Absorption spectroscopy in microfluidic flow cells using a metal clad leaky waveguide device with a porous gel waveguide layer
Ruchi
Gupta
a,
Behnam
Bastani
a,
N. J.
Goddard
a and
Bruce
Grieve
b
aSchool of Chemical Engineering and Analytical Science, University of Manchester, Manchester, UK
bSchool of Electrical and Electronic Engineering, University of Manchester, Manchester, UK
First published on 31st October 2012
Abstract
Broadband absorption spectroscopy is advantageous because the full spectral profile of an analyte can permit identification of species. This work for the first time investigates the feasibility of a metal clad leaky waveguide (MCLW) device to obtain an absorption spectrum of an analyte of interest, methylene blue, using a white light source in a microfluidic flow cell. The MCLW device comprises a porous low refractive index gel, agarose, deposited on a titanium coated glass slide. The device was capable of detecting 2.3 μM of methylene blue at a wavelength of 650 nm. The corresponding minimum detectable absorbance is 1.6 × 10−1 cm−1. In comparison to commonly used detection devices the MCLW is simpler, robust, easier to fabricate and can be easily interfaced to microfluidic devices. It was also possible to store the MCLW devices dry for up to a year and rehydrate them in 30 s to a working condition.
1. Introduction
Absorption spectroscopy is a commonly used analytical technique for identification, detection and quantitation of an analyte in microfluidic devices.1,2 The use of absorption spectroscopy as a detection technique requires identifying a spectral region in which only the analyte of interest absorbs, with no significant overlapping absorption bands from solvent or other species present. Absorption spectroscopy can also be used to determine the concentration of an analyte through the application of the Beer–Lambert law. According to the Beer–Lambert law, the wavelength-dependent absorbance, A, is given by3 | (1) |
A number of schemes have been investigated to perform absorption spectroscopy in microfluidic flow cells. Each of these schemes have their benefits and limitations. For example, in case of single pass absorption spectroscopy, there is a trade-off between simplicity and poor limit of detection (LOD).4,5 Techniques such as cavity enhanced absorption spectroscopy (CEAS), in which a microfluidic flow cell is placed within an optical cavity comprising two highly reflecting mirrors, can potentially improve the LOD by increasing the effective optical path length by several orders of magnitude.6–8 CEAS, however, has only been effectively used for flow cells made of high optical quality glass. Glass has the disadvantages of fragility, expense and difficulty of processing.9,10 Some other limitations of CEAS include difficulties in the alignment of optical components and large footprint of the experimental set-up. In order to address the latter issues, mirrors have been integrated with microfluidic flow cells.11,12 It is, however, difficult to fabricate high quality mirrors integrated with microfluidic flow cells. Fiber-loop cavities have also been used for absorbance measurements in microfluidic devices,13–15 but demand complicated fiber alignment procedures and careful design of microfluidic devices.16
Liquid core waveguides (LCW) can increase the distance travelled by light through the sample by total internal reflection. LCWs, however, require using either high refractive index liquids (e.g. aromatics, carbon disulphide and halogenated compounds),17–19 or coating low refractive index polymers on the walls of flow cells.20 While the former places severe limitations on the type of chemistry that may be used, the latter suffers from poor long term performance as a result of degradation of the polymer coating. Low refractive index Teflon tubing can be used in place of coated flow cells, but the integration of the tubing with other microfluidic systems is difficult.21 These limitations can be overcome by the use of anti-resonant reflecting optical waveguide (ARROW), where dielectric mirrors on the walls of flow cells confine light into the sample liquid, but ARROWs place constraint on the dimensions of microfluidic flow cells and are difficult to fabricate.22,23
The feasibility of evanescent wave techniques such as attenuated total reflection (ATR)24,25 and evanescent wave CEAS26–29 to perform absorbance spectroscopy in microfluidic flow cells has also been investigated.24,25 The microresonators can in general only be used to record absorbance over a relatively narrow range of wavelengths governed by the availability of tuneable laser sources. In addition, microresonators are point detectors and are often difficult to fabricate. Whispering gallery mode (WGM) resonators30–32 and high refractive index waveguides33–35 have been interfaced with microfluidic devices to perform absorption spectroscopy with improved LOD. Other notable contributions in the field have been made by Campbell et al.36 and Zhang et al.37 However, coupling light into conventional waveguides is usually achieved via the end-fire technique and has been extremely difficult because of the critical alignment of the light beam with the sub-micron thickness of the waveguide.38 In contrast, in the metal clad leaky waveguide (MCLW), a prism can be used to couple light in and out of the device. In addition, the presence of the metal layer results in a peak in reflectivity around the resonance angle, providing a direct visualisation of the device output. Therefore, MCLW can be easily interfaced with microfluidic flow cells to perform absorption spectroscopy in such devices.
