A dissymmetric molecular capsule with polar interior and two mechanically locked hemispheres†
Marcos
Chas
a and
Pablo
Ballester
*ab
aInstitute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007, Tarragona, Spain. E-mail: pballester@iciq.es; Fax: +34 977920224; Tel: +34 977920206
bCatalan Institution for Research and Advanced Studies (ICREA), Passeig Lluís Companys, 23, 08018, Barcelona, Spain
First published on 30th September 2011
Abstract
The synthesis and chiral resolution of a dissymmetric tetraurea-calix[4]pyrrole/tetraurea-calix[4]arene molecular capsule with two hemispheres mechanically locked is described. The assembly features a bis-[2]catenated topology and shows reversible molecular co-encapsulation of two different neutral molecules or two oppositely charged ions.
Introduction
In the mid-1990s, Shimizu and Rebek reported the first example of self-assembly of supramolecular dimeric capsules based on tetra-urea calix[4]arene scaffolds.1 Böhmer et al. subsequently published a closely related capsular system2 and provided its X-ray crystal structure.3–5 Since then, the study of such assemblies and their applications6 have constituted an active field of investigation.7,8 The serendipitous discovery of exclusive heterodimerization in the self-assembly of capsules involving tetra-aryl 1a and tetra-tosyl ureas 29 paved the way to the high yield synthesis of elaborate bis-loop tetraurea calix[4]arenes such as 3 (Fig. 1 and Scheme 1).10 The use of the tetratosyl urea 2 as template prevents undesired intermolecular reaction between alkenyl residues belonging to two different calix[4]arenes 1b because no homo dimers are formed under these conditions. In turn, bis-loop 3 can be employed for the efficient synthesis of interlocked structures like catenanes11 and rotaxanes.12 Bis-loop tetra-urea calix[4]arenes 3 cannot dimerize and in mixtures with alkenyl tetra-ureas 1b, they assemble exclusively and quantitatively into heterodimeric capsulesi.e.1b·3. Application of olefin metathesis to these heterodimers allowed the isolation of chiral bis[2]catenanes 3·3 in acceptable to good yields.13![Template syntheses of bis-loop 3 starting form heterodimeric capsule 1b·2 and bis[2]catenane 3·3 starting from pseudo-[2]rotaxane homodimer 1b·3.13](/image/article/2012/SC/c1sc00668a/c1sc00668a-s1.gif) | ||
| Scheme 1 Template syntheses of bis-loop 3 starting form heterodimeric capsule 1b·2 and bis[2]catenane 3·3 starting from pseudo-[2]rotaxane homodimer 1b·3.13 | ||
![Line drawing structures of the tetra-urea calix[4]arene derivatives 1, 2, and 3 with their schematic representations.](/image/article/2012/SC/c1sc00668a/c1sc00668a-f1.gif) | ||
| Fig. 1 Line drawing structures of the tetra-urea calix[4]arene derivatives 1, 2, and 3 with their schematic representations. | ||
To overcome some inherent limitations of tetra-urea calix[4]arene dimers, we introduced related tetra-urea calix[4]pyrrole capsules 4a·4a (Fig. 2).14 The size of the internal cavity of calix[4]arene capsules is increased by 62% in the calix[4]pyrrole analogs and this allows pair-wise guests encapsulation. In addition, tetra-urea calix[4]pyrrole dimeric capsules feature two endohedral hydrogen bonding sites that are appropriate for the inclusion of guests through polar interactions.15 This latter characteristic contrasts strongly with the absence of polar binding sites in the calix[4]arene capsule's interior.16 Recently, we discovered that in dichloromethane solution and in the presence of 1 equivalent of an appropriate N-oxide, tetra-urea calix[4]arene 1a and tetra-urea calix[4]pyrrole 4a exclusively heterodimerize to give capsule 1a·4a.17 We imagined that this new exclusive heterodimeric assembly strategy could be combined with the two other known assembly phenomena (aryl with tosyl ureas, bisloop with aryl ureas) to access the mechanically locked molecular capsule 3·5 having two “hemispheres” of completely different polarity. Dimeric capsules interlocked with two loops experience reversible hydrogen bonding dynamics,18 so we expect them to be more flexible and promiscuous in guest encapsulation. Moreover, the bis-[2]catenanes derived from calix[4]arenes and calix[4]pyrroles should perform in a wider variety of conditions than their strictly supramolecular counterparts. To the best of our knowledge, molecular encapsulation experiments with bis-[2]catenanes derived from calix[4]arenes have not been reported. Here we describe the synthesis and chiral resolution of bis[2]catenane 3·5. We also present preliminary encapsulation experiments of interlocked dimer 3·5 with neutral molecules, ion-pairs and discrimination of chiral guests.
