Quantification by SIFT-MS of acetaldehyde released by lung cells in a 3D model
Abigail V.
Rutter
a,
Thomas W. E.
Chippendale
a,
Ying
Yang
a,
Patrik
Španěl
ab,
David
Smith
a and
Josep
Sulé-Suso
*ac
aInstitute for Science and Technology in Medicine, School of Medicine, Keele University, Thornburrow Drive, Hartshill, Stoke-on-Trent, ST4 7QB, UK
bJ. Heyrovský Institute of Physical Chemistry, Academy of Sciences of the Czech Republic, Dolejškova 3, 182 23 Prague 8, Czech Republic
cCancer Centre, University Hospital of North Staffordshire, Newcastle Rd, Stoke-on-Trent, ST4 6QG, UK
First published on 23rd October 2012
Abstract
Our previous studies have shown that both lung cancer cells and non-malignant lung cells release acetaldehyde in vitro. However, data from other laboratories have produced conflicting results. Furthermore, all these studies have been carried out in 2D models which are less physiological cell growth systems when compared to 3D models. Therefore, we have carried out further work on the release of acetaldehyde by lung cells in 3D collagen hydrogels. Lung cancer cells CALU-1 and non-malignant lung cells NL20 were seeded in these hydrogels at different cell concentrations and the release of acetaldehyde was measured with the Selected Ion Flow Tube Mass Spectrometry (SIFT-MS) technique. The data obtained showed that the amount of acetaldehyde released by both cell types grown in a 3D model is higher when compared to that of the same cells grown in 2D models. More importantly, acetaldehyde from the headspace of lung cancer cells could be measured even at a low cell concentration (105 cells per hydrogel). The differential of acetaldehyde release could be, depending on the cell concentration, more than 3 fold higher for cancer cells when compared to non-malignant lung cells. This pilot study is the first to study acetaldehyde emission from albeit only two cell types cultured in 3D scaffolds. Clearly, from such limited data the behaviour of other cell types and of tumour cells in vivo cannot be predicted with confidence. Nevertheless, this work represents another step in the search for volatile biomarkers of tumour cells, the ultimate goal of which is to exploit volatile compounds in exhaled breath and other biological fluids as biomarkers of tumours in vivo.
1. Introduction
Lung cancer is a worldwide medical problem associated with poor prognosis. The survival rates are around 5–15% at 5 years and have not changed markedly for the last few years in spite of the development of new drugs.1 Furthermore, screening for lung cancer using sputum cytology and imaging (Chest X Rays, CT scan) can, in some cases, increase survival due to earlier diagnosis, but it is not clear yet whether screening translates into decreased lung cancer death rates.2 On the other hand, assessment of tumour response to treatment still relies heavily on imaging techniques such as CT scan. It is therefore obvious that a cheaper, on-line, radiation-free and non-invasive system is desirable in the management of lung cancer.An area of research that has attracted much attention over recent years is breath analysis. It has been hypothesized that lung tumours might release volatile substances that could be detected in the exhaled breath of patients with this disease. If such biomarkers could be identified in breath, then, in principle, they could be exploited both as a diagnostic tool and to assess tumour response to treatment. Reviews of the progress in breath analysis research up to 2005 are given in the book “Breath Analysis for Clinical Diagnosis and Therapeutic Monitoring”3 in which several techniques used to analyse exhaled breath are described, including gas chromatography mass spectrometry (GC-MS), proton transfer reaction mass spectrometry (PTR-MS) and selected ion flow tube mass spectrometry (SIFT-MS), the latter of which has been developed in our laboratory and which is the analytical tool chosen for the studies described in this paper.