To our knowledge there are reports on the use of MCLWs for refractive index measurements,39 particle detection40,41 and (bio)chemical sensing,42 but none on measuring the absorption spectrum of liquid samples. This paper for the first time demonstrates the use of a MCLW device to obtain an absorption spectrum of an analyte of interest, methylene blue, using a white light excitation source. This work has also explored the suitability of a MCLW device comprising of porous low refractive index agarose waveguide layer for absorbance spectroscopy. Zourob and Goddard42 on the other hand, utilised a MCLW device consisting of a waveguide layer made of sol–gel. In comparison to sol–gel (n = 1.42), the refractive index contrast between agarose (n = 1.337) and the glass substrate (n = 1.517) is higher. As a result, a MCLW device consisting of agarose waveguide layer has sharper reflectivity peak and hence lower LOD than a structure with sol–gel waveguide layer. The devised MCLW device consisted of a thin titanium metal layer and a porous low refractive index gel, agarose, on a glass slide and was found to be able to detect 2.3 μM methylene blue, which corresponds to a minimum detectable absorbance of 1.6 × 10−1 cm−1. The average response time of the device was 35 s. The waveguide layer (i.e. agarose) was deposited by spin coating and an injection moulded polystyrene flow cell was used. The agarose layer was stable and the device was used for 40 times without a noticeable change in its performance. In addition, the device could be dry stored at room temperature for at least a year and used after rehydration for 30 s in de-ionised water or phosphate buffer.
2. Experimental
2.1 Chemicals
100 mM, pH 7 phosphate buffer was made by adding 42.29 mM of sodium phosphate monobasic monohydrate (99.6%, Sigma-Aldrich, Gillingham, UK) and 57.71 mM of disodium hydrogen orthophosphate dodecahydrate (99.85%, Fisher Scientific, Loughborough, UK). A stock solution of 4 mM methylene blue (>82%, Sigma-Aldrich, Gillingham, UK) was prepared in phosphate buffer which was diluted with the buffer to obtain solutions of desired concentrations. Unless stated otherwise, all solutions were prepared using 18.2 MΩ water (Elga Maxima Ultra Pure, Vivendi Water Systems, Buckinghamshire, UK).The chemicals required to fabricate the MCLW device are as follows: hydrogen peroxide (30%), sulphuric acid (98%), titanium (>99.99%), ethanol (>99.5%), 3-aminopropyl-triethoxysilane (APTS, 99%), agarose type VII-A, glutaraldehyde (50% in water, Sigma-Aldrich, Gillingham, UK) and toluene (>99%, Acros Organics, Loughborough, UK). 1.2 mm thick glass slides were bought from VWR International (Leicestershire, UK).
2.2 MCLW device fabrication
The glass slides were cleaned in piranha solution (H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4 = 1
H2SO4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and dried overnight in an oven (Memmert, Schwabach, Germany) at 110 °C. The cleaned glass slides were coated with 9 nm thick titanium using electron-beam deposition (Edwards, West Sussex, UK) at a rate of 0.2 nm s−1 and under a pressure of 10−6 mbar. Film thickness was monitored by a quartz crystal oscillator (E08668000, Edwards, West Sussex, UK).
3) and dried overnight in an oven (Memmert, Schwabach, Germany) at 110 °C. The cleaned glass slides were coated with 9 nm thick titanium using electron-beam deposition (Edwards, West Sussex, UK) at a rate of 0.2 nm s−1 and under a pressure of 10−6 mbar. Film thickness was monitored by a quartz crystal oscillator (E08668000, Edwards, West Sussex, UK).
Titanium coated glass slides were silanized using the procedure described in.42 Briefly, the substrates were cleaned with ethanol and toluene, following which they were immersed in 5% APTS in toluene for 30 min at room temperature to form a silane layer via Ti–O–Si covalent bonds. The substrates were then rinsed with ethanol and water. Finally, the substrates were dried under nitrogen and placed in the oven at 110 °C for 1 hour.
A 4% (w/v) agarose solution was prepared by adding 0.4 g of agarose type VII-A in 10 ml of de-ionised water. The solution was heated on a hot plate (Fisher Scientific, Loughborough, UK) at 150 °C for 1 hour in a closed vial while stirring at 125 rpm. 12.5 μl of 25% (v/v) glutaraldehyde was added and the solution was heated and stirred for another 15 min. 1000 μl of gel was spin coated onto a silanised substrate at 4000 rpm for 30 s.