![Line drawing structures of aryl extended tetra-urea calix[4]pyrroles 4a and 4b, bis[2]catenane 3·5 and the guests used in this study.](/image/article/2012/SC/c1sc00668a/c1sc00668a-f2.gif) | ||
| Fig. 2 Line drawing structures of aryl extended tetra-urea calix[4]pyrroles 4a and 4b, bis[2]catenane 3·5 and the guests used in this study. | ||
Results and discussion
Following the chemistry developed by Böhmer,13 racemic bis[2]catenane 3·5 was synthesized by metathesis. In this reaction bis-loop 3 doubles as a template and the reaction partner for alkenyl calix[4]pyrrole 4b through the heterodimeric pseudo-[2]rotaxane 3·4b (Scheme 2). The required tetra-urea alkenyl calixpyrrole 4b was prepared by acylation of the corresponding tetraamine14 with the activated urethane.19 The dimerization of 3·4b can be easily followed by 1H NMR but requires the addition of a guest (N-oxide 6 or 8); bis-loop 3 alone does not induce the heterodimer assembly 3·4b. After metathesis and catalytic hydrogenation, the racemic bis[2]catenane 3·5 was isolated by column chromatography in 65% yield.![Synthesis of bis[2]catenane 3·5 as racemate.](/image/article/2012/SC/c1sc00668a/c1sc00668a-s2.gif) | ||
| Scheme 2 Synthesis of bis[2]catenane 3·5 as racemate. | ||
The 1H NMR spectrum of 3·5 in CHCl3-d solution showed both broad and sharp proton signals. The sharp signals correspond to hydrogen bonded urea NHs (Hb, Hc) appearing at δ = 9.2 ppm. Free NH pyrrole protons could easily be detected at δ = 8.6 ppm. Most likely, the filling of the capsule's cavity by CHCl3-d molecules alone or in combination with solvent impurities does not produce a single hydrogen bonded structure (Fig. 3c). The existence of dynamic equilibria between conformers, which are slow or intermediate on the NMR timescale, produces the observed complexity in the 1H NMR spectrum of “free” 3·5.
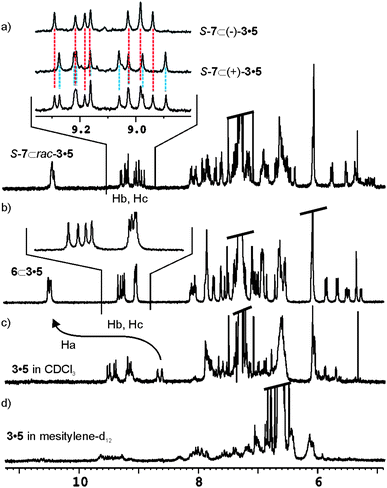 | ||
| Fig. 3 Downfield region of the 1H NMR spectra of a) SSS-7 ⊂ rac-3·5 in CHCl3-d, the inset contains an expansion comparing the urea NHs (Hb, Hc) in the racemic complex with those of SSS-7 ⊂ (+)-3·5 and SSS-7 ⊂ (−)-3·5, b) 6 ⊂ 3·5 in CHCl3-d, c) catenane 3·5 in CHCl3-d solution and d) catenane 3·5 in mesitylene-d12 solution. | ||
When 1 equivalent of N-oxide 6 or 8 is added to the above CHCl3-d solution, the proton signals of 3·5 simplify as a result of the formation of a unique and well defined hydrogen-bonded capsular structure induced by guest encapsulation (Fig. 3b). The pyrrole NH (Ha) protons move downfield from δ = 8.6 ppm to δ = 10.5 ppm, a change indicative of hydrogen bonding between the pyrrole NH and the oxygen atom of the encapsulated guest. Characteristic signals for the methyl groups of the encapsulated guests are also visible in the upfield region of the spectrum. The explanation to the high number of signals observed for certain protonsi.e.urea NHs, Hb and Hc, in the 1H NMR of the encapsulation complexes of 3·5 requires analysis of its symmetry properties.
