Sustainable production of chemicals – an educational perspective†‡
Marco
Eissen
*
Gymnasium Ganderkesee, Am Steinacker 12, 27777 Ganderkesee, Germany. E-mail: marco.eissen@web.de; Tel: +49 4222 920197
First published on 21st March 2012
Abstract
Sustainability is a very general term and the question arises how to specify it within daily laboratory work. In this regard, appropriate metrics could support a socially acceptable, ecological and economic product development. The application of metrics for sustainability should be strengthened in education, because they do not belong to standard considerations in synthesis design so far. This article describes educational material (online resource) designated to students and chemists who are willing to quantify the benefits of green chemistry in the lab and to search for an optimization potential. As an example, bromination reactions are compared by considering mass balance, raw material costs and qualitative aspects such as, e.g., toxicology and combustibility. While keeping in mind the three dimensions of sustainability – economy, environment and social affairs – the idea of the concept of sustainable chemistry, i.e., green chemistry, takes shape in daily laboratory work.
Introduction
In the educational literature a couple of suggestions are made how metrics can be applied in synthesis design to let green chemistry become a habit of mind (Table 1). The term ‘metric’ denotes a ratio or number which indicates the performance of an object of study.| Chemical synthesis | Metrics | Literature | ||
|---|---|---|---|---|
| AEa | Eb | other | ||
| a Atom economy (Trost, 1991, Eissen et al., 2004b). b Environmental factor (Sheldon, 1994). | ||||
| Bromination | ✓ | ✓ | ✓ | (McKenzie et al., 2005) |
| Nitrosation | ✓ | ✓ | ✓ | (Van Arnum, 2005) |
| Grignard (and many others) | ✓ | ✓ | ✓ | (Andraos and Sayed, 2007) |
| Friedel–Crafts | ✓ | (✓) | (Ranke et al., 2008) | |
| Suzuki | ✓ | (Aktoudianakis et al., 2008) | ||
| Biginelli | ✓ | (Aktoudianakis et al., 2009) | ||
| Pechmann condensation | ✓ | ✓ | (Young et al., 2010) | |
| Ionic liquids | ✓ | ✓ | ✓ | (Stark et al., 2010) |
| Metal-acetylacetonate | ✓ | ✓ | ✓ | (Ribeiro and Machado, 2011) |
Whereas the concept of atom economy (AE) (Trost, 1991, Eissen et al., 2004b) takes only a look at the efficiency of the reaction stoichiometry, the environmental factor (E-factor; Sheldon, 1994; relation waste/product) which is similar to the mass intensity (MI; Constable et al., 2002; relation raw material/product) considers all substance masses. As mass intensity does not correlate with atom economy, the latter alone will not deliver the desired ‘cleaner’ process (Curzons et al., 2001). In this regard it is noteworthy that two of the listed examples (Table 1) admittedly use the label ‘green’ in the article title, but confine themselves to atom economy and ignore catalyst and solvent amounts. Apparently, calculations that go beyond atom economy necessitate a certain expenditure. This might be a reason for a preferred reference in professional literature to rather qualitative criteria (if at all) such as the twelve principles of green chemistry (Anastas and Warner, 1998) than to substantiate (Andraos, 2009) claims of ‘greenness’ with some kind of metrics analysis, i.e., to substantiate the validity of the principles (Winterton, 2001). E.g., efficiency of prinicple 9 by Anastas and Warner (1998) “Catalytic reagents are superior to stoichiometric reagents” can simply be expressed (together with principles 2 and 8) by the E-factor because the amount of (catalyst, etc.) waste objectively and comparable reflects the efficiency of (alternative) syntheses (Eissen et al., 2003a). The industrial efforts for both environmental protection and economic profitability by means of production-integrated measures assembled to a conceptional picture around 1990 (Wiesner et al., 1995) and principles partly being similar to the green chemistry ones were formulated. In academia, however, a green look on chemical synthesis that rests upon a quantitative assessment seems to have increasingly emerged only recently (Table 2). Regarding a so-called life cycle assessment (systematic analysis of the environmental impacts of products during their total lifespan), efforts for its integration are even sparser because much more information, e.g., concerning energy demand, have to be collected and interpreted.
Besides environmental issues, material flow management (not only for the chemical but also for textile, automobil, etc., sectors) has to be concerned with social and economic aspects too (see, e.g., Enquete Kommission “Schutz des Menschen und der Umwelt” des Deutschen Bundestages, 1994).