2.3 Instrumentation
Fig. 1 shows a schematic of the optical arrangement used in this work. | ||
| Fig. 1 Schematic of the set-up used to test the MCLW device (where the angle of incidence, θ, was scanned). | ||
The light was coupled into the device using a N-BK7 equilateral prism (Qioptic Photonics, Denbighshire, UK). In order to ensure good optical coupling between the prism and the device substrate, a drop of index matching liquid (Type A, Cargille Labs, New Jersey, USA) was placed on the surface of the prism. A polystyrene flow cell, which was produced using a Babyplast 6/10 (Cronoplast S.L., Abrera, Spain) injection moulding machine, was placed over the MCLW device and clamped firmly in place from above and sealed with a nitrile O-ring. The light source and the detector were mounted on rails to allow positional freedom. The rails were connected to two computer-controlled goniometers of high angular precision; one of which was used to control the angular position of the light source, and the other to synchronise the detector to the corresponding specular reflection angle.
The absorption spectroscopy was performed using a tungsten halogen lamp (HL-2000, 360–2000 nm, 7 W, Ocean Optics, Duiven, Netherlands) connected to an optical fiber (FC-UV400-2, Ocean Optics, Duiven, Netherlands). The other end of the fiber was mounted in a 25 mm diameter tube using an adaptor and passed through a variable diameter iris (Comar Instruments, Cambridge, UK). The iris was placed between the fiber end and the collimating achromatic doublet lens (Comar Instruments, Cambridge, UK), which had a focal length of 63 mm and a diameter of 25 mm. Finally, the beam passed through a polariser (Comar Instruments, Cambridge, UK) to obtain TE-polarised light which was prism coupled into the MCLW device. The output beam from the MCLW device was focused using a 10 mm focal length, 6.3 mm diameter plano-convex lens onto a second optical fiber with a core diameter of 230 μm (Ocean Optics, Duiven, Netherlands) and coupled via the fiber to a spectrometer (USB 4000, Ocean Optics). The spectrometer was USB interfaced to a computer and data was acquired via the SpectraSuite software (Ocean Optics, Duiven, Netherlands). At each angle of incidence of the collimated white light source, the output spectra of the reference and sample solutions were recorded over the wavelength range from 360 nm to 890 nm with an acquisition time of 500 ms and averaged over 5 traces. This data was then converted to absorption spectrum of the sample using eqn (1).
In order to measure the LOD of the MCLW device, a TE-polarised laser source (Acculase, RS Components, Northamptonshire, UK) with a peak wavelength of 650 nm and a power of 5 mW and a photodiode detector (OSD100-6, Centronic, Surrey, UK) were used. The angle of incidence (marked as θ in Fig. 1) of the monochromatic light source was scanned using the computer-controlled goniometers to obtain the angular spectrum of reflectivity. The data were collected using a 16-bit analogue-to-digital converter connected to an IBM PC compatible computer via a USB port. The data were acquired via software written in-house using Borland C++ Builder version 6.
In order to measure the response time of the MCLW device, the angle of incidence of the laser light source was fixed at the angle of peak reflectivity and reflectivity data were recorded as a function of time at a fixed wavelength. The plastic syringes filled with 100 mM, pH 7 phosphate buffer and 10 μM methylene blue were connected to the inlet of the flow cell via a three-way valve. This allowed switching between the buffer and the dye, and vice versa, without introducing air bubbles.
