Predictably tuning the frontier molecular orbital energy levels of panchromatic low band gap BODIPY-based conjugated polymers†
Abstract
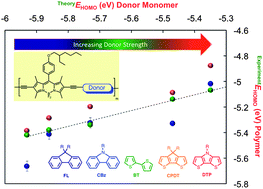
Here we report the synthesis, electrochemical properties, and optical properties of five novel π-conjugated alternating copolymers based on the BODIPY core. These polymers were synthesized via the Sonogashira polymerization reaction and contain BODIPY units alternating with comonomers such as 9,9-bis(2-ethylhexyl)-9H-fluorene (FL), 9-(2-ethylhexyl)-9H-carbazole (CBz), 2,2′-bithiophene (BT), 4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b']dithiophene (CPDT) and 4-(2-ethylhexyl)-4H-dithieno[3,2-b:2',3'-d]pyrrole (DTP). These comonomers were rationally chosen based on their gas phase ionization potential (IP) values estimated by density functional theory (DFT) calculations. Cyclic voltammetry on drop-cast thin films, as well as solutions of these polymers, revealed that the highest occupied molecular orbitals (HOMOs) of the resulting polymers correlated well with the ionization potentials (donor strength) of the comonomers. On the contrary, the lowest unoccupied molecular orbital (LUMO) energy levels of all copolymers were fairly invariant, independent of the comonomer used. This suggests that the BODIPY moiety provides the primary influence on the LUMO levels of the polymer. In addition to the experimentally determined HOMO/LUMO energy levels bearing good correlation with theoretical estimates, all polymers were found to possess broad absorption spectra covering the entire visible range, thus making them truly panchromatic. These polymers provide us with a toolset to tune the frontier molecular orbital energy levels, while retaining the low band gap and broad absorption of these polymers.

 Please wait while we load your content...
Please wait while we load your content...