Therefore, the objective of the material discussed in this article is to create a learning situation in which a student is prompted to weigh up these three dimensions for a concrete synthetic example (bromination reactions). Thus, she or he will have to understand, recognise and interpret the interconnections of the different dimensions in dialogue within a cooperative learning scenario (see Wheeler, 2000 cited according to Burmeister et al., 2012) – this training can be understood as an education for sustainability rather than about sustainability (McKeown, 2006). As a prerequisite, raw material demand and waste production will have to be quantified and related calculations have to be understood. Metrics should become a natural part in synthesis design in order to back up claims of synthetic efficiency in research (Andraos, 2009) as well as in education. This article will provide the reader teaching material that can be employed in upper secondary and higher education and will report about its application in initial educational experiences. See Table 2 for a range of examples in chemical research.
Mass efficiency and life cycle assessment for sustainability
According to the Brundtland Report (United Nations, 1987) sustainable development means: “Sustainable development is development that meets the needs of the present without compromising the ability of future generations to meet their own needs.”As the development and safeguarding of wealth in a sustainable manner is conceivable only in the framework of social harmony, only in accordance with a sound environment and on the basis of stable resource availability, are the three dimensions of environment, economy and social affairs equally involved. Chemistry, as one of the most important branches, can contribute to a sustainable development, because it represents a starting point for important mass flows (Eissen et al., 2002, Böschen et al., 2003, Eissen et al., 2004a). The “Conservation and management of resources for development” is one of the main foci of interest in Agenda 21 (United Nations, 1992). The utilization of resources must not exceed the regeneration rate just as little as the emission of substances should exceed the degradation/processing rate within the environment.
However, this goal can only be measured and controlled in a few areas of life. Therefore, a pragmatic approach is to choose the least harmful path. Comparing several products, one should prefer the option which
| - has low costs | e.g. for facilities, raw material extraction, product use → economy |
| - is more social | e.g. by fewer accidents, more jobs, no children's work → social affairs |
| - protects the environment | e.g. by less waste, emissions, raw materials, land use, by advantageous product properties → environment |
Balancing and evaluating potentially opposing results is time consuming and in most cases practically impossible in class, especially against the background of the “green chemistry paradox” of using hazardous chemistry to develop improved production-scale processes (Ritter, 2010). A life cycle assessment (LCA), which highlights only one dimension of the three aspects, will hardly be conducted in industry due to the effort and costs. There are some essential approaches in large enterprises such as the eco-efficiency analysis (Saling et al., 2005, Saling, 2008) at BASF and the fast life cycle assessment of synthetic chemistry (FLASC) at GSK (Curzons et al., 2007). However, they are too complex and time consuming to be applied in secondary or undergraduate chemistry teaching.
Anyhow, in order to gain an initial overview, a simple mass balance can help, i.e., an illustration of how much raw material or waste can be recorded per product unit. Two alternative syntheses in the production of (R)-2-hydroxy-4-phenylbutyric acid (ethylester) (Scheme 1) will serve as an example. The mass balance of the reaction (Fig. 1) shows a relatively high auxiliary material demand and a high amount of sewage (in kilogram per kilogram product) for variant A, while it reveals that variant B is overall more advantageous. Remarkably, a complete life cycle assessment (Fig. 2) according to the method of EcoIndicator 95 shows the same result. As can be seen here, extraction (M4) and the subsequent recycling (M8) most adversely affects the sum of eco points: at the end the stepped line shows more points (Eco 95) for variant A than for variant B. There is also another case study showing that an LCA result is also obtained by a simpler approach (Ravelli et al., 2010). Another LCA examination (Jimenez-Gonzalez et al., 2011) provides support by the estimate that mass related metrics such as the process mass intensity (PMI), which, due to E-factor = PMI–1, is a kind of ‘brother’ of the E-factor, seems to be very reliable.