The solutions were pumped through the flow cell manually with a 5 ml disposable syringe. 100 mM phosphate buffer of pH 7 was used as a reference solution to perform all measurements. Unless stated otherwise, before pumping each solution into the flow cell, an air bubble was injected to avoid mixing of liquids introduced successively. The flow cell was flushed with 5 ml of phosphate buffer after each set of measurements for different dye concentrations and data (as a function of wavelength and angle in case of white and laser light source respectively) was recorded. The order in which the dye solutions were pumped was from high to low concentration. The LOD was calculated as the sum of the y-intercept of the calibration curve and three times the standard deviation, sB, associated with that value. The standard deviation, sB, was calculated as follows:43
 | (2) |
3. Results and discussion
3.1 Design of the MCLW device
In order to perform high sensitivity absorption spectroscopy using a MCLW device, it is essential to increase (1) the fraction of optical mode power interacting with the absorbing sample and (2) the distance for which light travels in the waveguide (i.e. propagation distance). To achieve these aims, the use of a porous low refractive index material, agarose, as a waveguide layer was found to be suitable. The porous nature of the agarose waveguide layer implies that the absorbing sample molecules can diffuse into the waveguide and interact with the optical mode propagating in that layer. In addition, the absorbing sample layer just above the waveguide can interact with the evanescent field in that region. This means that all the light coupled into the waveguide can interact with the sample. This is quite different to, for example, non-porous waveguides, where the sample can only interact with the evanescent field. This property of the porous waveguide means that the main determinant of the sensitivity is the propagation distance in the waveguide layer. The optical mode profile of the MCLW with agarose waveguide obtained using a transfer matrix modelling package, L-Pro version 4.0,44 is shown in Fig. 2. The 1/e propagation distance of light in the MCLW device with a 2 μm thick agarose waveguide was calculated to be 480 μm, which is at least two orders of magnitude higher than the structure reported in.39–42 | ||
| Fig. 2 Mode profile of the MCLW device designed for high sensitivity absorption spectroscopy at a wavelength of 650 nm. | ||
In the case of the MCLW with a porous waveguide layer, the response of the sensor to the absorbing sample molecules in the evanescent field is instantaneous, but to interact with the mode in the waveguide requires the sample molecules to diffuse into that layer. Thus, it was expected that the response of the sensor should show two distinct phases; a very rapid change caused by absorbing sample molecules entering the evanescent field and a slower response as the absorbing sample molecules diffuse into the waveguide. To reduce the diffusion time, a thin waveguide layer is required, as shown by the equation relating diffusion time, t, to the average diffusion distance, ![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) :
:
 | (3) |
Transfer matrix modelling of the structure showed that the intensity of the reflectivity peak is related to the absorbance of the sample fluid placed on top of the waveguide layer at a chosen wavelength of the light source (data not shown). This means that the absorption spectrum of the sample can be obtained by illuminating the MCLW device with a white light source at the angle of maximum reflectivity and recording the wavelength-dependent intensity of the reflected beam. The peak intensities were recorded at five angles (65.0, 67.5, 68.0, 68.5 and 70.0°) to determine the optimum range of angles for measuring the spectrum of the dye in the wavelength range 500–750 nm. The optimum angle range was found to be 67.5 to 68.5°, as shown in Fig. 4. The apparent absorption spectrum of methylene blue depends on the angle of incidence of the white light source because the observed response is a product of the absorption spectrum of methylene blue with the wavelength dependence of the MCLW at a fixed angle of incidence (see Fig. 3). The final spectrum at each dye concentration was the average of the spectra obtained at 67.5, 68.0 and 68.5°.
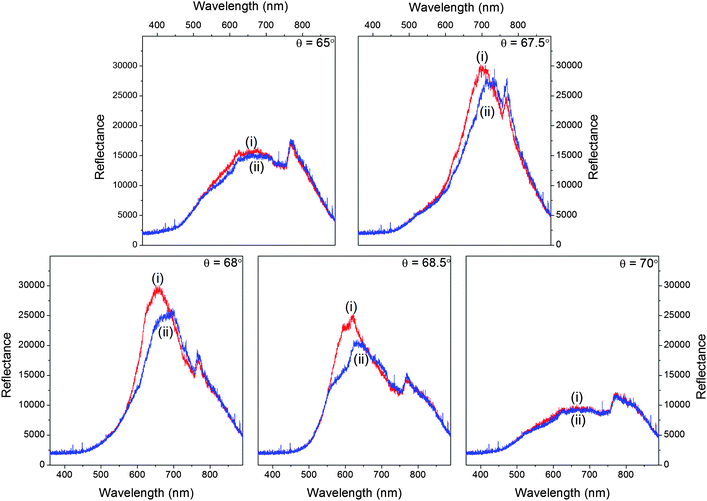 | ||
| Fig. 3 Reflectivity curves of the MCLW device for (i) water and (ii) methylene blue at five angles of incidence of the white light source (65.0, 67.5, 68.0, 68.5 and 70.0°). | ||
3.2 Measurement of absorption spectrum of methylene blue using the MCLW device
The suitability of the designed MCLW device to obtain the absorption spectrum of an analyte was investigated using methylene blue. This is because methylene blue absorbs between 500 nm and 750 nm with absorption maxima at 662–664 nm and 605–610 nm for monomeric and dimeric forms respectively.45,46The output light intensity versus wavelength for phosphate buffer and 4 mM dye solution at selected angles of incidence was recorded (data not shown). A comparison of spectra of phosphate buffer before and after the injection of methylene blue of different concentrations showed no noticeable difference (data not shown). This showed that the dye could be completely desorbed from the waveguide layer by flushing the MCLW device with phosphate buffer without leaving any entrapped dye.