Bis[2]catenane 3·5 has C2ν symmetry and is dissymmetric. The C2 axis coincides with the molecular axis passing through two centroids defined by the four meso carbons and the four methylene bridges, respectively (Fig. 4a).
![a) Top and side views of energy minimized hydrogen bonded capsuleS-(M,P)-3·5, non-chemically equivalent urea NHbprotons in the calix[4]pyrrole hemisphere are shown as yellow and purple balls and the C2 axis is indicated as a line; b) dynamic equilibria involving the two enantiomeric pairs of cyclodiastereoisomers of 3·5, the C2 axis and the directionality of the hydrogen-bonded belt are indicated.](/image/article/2012/SC/c1sc00668a/c1sc00668a-f4.gif) | ||
| Fig. 4 a) Top and side views of energy minimized hydrogen bonded capsuleS-(M,P)-3·5, non-chemically equivalent urea NHbprotons in the calix[4]pyrrole hemisphere are shown as yellow and purple balls and the C2 axis is indicated as a line; b) dynamic equilibria involving the two enantiomeric pairs of cyclodiastereoisomers of 3·5, the C2 axis and the directionality of the hydrogen-bonded belt are indicated. | ||
The sense of rotation of the urea groups in the hydrogen bonded belt of the capsule is unidirectional. For kinetically stable hydrogen bonds of the urea belt, the change in the directionality of the urea groups between clockwise and anticlockwise orientations is slow on the 1H NMR timescale. As a result, the C2v symmetry is reduced to C2 and an independent element of chirality is added to the assembly. Accordingly, racemic 3·5 and its encapsulation complexes (with achiral guests) are mixtures of two cyclo-diastereoisomers pairs that are enantiomers (Fig. 4b).This becomes clear in the analysis of the eight singlets (4 + 4), assigned to the urea NH protons adjacent to the m-substituted phenyl groups, Hb and Hc (see Fig. 2 for assignment). These resonate at low field (∼ δ = 9.2 ppm) in the 6 ⊂ 3·5 encapsulation complex (Fig. 3b). The change of senses of rotation of urea groups is responsible for the interconversion between cyclo-diastereoisomers, i.e. S-(P,M)-3·5 to S-(M,P)-3·5 (Fig. 4b).‡ Likewise, it also results in exchange between chemically non-equivalent NH protons in the same hemisphere. It is worth noting that even in THF-d8 solution at 333 K, the characteristic signals for the encapsulation complex 8 ⊂ 3·5 persist. This testifies to the considerable increased thermodynamic stability achieved by the mechanically interlocked components of the capsule.
As expected, the encapsulation of enantiopure N-oxide SSS-7 in racemic 3·5 produces 16 singlets (10 signals, of which two have triple intensity and two double) for the same urea proton NHs (Hb and Hc). This observation suggests that the mixture is composed by four diastereomeric encapsulation complexes as a consequence of the additional chiral element introduced by encapsulated SSS-7 (Fig. 3a).
The chiral resolution of the [2]bis-catenane 3·5 was achieved by semi-preparative HPLC on the chiral stationary phase Chiralpack IB with a mixture of MeOH/DCM/EtOH (45/10/45) containing 0.5% Et2NH as eluent. Enantiomer (−)-3·5 was first to elute from the column. The separated enantiomers show weak and opposite CD spectra and specific rotation values of [α]22435 = + 5° (c = 0.145 DCM) and [α]22435 = −4°.§ The isolation of the two enantiomers (+)/(−) 3·5 allowed the deconvolution of the 10 signals assigned to the urea NHs, Hb and Hc, in the encapsulation complex of racemic 3·5 with SSS-7 (Fig. 3a inset). The mixture composed of cyclo-diastereoisomers SSS-7 ⊂ (+)-(M·P)-3·5 and SSS-7 ⊂ (+)-(P·M)-3·5 produced 8 different signals for the NH protons. While the mixture of cyclodiastereoisomers constituted by SSS-7 ⊂ (−)-(M·P)-3·5 and SSS-7 ⊂ (−)-(P·M)-3·5 gave 7 different signals for the same NH protons, one with double intensity. The sum of the two different groups of protons coincides with the 10 signals obtained in the experiment with racemic 3·5 (Fig. 3a).
