love to see in the future is biomedical vibrational spectroscopy creating its own niche, for example in functional imaging, catching up on its rival, functional magnetic resonance imaging. Considering the difference in the respective time scales, functional vibrational imaging (10−12 s) should detect much faster changes than functional magnetic resonance imaging (10−6 s). But then perhaps I am only dreaming!In an Editorial such as this one it is difficult to acknowledge the names of all the spectroscopists who have made important contributions to our subject. Therefore I must apologize to the vibrational spectroscopic community at large and to those present at the SPEC 2012 meeting for my omissions. I had to be selective rather than exhaustive and no doubt I may have given undue weight to my own involvement and my personal experience with the history of vibrational biospectroscopy. Last, but not least, we should not forget the many unnamed graduate and post-graduate students who, either directly or indirectly, have made important contributions to the history of vibrational biospectroscopy.
References
- A. Barth and P. Haris, Infrared Spectroscopy – Past and Present, in Biological and Biomedical Infrared Spectroscopy, ed. P. I. Haris and A. Barth, IOS Press, Amsterdam, 2009 Search PubMed.
- D. A. Long, 80th Anniversary of the Discovery of the Raman Effect: A Celebration, J. Raman Spectrosc., 2008, 39, 316–321 CrossRef CAS.
- G. H. Herzberg, Infrared and Raman Spectra of Polyatomic Molecules, Van Nostrand Reinhold Company Inc., New York, 1st edn, 1945 Search PubMed.
- E. R. Blout and R. C. Mellors, Science, 1949, 110, 137–138 CAS.
- D. I. Woernley, Cancer Res., 1952, 12, 516–523 CAS.
- L. J. Bellamy, The Infrared Spectra of Complex Molecules, Methuen and Co. Ltd., London, 1954 Search PubMed.
- W. Bruegel, Einfuehrung in die Ultrarotspektroskopie, German edn, Steinkopff, Darmstadt, 1954 Search PubMed.
- R. N. Jones and C. Sandorfy, The Application of IR and Raman Spectroscopy to the Elucidation of Molecular Structure, in Chemical Applications of Spectroscopy, ed. W. West, Interscience Publishers, New York, 1956, pp. 247–581 Search PubMed.
- G. Roberts, B. S. Gallagher and R. N. Jones, Infrared Absorption Spectra of Steroids: An Atlas, Interscience Publ. Inc., New York, 1953, vol. 1 Search PubMed.
- R. N. Jones, H. H. Mantsch and D. J. Moffatt, J. Mol. Struct., 1989, 200, 289–299 CrossRef.
- N. Kaiser, Ger. Pat., 1958, DBP no. K 36308 IX/42 1.
- H. H. Mantsch, J. Mol. Struct., 2007, 834–836, 2–6 CrossRef CAS.
- D. Naumann, The ultra rapid differentiation and identification of pathogenic bacteria using FT-IR techniques, in Proc. SPIE, ed. J. G. Grasselli and D. G. Cameron, 1985, vol. 553, pp. 268–269 Search PubMed.
- M. Diem, M. Miljković, B. Bird, T. Chernenko, J. Schubert, E. Marcsisin, A. Mazur, E. Kingston, E. Zuser, K. Papamarkakis and N. Laver, J. Spectrosc., 2012, 27, 463–496 CrossRef CAS.
- Infrared and Raman Spectroscopy of Biological Materials, ed. H. U. Gremlich and B. Yan, Marcel Dekker, New York, 2001 Search PubMed.
- Biomedical Vibrational Spectroscopy, in Analytical and Bioanalytical Chemistry, ed. D. Naumann, W. Petrich and W. Schmitt, 2007, vol. 387, pp. 1589–1831 Search PubMed.
- Biomedical Vibrational Spectroscopy, ed. P. Lasch and J. Kneipp, John Wiley & Sons, Hoboken, New Jersey, 2008 Search PubMed.
- Vibrational Spectroscopy for Medical Diagnosis, ed. M. Diem, P. R. Griffiths and J. M. Chalmers, John Wiley & Sons, Chichester, 2008 Search PubMed.
- Vibrational Spectroscopy for Medical Diagnosis, ed. M. Diem, P. R. Griffiths and J. M. Chalmers, John Wiley & Sons, Chichester, 2008 Search PubMed.
- Infrared and Raman Spectroscopic Imaging, ed. R. Salzer and H. W. Siesler, Wiley, 2009 Search PubMed.
- Biomedical Applications of Synchrotron Infrared Microspectroscopy, ed. D. Moss, RSC Publishing, Cambridge, 2011 Search PubMed.
Footnote |
| † Based upon the Inaugural Plenary Lecture “History of Vibrational Biospectroscopy” given at the Biennial International Conference on Shedding New Light on Disease – SPEC 2012, in Chiang Mai, Thailand, on November 12th 2012. |
| This journal is © The Royal Society of Chemistry 2013 |