The intensity data was used to calculate the absorbance of 4 mM methylene blue at different angles of incidence viaeqn (1) (as shown in Fig. 4(a)), which were then used to obtain the absorption spectrum of the dye. It is clear from Fig. 4(a) and (b) that the absorption spectrum of methylene blue can be obtained by varying the angle of incidence of the white light source over a narrow range (∼1°) because the dispersion of the MCLW was quite small over the wavelength range in which the dye absorbs. This can be seen clearly in Fig. 3, where at an incident angle of 65° the MCLW gives a significant signal from ∼500 to 850 nm.
 | ||
| Fig. 4 Absorbance versus wavelength of (a) 4 mM methylene blue at different angles of incidence and (b) different concentrations of methylene blue. | ||
The procedure was repeated to obtain the absorption spectrum of methylene blue at different concentrations, which are shown in Fig. 4(b). The absorption spectrum of methylene blue in Fig. 4(b) has an absorption maximum at 610 nm, which is consistent with the results reported in previous studies on evanescent wave absorbance spectroscopy of methylene blue.46,47 As shown in Fig. 5(a), the relationship between absorbance versus the concentration of methylene blue was linear in the concentration range between 0.5 mM and 3 mM (A = 0.086 + 0.128c, where A is absorbance and c is concentration) with r2 = 0.996 at 610 nm. In this case, the LOD of methylene blue was 0.38 mM. The use of a white light source demonstrated that the designed MCLW device is suitable to obtain the absorption spectrum, but the LOD was limited by the intensity of the tungsten halogen lamp used. The LOD of the MCLW device was therefore investigated using a laser light source.
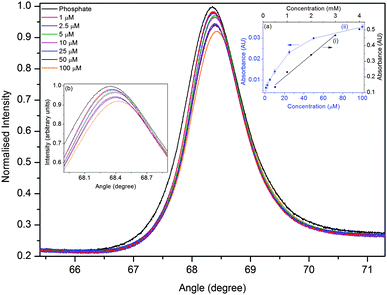 | ||
| Fig. 5 Plot of angular reflectivity spectrum for different concentrations of methylene blue at a wavelength of 650 nm (inset (a) compares the calibration curve obtained using a (i) white and (ii) laser light source and inset (b) is the amplified view of the reflectivity curve). | ||
3.3 LOD and response time of the MCLW device
In order to investigate the LOD of the designed MCLW device, it was probed using a laser light source operating at a wavelength of 650 nm. The angle of incidence of the light source was varied to obtain the angular reflectivity spectrum for different concentrations of methylene blue. Fig. 5 shows a plot of reflectivity versus the angle of incidence for different concentration of methylene blue. Eqn (1) was then used to convert the intensity data for various concentrations of methylene blue to absorbance, thereby obtaining a calibration curve. Linear absorbance response was observed in the range of 1–10 μM methylene blue. The calibration curve shows saturation at concentrations greater than 10 μM methylene blue because as the concentration of the absorbing species increases, in addition to a change in the intensity of the reflectivity peak, the distance over which light travels in the waveguide layer also reduces. The relationship between absorbance and the concentration of methylene blue in the linear range is given by A = 0.006 + 0.860c with r2 = 0.998. The LOD of methylene blue using the laser light source was 2.3 μM at 650 nm, which is two orders of magnitude better than the typical LOD obtained via single pass absorbance spectroscopy in microfluidic flow cells.4A comparison of the LOD and the minimum detectable absorbance of analytes for the designed MCLW and some of the existing devices in microfluidic flow cells is provided in Table 1. While the minimum detectable absorbance of MCLW is comparable to multireflection cells, it is worse than CEAS, fiber loop cavities, LCW, ATR and evanescent wave CEAS. The minimum detectable absorbance of the MCLW device is also poorer than the WGM resonators, which have also been used to perform absorption spectroscopy in microfluidic flow cells.32 The use of WGM resonators to measure absorbance at different points in microfluidic flow cells, however, requires fabricating the resonators at the desired locations. In contrast, the MCLW is not a point detector and can be used to monitor absorbance at different points in microfluidic flow cells by varying the spatial location of the light beam probing the device. In addition, it is quite difficult to perform absorption measurements using microresonators. The MCLW device can be constructed using a simple fabrication method, spin coating, and can be easily integrated with planar microfluidic flow cells. In addition, the MCLW device has a long shelf-life (at least a year) and can be used after 30 s rehydration in de-ionised water or phosphate buffer. The MCLW can be used for 40 times without a noticeable change in its performance.