For enantiopure (+) or (−) 3·5 with SSS-7, the relative intensities of the two sets of urea proton signals corresponding to the two cyclo-diastereoisomers present are equivalent. Apparently, the transfer of chiral information from the encapsulated guest to the sense of rotation of the ureas is completely ineffective. Similar encapsulation experiments performed with enantiopure (+)-3·5 in the presence of an excess of racemic 7 also produced signals for the urea NHs of the four diastereomeric complexes with identical relative intensity. This result points to the complete lack of chiral discrimination in the recognition of N-oxide 7 with capsule 3·5. Disappointingly, the combination of inherent chirality and cyclochirality built into the structure of 3·5 proved to be inefficient for chiral discrimination in the cavity.20
The size of the internal cavity in 3·5 is 284 Å3. The volumes of N-oxide 6 and SSS-7 (80 and 120 Å3, respectively) are too small to fulfil the 55% packing coefficient rule.21 Putative co-encapsulation with one CHCl3 (77 Å3) molecule yields packing coefficients of 55 and 69% respectively. In order to demonstrate the co-encapsulation of one molecule of CHCl3 we performed GOESY experiments in a CHCl3 solution of the encapsulation complex 6 ⊂ 3·5. Selective excitation of the signal for the free solvent produced a difference spectrum with an additional signal of low intensity resonating at δ = 3.36 ppm (Δδ = −3.9 ppm). This upfield signal is assigned to the proton of CHCl3 co-encapsulated in 6 ⊂ 3·5 (Fig. 5b) where it experiences the shielding effect of the aromatic rings that shape the cavity of the capsule. The encapsulated CHCl3 is involved in slow chemical exchange on the NMR timescale with CHCl3 molecules in the bulk solvent. Interestingly, when the experiment is repeated using SSS-7 as encapsulated guest, three different additional signals (one with double intensity), in the region of 3.4–3.6 ppm, are observed for the co-encapsulated CHCl3. We related this observation to the presence of the four diasteromeric capsules (discussed above) exhibiting an increase in the anisotropy of their interiors due to the encapsulation of the chiral guest. This result augurs well for the use of 3·5 in further chiral recognition studies assisted by co-encapsulation of a chiral guest.22 Conversely, a ROESY experiment performed using 8 as encapsulated N-oxide did not show any additional signal for co-encapsulated CHCl3. Guest 8 with a volume of 177 Å3 produces a packing coefficient of 62% making the co-encapsulation of a solvent molecule unlikely.
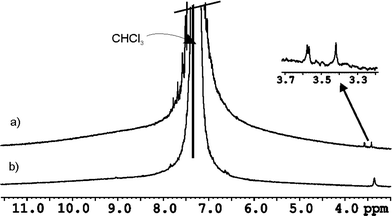 | ||
| Fig. 5 1H GOESY NMR spectra in non deuterated CHCl3 with selective excitation of the solvent signal: a) complex SSS-7 ⊂ rac-3·5 and b) complex 6 ⊂ 3·5. | ||
One of the most noticeable characteristics of the interlocked capsule 3·5 is its polarized inner space. The calix[4]arene pole consists of a shallow lipophilic cavity shaped by electron rich aromatic rings and is suitable for the inclusion of quaternary ammonium species.23 The calix[4]pyrrole pole features a deeper aromatic cavity closed by four pyrrole NHs and is suitable for the inclusion of anions24 and electron rich guests.25 It seemed to us that interlocked capsule 3·5 could be a perfect host for the encapsulation of ion-pairs of complementary size.26–28 Examples of ion-pair recognition in fully closed capsular assemblies are very scarce in literature.29,30 Molecular modelling studies (Fig. 6) suggested that tetramethyl ammonium chloride 9+·Cl−, tetramethyl phosphonium chloride 10+·Cl− or even tetraethyl phosphonium chloride 11+·Cl− were excellent fits for the aromatic cavity of the interlocked assembly 3·5. The 1H NMR spectra of a CHCl3-d solution containing 3·5 and one equivalent of 9+·Cl−, 10+·Cl− or 11+·Cl−, dissolved in MeOH-d3, show the earmarks of ion pair encapsulation. The pyrrolic NH (Ha) signals experience dramatic downfield shifts that are consistent with the formation of four hydrogen bonds to the chloride. We previously observed an analogous behaviour in the inclusion complexes of chloride with aryl extended calix[4]pyrroles.31 The methyl proton signal of cations 9+ and 10+ appear highly upfield shifted, at 0.9 and −0.4 ppm, respectively. The large upfield chemical shift (Δδ ≈ −1.4 ppm) is fully consistent with the encapsulation of the cation in the cavity of the calix[4]arene hemisphere. The 31P NMR spectrum of bound 10+ also reveals a strong upfield shift. DOSY 1H-NMR experiments indicated a considerable decrease in the diffusion coefficient of bound 10+ and a value coincident with that of the interlocked capsule 3·5 (see ESI, Figures S17–S19†).