The SIFT-MS technique and its applications in various fields of research have been reviewed at length and the reader is referred to several review articles on the subject.4–6 The basic features of SIFT-MS as an analytical tool are very briefly outlined in the Materials and methods section below. It should suffice to state and to emphasize at this point that accurate analyses of humid air samples for several compounds simultaneously can be achieved in real time by SIFT-MS down to concentrations of parts-per-billion by volume (ppbv), obviating sample preparation and collection that can compromise the sample. Thus, single breath exhalations, even donated by frail patients and patients with lung cancer, can be analysed for trace metabolites; the potential implications of such non-invasive analyses in medicine have been reviewed elsewhere.7
In a similar way, the humid headspace developed above in vitro cell and bacterial cultures can be analysed, and the rapid time response can be exploited to follow the temporal development of volatile compounds in the cultures.8 Our first entry to this field involved a study using SIFT-MS of the volatile metabolites released from cultures of lung cancer cell lines CALU-1 and SKMES, and the appearance of acetaldehyde (AA) at headspace concentrations that were closely related to the number of cells in the culture medium.9 These results raised great expectations that AA might be a possible breath biomarker of lung cancer. However, studies in another laboratory (see below)10 led to conflicting results, so these SIFT-MS studies on CALU-1 cells have been repeated and extended to include NL20 non-malignant lung epithelial cells and 35FL121 Tel+ telomerase positive lung fibroblast cells.11 In these studies, it was seen that AA was produced by the CALU-1 and NL20 cells, but absorbed from the medium by the 35FL121 Tel+ cells. This work has demanded a revisit of AA production and loss by cells in vitro, made more important by the observations of Filipiak et al.10 using GC-MS who observed a decrease of AA in the medium by CALU-1 cells. It is obvious that further work is required to better understand the release of volatiles by lung cells in vitro and how different experimental arrangements (cell growth and culture conditions) can influence the behaviour of these cells.
A common denominator in all of the studies of lung cancer cells growing in vitro is the fact that these cells are grown in 2D models, that is, on surfaces such as plastic or glass. This is far from the 3D situation of physiological tumour cell growth in vivo. In fact, it is not yet clear whether the release of volatile compounds such as AA could be modified by growing lung cells in a 3D situation. Thus, in the work described in this paper, we have studied by SIFT-MS the release of AA by both lung cancer and lung epithelial cells cultured in 3D scaffolds composed of collagen type I hydrogels, as a better approximation to the physiological situation of cells growing in vivo.
2. Materials and methods
2.1. Selected ion flow tube mass spectrometry, SIFT-MS
The measurements of volatile biomarkers in the headspace of the cell cultures (described below) were carried out using a Profile 3 SIFT-MS instrument (Trans Spectra Limited, UK). The principle of the SIFT-MS technique is well described in previous papers.4–6 In short, mixtures of reagent ions are produced in an electric discharge ion source (usually a microwave discharge) and from this mixture a current of reagent ions of a given mass-to-charge ratio, m/z, is obtained using a quadrupole mass filter. The reagent ions (H3O+ or NO+ ions) are injected into a fast-flowing inert carrier gas (helium at a pressure of ∼100 Pa and a flow rate of typically 400 ml min−1 at standard temperature and pressure) through a Venturi-type inlet (diameter typically 1 mm).12,13 Thus, a swarm of thermalised reagent ions is produced and convected along a flow tube at a velocity of about 100 m s−1. A sample of humid air (e.g. cell culture headspace) to be analysed is mixed with the ion swarm via a sample inlet port coupled to a heated sampling line, obviating any sample preparation as required with most other analytical techniques. The reagent ions react with the molecules of the sample in the flow tube for a well-defined reaction time. Bimolecular (binary) and termolecular (ternary) reactions occur that lead to conversion of a small fraction of the reagent ions to the product ions that are characteristic of the analytes. The remaining (large fraction) of the reagent ions together with the product ions are sampled from the flowing swarm via a pinhole orifice (∼0.3 mm diameter) located at the downstream end of the flow tube into a differentially pumped quadrupole mass spectrometer. After mass analysis they are detected and counted by an electron multiplier/pulse counting system and an on-board computer immediately calculates the concentrations of the trace gases (metabolites) in the sample with the aid of a kinetics library compiled from numerous studies of ion–molecule reactions.4,5Acetaldehyde is the primary biomarker analyte involved in this study. Its analysis by SIFT-MS has been discussed and explained in detail in previous papers.14,15 In particular, its analysis in the presence of carbon dioxide, which is inevitably present above growing cells, has received much attention and can be achieved with confidence.14 Thus, H3O+ precursor ions were primarily used for the analysis of AA resulting in product ions at m/z 45 and m/z 81, providing absolute concentration in real time above the cell cultures, whilst avoiding the overlap of ions at m/z 63 also due to the presence of CO2.