| Device | Analyte | Wavelength (nm) | LOD (μM) | Minimum detectable absorbance (cm−1) | Source |
|---|---|---|---|---|---|
| MCLW | Methylene blue | 650 | 2.3 | 1.6 × 10−1 | |
| CEAS | Potassium hexachloroiridate | 487 | 1.4 | 1.3 × 10−2 | 48 |
| Multireflection cells | Rhodamine B | 550 | 5 | 4.4 × 10−1 | 11 |
| Fiber loop cavities | Tartrazine | 405 | 5, ∼1 | 1.1 × 10−1, 2.0 × 10−2 | 16 and 49 |
| LCW | Bromothymol blue | 660 | 2 | 2.2 × 10−2 | 20 |
| E.g. iron, copper | 562, 484 | 1 × 10−4, 4 × 10−4, | 2.8 × 10−6, 5.5 × 10−5 | 50 and 51 | |
| WGM resonators | Methylene blue | 635 | 1.5 × 10−3 | 5.5 × 10−7 | 42 |
| ATR | Potassium ferricyanide | 420 | 8, 1 | 8.0 × 10−3, 1.0 × 10−3 | 24 and 25 |
| Evanescent wave CEAS | Potassium hexachloroiridate, iodine | 487, 520 | 5.4 × 10−4, 3.2 × 10−3 | 5.0 × 10−5, 2.2 × 10−6 | 27–29 |
| High refractive index waveguides | Methylene blue | 600 | 10 | 5.2 × 10−3 | 34 |
The response time of the MCLW device was then measured. The intensity of the reflected laser light was observed at the peak of reflectivity at 68.4° (as shown in Fig. 5) as the flow cell contents were cycled between dye and plain buffer. The response of the MCLW device was expected to consist of two distinct phases; caused by the entrance of methylene blue molecules in the evanescent field of the MCLW and the diffusion of the dye into the agarose waveguide. The two phases were, however, not clearly evident in Fig. 6. This observation can be explained by considering that the rapid response of the MCLW device was masked as a result of mixing in the flow cell and inlet tubing.
 | ||
| Fig. 6 Response time of the MCLW device when (a) 100 mM, pH 7 phosphate buffer was replaced with 10 μM methylene blue and (b) 10 μM methylene blue was replaced with 100 mM, pH 7 phosphate buffer. | ||
As shown in Fig. 6, the response time of the MCLW device when the buffer was replaced with the dye and vice versa was 36 s and 34 s respectively. An average response time of 35 s implies that the MCLW device is suitable for rapid testing of samples using, for example, colorimetric assays. The MCLW reported in this work, however, may not be a suitable to monitor electro-separation of species in microfluidic channels because such devices require detectors with a response time of less than a second.
By substituting an average diffusion time and distance as 35 s and 2 μm respectively in eqn (3), the diffusion coefficient of methylene blue in agarose was estimated to be 5.7 × 10−10 cm2 s−1, which is approximately four orders of magnitude lower than the dye's diffusion coefficient in methanol.52
An example of a possible application of the MCLW device is in two-wavelength spectrometric measurements to determine, for example, the pH of a sample containing an indicator dye.53,54 The measurement of the absorbance at two wavelengths eliminate the need to know the amount of the indicator dye added to the sample, thereby increasing the accuracy with which the pH of the sample can be determined.55 The MCLW device can also be used for sensitive detection of organic (e.g. proteins56) and inorganic (e.g. iron57) species in microfluidic flow cells via colorimetric assays.
In this work, the absorption spectrum of methylene blue was obtained by scanning the angle of incidence of a collimated white light source and resolving the wavelength-dependent components of the reflected beam. It is, however, also possible to obtain the absorption spectrum of an analyte of interest by probing the MCLW device using a white light source with a wedge beam at a fixed angle of incidence and resolving the angular and wavelength components of the reflected beam via a combination of a dispersive element and a camera. Previously, attempts have been made to integrate optical components and systems, including spectrometers, with microfluidic devices.58–60 Similarly, the optical components required to probe the MCLW device can be fabricated on-chip to achieve an integrated and portable analytical platform for on-site analysis of samples.
4. Conclusions
A metal clad leaky waveguide (MCLW) device consisting of a porous low refractive index waveguide layer made of agarose on a titanium-coated glass substrate was devised in this work. This work also demonstrated that the developed MCLW device could successfully be used for high sensitivity absorbance detection in microfluidic flow cells. In particular, the device could be used to detect 2.3 μM methylene blue at 650 nm. Some potential applications of the MCLW device is in multi-wavelength spectrometric measurements to determine a physical quantity (e.g. pH) with high accuracy and high sensitivity detection of (bio)chemical species in microfluidic flow cells.The reported MCLW device offers advantages such as good LOD and reliable long term performance. The other factors in the favour of the MCLW device for detection in microfluidic flow cells include its ease-of-integration, simple fabrication and ability to permit measurements at different spatial locations. This is in addition to the device's long shelf life of at least a year.
References
- W. W. Buchberger, J. Chromatogr., A, 2000, 884, 3 CrossRef CAS.