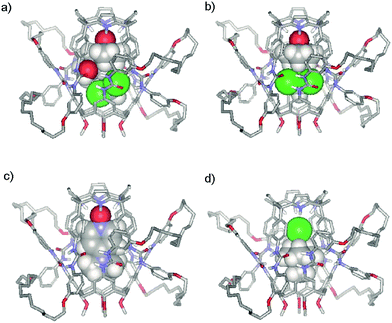 | ||
| Fig. 6 Energy minimized (CAChe/MM3) structures of encapsulation complexes: a) SSS-7·CHCl3 ⊂ 3·5, b) 6·CHCl3 ⊂ 3·5, c) 8 ⊂ 3·5 and d) 9+·Cl− ⊂ 3·5. Non polar hydrogen atoms of 3·5 have been removed for clarity. 3·5 is shown in stick representation and the included guests as CPK models. | ||
Fig. 7 depicts selected upfield and downfield regions of the 1H NMR spectrum of the encapsulation complex 11+·Cl− ⊂ 3·5 in which the same general trends are observed. When more that 1 equivalent of salt is added, separate signals for free and bound cation are detected. The slow chemical exchange experienced by the cation is indicative of its sequestration in a closed aromatic cavity.
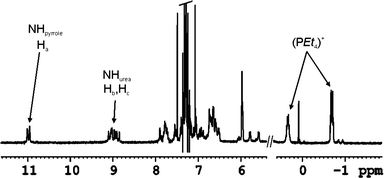 | ||
| Fig. 7 Selected regions of the 1H-NMR in CHCl3-d of the complex 11+·Cl− ⊂ 3·5. | ||
The 1H-NMR spectrum of bis-[2]catenane 3·5 in mesitylene-d12 solution shows very broad and undefined proton signals (Fig. 3d). Probably, mesitylene-d12 is worse than CHCl3-d for properly filling the internal cavity of 3·5. The reduced complementarity in size and shape that exists between solvent molecules and the interior of the molecular vessel produces an increase in the number of structures involved in conformational equilibria for free bis-[2]catenane 3·5. The larger N-oxide 8 was added as solid to the mesitylene-d12 solution of 3·5 and the resulting suspension was sonicated for several minutes. The 1H-NMR spectrum of the solution is very similar to the one recorded for the 8 ⊂ 3·5 encapsulation complex in CHCl3-d. In CHCl3-d or mesitylene-d12 solutions, the encapsulation of a single N-oxide 8 induces host 3·5 to adopt a defined and single hydrogen bonded structure due to the good match between volume sizes. In contrast, when a similar experiment is performed using N-oxide 6, the 1H NMR spectrum still shows broad and ill-defined signals. It is necessary to add a small amount of CHCl3 to obtain a 1H NMR spectrum showing proton signals diagnostic of capsule formation and co-encapsulation of both guests, 6 and CHCl3 (ESI, Fig. S21).
The co-encapsulation of both guests is required to complement 55% of the volume of the internal cavity of 3·5 inducing the formation of a single hydrogen bonded structure for the three molecule assembly. ROE experiments with selective excitation in the proton signal of free CHCl3 showed chemical exchange with a proton signal resonating a δ = 3.7 ppm (Fig. 8). We assigned this signal to the CHCl3 molecule co-encapsulated with trimethylamine N-oxide 6. While the proton chemical shift of free CHCl3 (δ = 5.9 ppm) in mesitylene-d12 is very different from the one measured in CHCl3-d (δ = 7.2 ppm), the chemical shift values for the co-encapsulated CHCl3 molecule compare well in both solvents (3.7 vs. 3.4 ppm). This result demonstrates that the capsular assembly is very effective in isolating the encapsulated CHCl3 molecule from the anisotropic effects produced by the bulk solvent. In short, the results described above clearly reveal the co-encapsulation of two electronically different neutral molecules or oppositely charged ions in the internal cavity of interlocked capsule 3·5.