2.2. Cell lines, cell growth and cultures
A non-small cell lung cancer cell line CALU-1 (European Collection of Cell Cultures, Salisbury, UK) and a non-malignant lung epithelial cell line NL20 (ATTCC, UK) were used in this study. The CALU-1 cell line was cultured in Dulbecco's Modified Eagle Medium (DMEM) with added 10% Foetal Calf Serum (FCS) and glucose (4.5 g L−1). The NL20 cell line was cultured in Ham's F12 medium supplemented with FCS (4%), antibiotics, glucose (2.7 g L−1) and growth factors according to the provider's instructions. The cells were cultured in tissue culture flasks (Sarstedt, UK) at 37 °C in an atmosphere containing 5% CO2. The culture medium was changed every 3–4 days during the culture period.Prior to performing the SIFT-MS measurements, the cells were detached from the culture flasks using trypsin/EDTA (CALU-1) and dissociation media (NL20) according to the provider's instructions before reaching confluence. Cell suspensions were then spun at 1200 rpm for 5 minutes. The cells were counted and appropriate number of cells were resuspended in rat tail collagen type I (BD Life Sciences, UK) at a concentration of 1.5 mg ml−1 as previously described.16 Thus, 20 ml of neutralised collagen solution containing varying cell number concentrations was transferred to 150 ml glass bottles sealed with lids and septa. The scaffolds were allowed to solidify by incubating them at 37 °C for 2 hours. Following this incubation period, 20 ml of freshly prepared medium (as indicated above) was added to the scaffold surface. For each experiment, a control scaffold containing no cells was also formulated. Following the addition of the media, the cells were incubated at 37 °C for 24 hours with the lid of each bottle left slightly open to allow the air to exchange with the incubator environment (5% CO2 in air), thereby ensuring that the cells remained suitably oxygenated during this initial attachment period. Following this stage, the space in each bottle was purged with dry cylinder air and the lids were closed tightly. The cells were then incubated for a further 16 hours at 37 °C in order to allow the headspace to develop and equilibrate, as previously described.11
2.3. SIFT-MS headspace analyses
SIFT-MS analysis was carried out as outlined above and as previously described in detail.11 Briefly, bottles with both cells and hydrogel scaffold, and control scaffolds containing no cells were transferred to a temperature controlled water bath at 37 °C. The hypodermic needle connected to the SIFT-MS sample inlet line punctured the septum that sealed the bottles, allowing the cell/scaffold headspace to flow directly into the SIFT-MS carrier gas. The headspace AA concentration was then measured using SIFT-MS in the multiple ion monitoring mode, which has been well described in the literature.4–6 Again, the important point to note is that no headspace sample manipulation or pre-concentration is required and the AA concentrations are immediately available in real time. The headspace concentration can be measured repeatedly after venting each time with dry air and allowing time for the liquid phase/vapour phase equilibrium to be re-established. Absolute accuracy of the concentration measurements is better than 15% (ref. 17) and reproducibility (precision) is calculated for each measurement from the ion counting statistics and from the variation of the real time data18 and was better than ±5% for all analyses.Concomitant with the AA measurements, the concentrations of methanol, ethanol and acetone in the headspace samples were measured simultaneously. We have no evidence from previous experiments that these compounds are produced by CALU-1 and NL20 cells under the incubation conditions pertaining to these studies. Yet these three compounds are always detected and are at readily measurable concentrations and we have traced their origin to the FCS that is used to supplement the DMEM and Ham's F12 medium. Thus, we have used these compounds as indicators of the consistency of the media used throughout these studies and to check if the hydrogel scaffold material was introducing more of these compounds. In fact, no further addition was seen and the headspace concentration of these compounds was remarkably steady throughout the overall measurements.
It is also important to note that AA was present at a concentration of about 100 ppbv in the headspace of the medium and scaffolds alone, as was the case when no scaffolds were present; this is referred to again below.