- K. B. Mogensen, H. Klank and J. P. Kutter, Electrophoresis, 2004, 25, 3498 CrossRef CAS.
- R. W. Ricci, M. A. Ditzler and L. P. Nestor, J. Chem. Educ., 1994, 71, 983 Search PubMed.
- B. Kuswandi, N. Nuriman, J. Huskens and W. Verboom, Anal. Chim. Acta, 2007, 601, 141 CrossRef CAS.
- P. J. Viskari and J. P. Landers, Electrophoresis, 2006, 27, 1797 CrossRef CAS.
- M. Islam, L. N. Seetohul and Z. Ali, Appl. Spectrosc., 2007, 61, 649 CrossRef CAS.
- S.-S. Kiwanuka, T. Laurila and C. F. Kaminski, Anal. Chem., 2010, 82, 7498 CrossRef CAS.
- S. E. Fielder, A. Hese and A. A. Ruth, Rev. Sci. Instrum., 2005, 76, 023107 CrossRef.
- H. Becker and L. E. Locascio, Talanta, 2002, 56, 267 CrossRef CAS.
- T. McCreedy, TrAC, Trends Anal. Chem., 2000, 19, 396 CrossRef CAS.
- L. Billot, A. Plecis and Y. Chen, Microelectron. Eng., 2008, 85, 1269 CrossRef CAS.
- H. Salimi-Moosavi, Y. Jiang, L. Lester, G. McKinnon and D. Jed Harrison, Electrophoresis, 2000, 21, 1291 CrossRef CAS.
- A. L. Gomez, J. A. Fruetel and R. P. Bambha, Proc. SPIE, 2009, 7397, 739706 Search PubMed.
- A. L. Gomez, R. F. Renzi, J. A. Fruetel and R. P. Bambha, Appl. Opt., 2012, 51, 2532 Search PubMed.
- M. Andachi, T. Nakayama, M. Kawasaki, S. Kurokawa and H.-P. Loock, Appl. Phys. B: Lasers Opt., 2007, 88, 131 Search PubMed.
- H. Waechter, J. Litman, A. H. Cheung, J. A. Barnes and H.-P. Loock, Sensors, 2010, 10, 1716 CrossRef CAS.
- O. J. A. Schueller, X.-M. Zhao, G. M. Whitesides, S. P. Smith and M. Prentiss, Adv. Mater., 1999, 11, 37 CrossRef CAS.
- C. Gooijer, G. P. Hoornweg, T. de Beer, A. Bader, D. J. van Iperon and U. A. T. Brinkman, J. Chromatogr., A, 1998, 824, 1 CrossRef CAS.
- K. Fujiwara, J. B. Simeonsson, B. W. Smith and J. D. Winefordner, Anal. Chem., 1988, 60, 1065 CrossRef CAS.
- A. Datta, I. Y. Eom, A. Dhar, P. Kuban, R. Manor, I. Ahmad, S. Gangopadhyay, T. Dallas, M. Holtz, F. Temkin and P. K. Dasgupta, IEEE Sens. J., 2003, 3, 788 CrossRef CAS.
- M. P. Duggan, T. McCreedy and J. W. Aylott, Analyst, 2003, 128, 1336 RSC.
- N. J. Goddard, J. Hulme, C. Malins, K. Singh and P. R. Fielden, Analyst, 2002, 127, 378 RSC.
- F. Prieto, L. M. Lechuga, A. Calle, A. Llobera and C. Domínguez, J. Lightwave Technol., 2001, 19, 75 Search PubMed.
- Y. Shi, A. F. Slaterbeck, C. J. Seliskar and W. R. Heineman, Anal. Chem., 1997, 69, 3679 CrossRef CAS.
- A. F. Slaterbeck, T. H. Ridgway, C. J. Seliskar and W. R. Heineman, Anal. Chem., 1999, 71, 1196 CrossRef CAS.
- M. Mazurenka, L. Wilkins, J. V. Macpherson, P. R. Unwin and S. R. Mackenzie, Anal. Chem., 2006, 78, 6833 CrossRef CAS.
- L. van der Sneppen, G. Hancock, C. F. Kaminski, T. Laurila, S. R. Mackenzie, S. R. T. Neil, R. Peverall, G. A. D. Ritchie, M. Schnippering and P. R. Unwin, Analyst, 2010, 135, 133 RSC.
- A. C. R. Pipino, Phys. Rev. Lett., 1999, 83, 3093 CrossRef CAS.
- A. C. R. Pipino, Appl. Opt., 2000, 39, 1449 CrossRef CAS.
- A. Nitkowski, L. Chen and M. Lipson, Opt. Express, 2008, 16, 11930 CrossRef CAS.