 | ||
| Scheme 1 Two possible syntheses (variants A and B) of (R)-2-hydroxy-4-phenylbutyric acid (ethylester); a substance which is used in the production of pharmaceuticals (Blaser et al., 2003). | ||
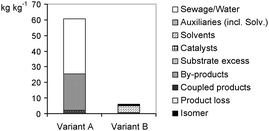 | ||
| Fig. 1 waste (kg) of both variants per kg product. | ||
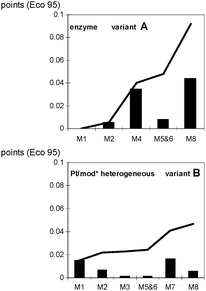 | ||
| Fig. 2 Eco-points for both variants of Scheme 1. M1 = catalyst; M2 = reduction; M3 = catalyst removal; M4 = extraction; M5 = solvent drain off; M6 = rectification; M7 = purification; M8 = solvent recycling. Reprinted from Jödicke et al., 1999, with permission from Elsevier. | ||
Teaching approach
Background of the lessons
The above discussed aspects have been worked out into a lesson plan based on different worksheets given in the online resource. The lesson plans were structured for advanced upper secondary or undergraduate chemistry education.For conducting the lesson successfully, students need a basic knowledge in both practical and theoretical organic chemistry. Students should be familiar with basic lab operations such as distillation, extraction, washing and drying as well as with functional groups, such as double bonds and esters, and basic organic reactions such as hydrogenation and bromination. However, in principle these basic lab operations, as well as the test tube reaction of an aqueous solution of bromine with cyclohexene (in juxtaposition to the non-reaction with cyclohexane), can also be explained during the course. In case the curriculum envisages oxidation numbers as another topic, related material (Eissen et al., 2011b) could be considered beforehand.
The goal of the teaching material is to explain how mass related metrics (Commitee on Industrial Environmental Performance Metrics et al., 1999, Curzons et al., 2001, Constable et al., 2002, Andraos, 2005, Augé, 2008, Calvo-Flores, 2009, Augé and Scherrmann, 2012), such as the mass index (MI, PMI or S−1; Constable et al., 2002)
can both be used in order to evaluate the environmental performance of alternative synthetic pathways on an introductory level.
Aside from the overall number it is more important which category (e.g., solvents, auxiliary materials, coupled product, etc.) and especially which substance(s) contribute most to waste production in order to detect the optimization potential to save raw materials and prevent waste production. The combination of mass index (1) with substance prices delivers the costs in, e.g., Dollar or Euro per kg product. At least raw material costs can thereby be considered as one aspect of the economic dimension. One can consider health and safety aspects as part of the social dimension of a production process, namely the physical integrity of employees and population. Instead of utilizing metrics as, e.g., suggested by Eissen and Metzger (2002b), however, the qualitative discussion of, e.g., toxicity and combustibility is initially considered sufficient within the material offered.
The specific exemplary juxtaposition of mass balance and life cycle assessment shows the existence of different assessment qualities on one hand and, on the other, that a simple mass balance can, of course with due care, already deliver a sensible statement in the early phase of synthesis design. This is in line, e.g., with the German Federal Environmental Agency (Umweltbundesamt, 2009) which demands integrating an economical utilization of energy and material resources in the planning of syntheses. Regarding education, the agency points to the materials made available online by the internet organic chemistry lab course (Bahadir et al., Ranke et al., 2008). Insofar, the opportunity for a student to collect basic and partially crucial information about alternative syntheses should motivate an independent grapple with a case study.
Considering a familiar reaction (bromination of alkenes), alternative variants can be examined and discussed (Scheme 2). Surely, the information will not make a final decision possible. However, in this case the journey is the reward: qualitative differences, e.g., regarding toxicology or waste production, support the students' assessment competence, sensitize them for different environmental categories and provide insight into the activities of a chemist in the laboratory, who has to consider all these aspects and discusses them with chemical engineers. A corresponding series of worksheets is available for this purpose (Table 3). An information sheet precedes them, presenting the context which has been mentioned in the preceding passage and which relativizes the validity of mass balances versus life cycle assessments to a certain extent by means of a further case study (Eissen et al., 2008).
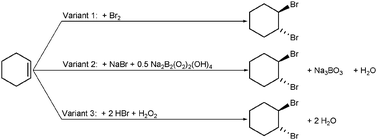 | ||
| Scheme 2 Bromination of alkenes by means of (1) bromine (Snyder and Brooks, 1943), (2) sodium bromide and perborate (Kabalka et al., 1998) and (3) hydrogen bromide and hydrogen peroxide (Barhate et al., 1999). | ||
The variants presented in Scheme 2 only represent a selection from many (Eissen and Lenoir, 2008). Further synthetic alternatives reverting to oxygen as oxidant can be found in the literature (Podgoršek et al., 2009a, Podgoršek et al., 2009b). The worksheets (Table 3) deal with metrics such as yield, mass index, environmental factor E (for variant 1 see eqn (1), Fig. 3) and cost index (Fig. 4).