3. Results
3.1. Acetaldehyde release
Firstly, it is important to summarise the results we obtained in previous measurements of AA released from CALU-1 cells cultured under very similar conditions to those pertaining to the present studies, but growing on glass bottle surfaces in DMEM with 10% FCS and in the absence of hydrogel scaffolds, that is, in the 2D situation.9,11 Thus, our earliest studies showed that the headspace AA concentration was directly proportional to the number of cells in the medium, the rate of AA production being about 106 molecules per minute of incubation time per cell.9,11 For 40 × 106 cells in 50 ml of the liquid medium, a partial pressure of AA of about 300 ppbv (greater than that in the headspace of the medium alone) resulted in a 150 ml of air/vapour headspace above the medium/cells. In a more recent study also involving CALU-1 cells, and again in the 2D situation, very similar results have been obtained with the headspace AA concentration due to 50 × 106 cells alone being typically 400 ppbv, and higher at about 500 ppbv for 80 × 106 cells, whilst the AA concentration above the DMEM alone was about 250 ppbv.11 Additionally, in these more recent 2D studies, the AA concentration above 50 × 106 NL20 cells alone has been measured to be about 300 ppbv, again increasing with the cell number in the medium while in the headspace above the Ham's F12 medium alone the AA concentration was typically 100 ppbv.11It is most important to note that in the 2D studies described above, the AA production continuously reduced at low cell numbers towards the medium alone headspace AA value. However, the situation in the presence of the scaffolds, the 3D situation, is dramatically different, as can be seen in the data given in Table 1. It was natural for us to begin with a cell number in the medium–scaffold composite similar to those used in our previous experiments, viz. 20 to 40 × 106. It was immediately obvious that AA production by CALU-1 cells was greater in this 3D situation than in the 2D situation actually by about a factor of 2 at these cell numbers. This prompted us to carry out measurements at much lower cell numbers and the results obtained are startling, as can be seen by inspecting the data in Table 1 and the representation of these data shown in Fig. 1. At cell numbers of only 106, the mean of 5 independent AA headspace concentrations (different bottles containing ostensibly the same medium volume, scaffold mass and cell numbers) is about twice that for a cell number of 40 × 106. Even at 105 cells the AA concentration is still high, but now the mean value of 3 separate measurements indicates a definite reduction below the 106 cell number situation. Furthermore, AA production is still apparent at cell numbers as low as 5 × 104. Thus, AA production apparently maximises in this situation at about 106 cells. The ratios of maximal to minimal concentrations obtained from experiments with the identical seeded cell numbers are in the range from a factor of 1.15 to a factor of 2.16. These variations are much greater than analytical uncertainty of the SIFT-MS quantifications (<5%).
| Cell type | Number of cells seeded (×106) | AA concentrations (ppbv) | Mean (ppbv) |
|---|---|---|---|
| CALU-1 | 0 | 81, 99, 103, 114, 135 | 106 |
| 0.01 | 113, 196, 218 | 176 | |
| 0.05 | 307, 310, 617 | 411 | |
| 0.1 | 1122, 1242, 1547 | 1304 | |
| 1 | 1862, 1909, 1946, 1996, 2154 | 1973 | |
| 5 | 1042, 1089, 1151, 1568 | 1213 | |
| 10 | 1060, 1157, 1170, 1411, 1842 | 1328 | |
| 20 | 550, 855, 869, 869, 1187 | 866 | |
| 40 | 687, 787, 931, 974, 1174 | 911 | |
| NL20 | 0 | 98, 113, 130, 133, 136 | 122 |
| 10 | 383, 385, 394, 402, 432 | 399 | |
| 20 | 456, 530, 595, 643, 755 | 596 | |
| 40 | 505, 698, 745, 830, 1995 | 955 |
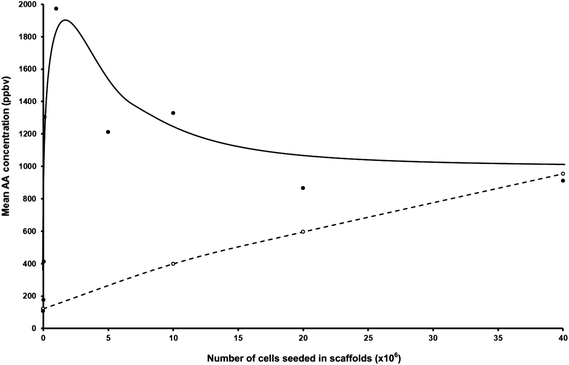 | ||
| Fig. 1 Plot of the mean acetaldehyde concentrations in parts-per-billion by volume, ppbv, taken from Table 1, in the headspace of CALU-1 lung cancer cell cultures (filled circles) and NL20 non-malignant lung cell cultures (open circles) seeded at different cell concentrations. | ||
The situation for the NL20 cells in the 3D situation conforms more to the data that were obtained in the previous 2D study11 in that AA production increased with increasing cell numbers. However, the efficiency of production for 40 × 106 cells is increased by about a factor of 2 in this 3D situation when compared to the 2D situation. Furthermore, AA production is still very evident even for only 106 NL20 cells, as can be seen in Table 1.
4. Discussion
Several questions arise from the results of these studies, the answers to which are still subject to debate; here we can only provide some comments and ideas.(i) How is AA produced and is it related to cell proliferation and/or cell death?