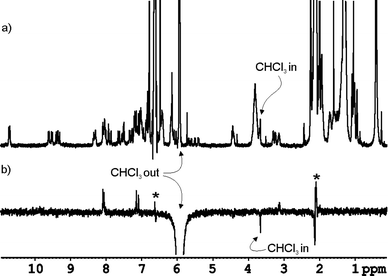 | ||
| Fig. 8 a) 1H NMR spectrum of 6·CHCl3 ⊂ 3·5 in mesitylene-d12 solution containing a reduced amount of free CHCl3, b) ROE experiment performed on the same sample by selective excitation of the free chloroform signal at 5.94 ppm. The inverted proton signal at 3.7 ppm is assigned to the encapsulated CHCl3 molecule being involved in slow chemical exchange with free CHCl3. Artifact signals are indicated with an asterisk. Positive proton signals are produced by NOE effect due to excitation of proton signals of the capsule buried under the CHCl3 peak. | ||
Conclusions
In summary, we have synthesized and resolved a dissymmetric mechanically locked capsule 3·5 with polar interior. The different functionalization of the inner space of the capsular assembly is provided by calix[4]pyrrole and calix[4]arene hemispheres. The former uses hydrogen bond interactions to recognize anions or N-oxide guests while the latter provides efficient cation–π and CH–π interactions for co-encapsulated guests. Capsule 3·5 is completely ineffective in chiral discrimination of N-oxide 7, however, it provides rare examples of ordered co-encapsulation in fully enclosed space. One large or two small, electronically different neutral molecules can be accommodated in the capsular space. Both oppositely charged ions of tetra-alkylammonium and phosphonium chloride salts are also sequestered in the cavity's interior. The anisotropic nature of the capsules' interior produces a single spatial arrangement of included guest or co-guests. The co-encapsulation of two guests equipped with additional reacting groups is currently under investigation to test the properties of 3·5 as a molecular vessel.32Acknowledgements
We are grateful for funding from MICINN (CTQ2008-00222/BQU, Consolider Ingenio 2010 Grant CSD2006-0003), DURSI (2009SGR6868) and ICIQ Foundation. M. C. thanks MICINN for a Juan de la Cierva contract. We also thank Prof. Julius Rebek Jr. (The Scripps Research Institute, La Jolla, USA) for assistance with editing the revised version of the manuscript.Notes and references
- K. D. Shimizu and J. Rebek, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 12403–12407 CrossRef CAS.
- O. Mogck, V. Bohmer and W. Vogt, Tetrahedron, 1996, 52, 8489–8496 CrossRef CAS.
- O. Mogck, E. F. Paulus, V. Bohmer, I. Thondorf and W. Vogt, Chem. Commun., 1996, 2533–2534 RSC.
- D. N. Reinhoudt, I. Higler, P. Timmerman and W. Verboom, Eur. J. Org. Chem., 1998, 2689–2702 Search PubMed.
- J. Scheerder, R. H. Vreekamp, J. F. J. Engbersen, W. Verboom, J. P. M. vanDuynhoven and D. N. Reinhoudt, J. Org. Chem., 1996, 61, 3476–3481 CrossRef CAS.
- M. Janke, Y. Rudzevich, O. Molokanova, T. Metzroth, I. Mey, G. Diezemann, P. E. Marszalek, J. Gauss, V. Bohmer and A. Janshoff, Nat. Nanotechnol., 2009, 4, 225–229 CrossRef CAS.
- J. Rebek, Chem. Commun., 2000, 637–643 RSC.
- V. Bohmer, Y. Rudzevich and V. Rudzevich, Macromol. Symp., 2010, 287, 42–50 CrossRef.
- R. K. Castellano, B. H. Kim and J. Rebek, J. Am. Chem. Soc., 1997, 119, 12671–12672 CrossRef CAS.
- M. O. Vysotsky, A. Bogdan, L. Y. Wang and V. Bohmer, Chem. Commun., 2004, 1268–1269 RSC.
- L. Y. Wang, M. O. Vysotsky, A. Bogdan, M. Bolte and V. Bohmer, Science, 2004, 304, 1312–1314 CrossRef CAS.
- A. Bogdan, Y. Rudzevich, M. O. Vysotsky and V. Bohmer, Chem. Commun., 2006, 2941–2952 RSC.