- A. Nitkowski, A. Baeumner and M. Lipson, Biomed. Opt. Express, 2011, 2, 271 Search PubMed.
- S. L. Westcott, J. Zhang, R. K. Shelton, N. M. K. Bruce, S. Gupta, S. L. Keen, J. W. Tillman, L. B. Wald, B. N. Strecker, A. T. Rosenberger, R. R. Davidson, W. Chen, K. G. Donovan and J. V. Hryniewicz, Rev. Sci. Instrum., 2008, 79, 033106 Search PubMed.
- G. Pandraud, T. M. Koster, C. Gui, M. Dijkstra, A. van den Ber and P. V. Lambeck, Sens. Actuators, A, 2000, 85, 158 CrossRef.
- A. Prabhakar and S. Mukherji, Lab Chip, 2010, 10, 748 RSC.
- A. G. Mignani, R. Falciai and L. Ciaccheri, Appl. Spectrosc., 1998, 52, 546 CrossRef CAS.
- P. L. Edmiston, D. P. Campbell, D. S. Gottfried, J. Baughman and M. M. Timmers, Sens. Actuators, B, 2010, 143, 574 CrossRef.
- L. Zhang, P. Wang, Y. Xiao, H. Yu and L. Tong, Lab Chip, 2011, 11, 3720 RSC.
- S. J. McNab, N. Moll and Y. A. Vlasov, Opt. Express, 2003, 11, 2927 CrossRef.
- M. Zourob, S. Mohr, P. R. Fielden and N. J. Goddard, Sens. Actuators, B, 2003, 94, 304 CrossRef.
- M. Zourob, S. Mohr, B. J. T. Brown, P. R. Fielden, M. B. McDonnell and N. J. Goddard, Anal. Chem., 2005, 77, 232 CrossRef CAS.
- M. Zourob, S. Mohr, B. J. T. Brown, P. R. Fielden, M. McDonnell and N. J. Goddard, Sens. Actuators, B, 2003, 90, 296 CrossRef.
- M. Zourob and N. J. Goddard, Biosens. Bioelectron., 2004, 20, 1718 Search PubMed.
- J. N. Miller and J. C. Miller, Statistics and Chemometrics for Analytical Chemistry, Pearson Prentice Hall, Harlow, Essex, 2010 Search PubMed.
- R. T. Kersten, Opt. Commun., 1973, 9, 427 CrossRef.
- K. Bergmann and C. T. O'Konski, J. Phys. Chem., 1963, 67, 2169.
- K. Fujita, K. Taniguchi and H. Ohno, Talanta, 2000, 65, 1066 Search PubMed.
- G. Ferrini, L. De Carlo, C. Giannetti and F. Parmigiani, Opt. Spectrosc., 2009, 107, 464 CrossRef CAS.
- S. R. T. Neil, C. M. Rushworth, C. Vallance and S. R. Mackenzie, Lab Chip, 2011, 11, 3953 RSC.
- H. Waechter, D. Munzke, A. Jang and H.-P. Loock, Anal. Chem., 2011, 83, 2719 CrossRef CAS.
- T. Dallas and P. K. Dasgupta, TrAC, Trends Anal. Chem., 2004, 23, 385 CrossRef CAS.
- R. N. M. J. Páscoaa, I. V. Tóth and A. O. S. S. Rangela, Anal. Chim. Acta, 2012, 739, 1 CrossRef CAS.
- E. A. Genina, A. N. Bashkatov and V. V. Tuchin, Med. Laser Appl., 2008, 23, 31 Search PubMed.
- D. W. King and D. R. Kester, Mar. Chem., 1989, 26, 5 Search PubMed.
- T. D. Clayton and R. H. Byrne, Deep-Sea Res., Part I, 1993, 40, 2115 Search PubMed.
- R. H. Byrne, Anal. Chem., 1987, 59, 1479 CrossRef CAS.
- C. V. Sapan, R. L. Lundblad and N. C. Price, Biotechnol. Appl. Biochem., 1999, 29, 99 CAS.
- L. L. Stookey, Anal. Chem., 1970, 42, 779 CrossRef CAS.
- Z. Hu, A. Glidle, C. N. Ironside, M. Sorel, M. J. Strain, J. Cooper and H. Yin, Lab Chip, 2012, 12, 2850 RSC.
- J. M. Ruano, A. Glidle, A. Cleary, A. Walmsley, J. S. Aitchison and J. M. Cooper, Biosens. Bioelectron., 2003, 16, 175 Search PubMed.
- J. M. Ruano, V. Benoit, J. S. Aitchison and J. M. Cooper, Anal. Chem., 2000, 72, 1093 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