It is generally considered that AA is formed via ethanol metabolism through alcohol dehydrogenase (ADH), which is present in the cells. On the other hand, AA is lost via the enzyme aldehyde dehydrogenase (ALDH) resulting in acetate. It has also been reported that saturated hydrocarbons (e.g. ethane, pentane) and aldehydes are formed during lipid peroxidation of fatty acid components of cell membranes.19 In the present experiments, ethanol is present in the headspace of the culture medium at a concentration of about 2000 ppbv, which equates to a liquid phase concentration of 170 μmol L−1; its origin is largely the FCS that is routinely added to the media.11 AA is also seen in the DMEM headspace alone at a level of about 100 ppbv (0.7 μmol L−1 in the liquid).11
(ii) Why is AA production more efficient in the 3D model compared to a 2D one, and is the 3D situation a realistic approximation to the in vivo situation?
It has been shown that 3D systems mimic more readily the in vivo setting than does the traditional 2D cell culture, and offers distinct advantages over the in vivo setting for the study of some organ systems.20 We have previously shown that CALU-1 cells can not only survive in collagen hydrogels but, more importantly, they proliferate and invade the hydrogel16 although it is not clear whether the cells proliferated significantly in the present 3D cultures. It is not possible at this stage to identify the factors involved in the decrease of AA production with increasing numbers of CALU-1 cells in culture above 106 cells. However, it could be hypothesised that increasing numbers of seeded cells might decrease cell proliferation and/or slow down metabolic processes that might be involved in acetaldehyde release due to consumption of nutrients. Further work is required to better understand whether the decrease of AA by CALU-1 cells seeded at 40 × 106 cells per hydrogel scaffold when compared to 106 cells per hydrogen scaffold is due to cell death, changes in their metabolism and/or proliferative activity.
(iii) Why should there be a wide variation in AA production for a given cell number?
Our previous work showed that there was a small variation in the release of AA by the same number of seeded cells.9,11 In the present experiments, cells were allowed to grow for a further 24 hours. This extra growth period might have amplified these initial differences.
(iv) What is the relevance of this work to cancer research?
Over the last few years, there has been increasing interest in AA not only for its involvement in cancer development but also as a biomarker for cancer diagnosis. AA has both mutagenic and carcinogenic effects; it binds to DNA forming stable adducts interfering with DNA synthesis.21 It also binds to proteins causing structural and functional alterations22 of enzymes involved in DNA repair.23 All these might result in tumour development. On the other hand, the total alcohol dehydrogenase activity has been found to be significantly higher in some cancer tissues compared to healthy organs suggesting that some cancer cells have a greater capability for ethanol oxidation. It would seem as well that some tumour cells have less ability to remove AA than normal tissues, although this is not a general finding.21 However, the present data show that the release of AA by lung cancer cells is higher when compared to normal lung cells. Further work is required to assess whether such a differential can also be seen in the breath of patients with lung cancer and control cases without lung cancer.
5. Concluding remarks
There are two very important points to highlight on the basis of the data presented here. Firstly, from the experimental point of view, the release of AA by lung cells varies according to the growth conditions. Our data strengthen the case for the study of volatile compounds released by lung cells under the more physiological 3D situation rather than the commonly used 2D situation. Secondly, the differential of AA release could be, depending on the cell concentration, more than 3 fold higher for cancer cells when compared to non-malignant lung cells. This differential in AA production between malignant and non-malignant lung epithelial cell lines is very marked and interesting, but clearly these results cannot be extrapolated to other cell lines. However, the results of the present study provide further stimulus to more studies involving a wider variety of malignant and non-malignant cell lines, work that is in train in our laboratory. An even greater extrapolation would be to assume that the behaviour of in vitro cell cultures, even 3D cultures, simply reflects the behaviour of cells in vivo. Nevertheless, the hope is that new insights into the differential emissions of volatile compounds by normal and tumour cells might ultimately reveal volatile biomarkers for the detection of tumours in the body, via, for example, exhaled breath analysis, and thus assist in the treatment and management of cancer.Acknowledgements
This work was partially supported by The North Staffordshire Respiratory Research Foundation (Breath of Life).References
- I. Gkiozos, A. Charpidou and K. Syrigos, Developments in the treatment of non-small cell lung cancer, Anticancer Res., 2007, 27, 2823 CAS.