- O. Molokanova, A. Bogdan, M. O. Vysotsky, M. Bolte, T. Ikai, Y. Okamoto and V. Bohmer, Chem.–Eur. J., 2007, 13, 6157–6170 CrossRef CAS.
- P. Ballester and G. Gil-Ramirez, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10455–10459 CrossRef CAS.
- P. Ballester, G. Gil-Ramirez and M. Chas, J. Am. Chem. Soc., 2010, 132, 2520–2521 CrossRef.
- A. Szumna and B. Kuberski, Chem. Commun., 2009, 1959–1961 Search PubMed . For other examples of molecular capsules with polar interiors see: J. L. Atwood, L. J. Barbour and A. Jerga, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4837–4841 CrossRef CAS.
- M. Chas, G. Gil-Ramirez and P. Ballester, Org. Lett., 2011, 13, 3402–3405 CrossRef CAS.
- G. Diezemann, T. Schlesier, T. Metzroth, A. Janshoff and J. Gauss, J. Phys. Chem. B, 2011, 115, 6445–6454 CrossRef.
- A. Bogdan, M. O. Vysotsky, T. Ikai, Y. Okamoto and V. Bohmer, Chem.–Eur. J., 2004, 10, 3324–3330 CrossRef CAS.
- A. Szumna, Chem.–Eur. J., 2009, 15, 12381–12388 CrossRef CAS.
- S. Mecozzi and J. Rebek, Chem.–Eur. J., 1998, 4, 1016–1022 CrossRef CAS.
- J. Rebek, A. Scarso, A. Shivanyuk and O. Hayashida, J. Am. Chem. Soc., 2003, 125, 6239–6243 CrossRef.
- J. M. Harrowfield, W. R. Richmond and A. N. Sobolev, J. Inclusion Phenom. Mol. Recognit. Chem., 1994, 19, 257–276 CrossRef CAS.
- P. A. Gale, J. L. Sessler, V. Kral and V. Lynch, J. Am. Chem. Soc., 1996, 118, 5140–5141 CrossRef CAS.
- W. E. Allen, P. A. Gale, C. T. Brown, V. M. Lynch and J. L. Sessler, J. Am. Chem. Soc., 1996, 118, 12471–12472 CrossRef CAS.
- J. L. Atwood and A. Szumna, Chem. Commun., 2003, 940–941 RSC.
- J. L. Atwood and A. Szumna, J. Am. Chem. Soc., 2002, 124, 10646–10647 CrossRef CAS.
- J. L. Sessler, S. K. Kim, D. E. Gross, C. H. Lee, J. S. Kim, V. M. Lynch, L. H. Delmau and B. P. Hay, J. Am. Chem. Soc., 2010, 132, 5827–5836 CrossRef.
- P. Ballester, Chem. Soc. Rev., 2010, 39, 3810–3830 RSC.
- P. Ghosh and M. Arunachalam, Chem. Commun., 2011, 47, 8477–8492 RSC.
- G. Gil-Ramirez, E. C. Escudero-Adan, J. Benet-Buchholz and P. Ballester, Angew. Chem., Int. Ed., 2008, 47, 4114–4118 CrossRef CAS.
- M. Fujita, M. Yoshizawa and J. K. Klosterman, Angew. Chem., Int. Ed., 2009, 48, 3418–3438 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedure for the synthesis of SSS-7, racrac-7, alkenyl tetra-urea calix[4]pyrrole 4b and bis-[2]catenane 3·5 together with their characterization data. 1H NMR spectra of the encapsulation complexes and other 1D and 2D NMR experiments described in the text. See DOI: 10.1039/c1sc00668a |
| ‡ The absolute configuration (R or S) assigned to the bis-[2]catenane enantiomers is based on the sense of the twist required to superimpose the loops facing the observer with the one in which they are threaded. The (P/M) notation defines the cyclochiral conformation of each binding unit viewed from a position located in the middle of the capsule and facing their respective cavities. In this paper, we always refer first the cyclochiral conformation of the calix[4]arene moiety. Due to the complementarity that exists between the senses of rotation of the urea groups of the two components of the capsule. The cyclochirality conformation of the calix[4]pyrrole unit is automatically defined as the reverse. |
| § The correlation between absolute configuration of the bis-[2]catenane 3·5 and sign of optical rotation is not known. |
| This journal is © The Royal Society of Chemistry 2012 |