- P. B. Bach, J. N. Mirkin, T. K. Oliver, C. G. Azzoli, D. A. Berry, O. W. Brawley, T. Byers, G. A. Colditz, M. K. Gould, J. R. Jett, A. L. Sabichi, R. Smith-Bindman, D. E. Wood, A. Qaseem and F. C. Detterbeck, Benefits and harms of CT screening for lung cancer: a systematic review, JAMA, J. Am. Med. Assoc., 2012, 307, 2418 CAS.
- Breath Analysis for Clinical Daignosis and Therapeutic Monitoring, ed. A. Amann and D. Smith, World Scientific Publishing Co. Pte. Ltd, Singapore, 2005 Search PubMed.
- D. Smith and P. Španěl, Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis, Mass Spectrom. Rev., 2005, 24, 661 CrossRef CAS.
- D. Smith and P. Španěl, Ambient analysis of trace compounds in gaseous media by SIFT-MS, Analyst, 2011, 136, 2009 RSC.
- P. Španěl and D. Smith, Progress in SIFT-MS: breath analysis and other applications, Mass Spectrom. Rev., 2011, 30, 236 CrossRef.
- S. C. Van't Westeinde and R. J. van Klaveren, Screening and early detection of lung cancer, Cancer J., 2011, 17, 3–10 CrossRef CAS.
- T. W. E. Chippendale, P. Španěl and D. Smith, Time-resolved selected ion flow tube mass spectrometric quantification of the volatile compounds generated by E. coli JM109 cultured in two different media, Rapid Commun. Mass Spectrom., 2011, 25, 2163–2172 CrossRef CAS.
- D. Smith, et al., Quantification of acetaldehyde released by lung cancer cells in vitro using selected ion flow tube mass spectrometry, Rapid Commun. Mass Spectrom., 2003, 17, 845–850 CrossRef CAS.
- W. Filipiak, et al., Release of volatile organic compounds (VOCs) from the lung cancer cell line CALU-1 in vitro, Cancer Cell Int., 2008, 8, 17 CrossRef.
- J. Sulé-Suso, et al., Quantification of acetaldehyde and carbon dioxide in the headspace of malignant and non-malignant lung cells in vitro by SIFT-MS, Analyst, 2009, 134, 2419–2425 RSC.
- D. Smith and P. Španěl, Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis, Mass Spectrom. Rev., 2005, 24(5), 661–700 CrossRef CAS.
- D. Smith, A. Pysanenko and P. Španěl, Ionic diffusion and mass discrimination effects in the new generation of short flow tube SIFT-MS instruments, Int. J. Mass Spectrom., 2009, 281(1–2), 15–23 CAS.
- P. Španěl and D. Smith, Quantification of trace levels of the potential cancer biomarkers formaldehyde, acetaldehyde and propanol in breath by SIFT-MS, J. Breath Res., 2008, 2 CrossRef.
- P. Španěl, Y. Ji and D. Smith, SIFT studies of the reactions of H3O+, NO+ and O2+ with a series of aldehydes and ketones, Int. J. Mass Spectrom. Ion Processes, 1997, 165–166, 25–37 CrossRef.
- Y. Yang, et al., Study of tumor cell invasion by Fourier transform infrared microspectroscopy, Biopolymers, 2005, 78, 311–317 CrossRef CAS.
- D. Smith, A. Pysanenko and P. Španěl, Ionic diffusion and mass discrimination effects in the new generation of short flow tube SIFT-MS instruments, Int. J. Mass Spectrom., 2009, 281, 15–23 CrossRef CAS.
- P. Španěl, K. Dryahina and D. Smith, A general method for the calculation of absolute trace gas concentrations in air and breath from selected ion flow tube mass spectrometry data, Int. J. Mass Spectrom., 2006, 249–250, 230–239 Search PubMed.
- P. J. Mazzone, Analysis of volatile organic compounds in the exhaled breath for the diagnosis of lung cancer, J. Thorac. Oncol., 2008, 3, 774–780 CrossRef.
- M. C. Petersen, et al., Tissue engineering: a new frontier in physiological genomics, Physiol. Genomics, 2007, 32, 28–32 CrossRef CAS.
- W. Jelski and M. Szmitkowski, Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) in the cancer diseases, Clin. Chim. Acta, 2008, 395, 1–5 CrossRef CAS.
- R. Guo and J. Ren, Alcohol and acetaldehyde in public health: from marvel to menace, Int. J. Environ. Res. Public Health, 2010, 7, 1285–1301 CAS.
- H. K. Seitz and F. Stickel, Molecular mechanisms of alcohol-mediated carcinogenesis, Nat. Rev. Cancer, 2007, 7, 599–612 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