 | (1) |
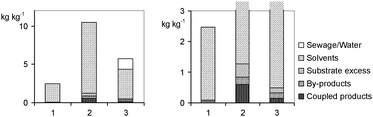 | ||
| Fig. 3 Environmental factor E for three syntheses of Scheme 2. A zoomed presentation can be found on the right hand side, in order to better identify coupled and by-products. | ||
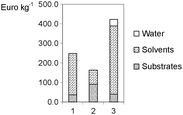 | ||
| Fig. 4 Raw material costs (see worksheet Table 3) for the production of dibromocyclohexane (Scheme 2). Water, which is presented in protocol 3, belongs to the solutions of hydrogen bromide and hydrogen peroxide. | ||
| Work sheet/Content | Problem/Content | Cognitive skills |
|---|---|---|
| 1 Three synthesis protocols of a bromination | 1 Oxidation reaction from bromide ions to bromine | Knowledge/Remembering (of oxidation state and electron donor and acceptor relations) |
| 2 Yield of a reaction | Knowledge/Remembering (of the product – key-substrate ratio) | |
| 3 Criteria for the selection of a synthesis protocol | Comprehension/Understanding (of the information sheet) | |
|
2 Concretisation of the three dimensions of sustainability with corresponding topics:
1. Resource efficiency 2. Production of waste materials 3. Raw material costs … (continued on worksheet 3) |
4 Ratio of substrate and product amount (→ Topic 1) | Application/Applying (of the mass index S−1) |
| 5 Amount of by-products and substrate excess (→ Topic 2) | Application/Applying (of stoichiometric substrate consumption and of the yield (see Problem 2)) | |
| 6 Ratio of waste and product amount (→ Topic 2) | Application/Applying (of environmental factor E) | |
| 7 Raw materials and waste of three protocols (→ Topics 1 and 2) | Evaluation/Evaluating (of substance amounts regarding synthetic efficiency) | |
| 8 Software support being available for Problems 4 to 6 in order to receive relevant data (Problems 7 and 9). | Application/Applying (of a new software tool) | |
| 9 Substance costs (→ Topic 3) | Simple multiplication procedure | |
| 3 … | 10 Environmental, health, safety and costs as parameters relevant for the assessment of the sustainability (→ all topics) | Evaluation/Evaluating (of substance amounts and cost as well as qualitative criteria (e.g., toxicity, flammability) regarding synthetic efficiency) |
| 4. Hazards of applied substances | ||
| R-phrases of considered chemical substances |
In principle, these calculations are not a prerequisite, since the results are indicated on the worksheets. Finally, the interpretations of results should remain at the centre of the worksheets and to a lesser extent the way in which they were obtained. Such examinations are so fundamental that they can be/are applied within research at universities, and in industry (see Box 1 in the literature (Eissen et al., 2008, Eissen et al., 2010)).
Course of the lessons
The comparison of the three variants represents the actual goal, which is linked with the preparation of a mass balance (Table 3, Problem 7). The raw material and waste amounts have to be interpreted sensibly. Here, as in reality, the varying data quality plays a role: for two variants the quantitative information regarding the work up is missing. It has to be pointed out that the result of a mass balance is already given. This is provided on the basis that a discussion of the synthesis variants is available and does not have to be created only by (software assisted) calculation. The environmental factor and the mass intensity can be viewed in a more differentiated fashion than simply juxtaposing the metrics. The sample solution to Problem 7 highlights the waste treatment – a perspective which is rather unfamiliar to young students. Therefore, the worksheet promotes an early sensitization for the isolation part of a synthesis and, in this regard, is considered to be an important contribution to a chemists' education.
After all, the calculation is only a means to an end. The same is valid for Problem 9 (Table 3), in which the costs per kilogram raw material and the used substance amounts are already given in order to compile a cost index (Fig. 4). Determination of substance amounts can be tracked by applying the software EATOS (Table 3; Problem 8). List prices, which are usually not noted in Euro/kg but, for example, in Euro per 2.5 L (see sample solution), can be recorded in EATOS, which converts automatically to Euro/kg. However, the tasks are designed in a way that a comparison of the given synthetic protocols is possible, so that Problem 8 (application of EATOS) could simply be ignored.
Experiences and evaluation
The described teaching was tested in advanced upper secondary chemistry education. Three learning groups of about 8 to 15 students respectively worked with the lesson material (total number N = 35).The information sheet was read and discussed within half an hour. The time demand was about 40–50 min for worksheet 1 (Problem 1–3), 60 min for worksheet 2 (Problem, 4–9, Problem 8 was left out) and 45 min for worksheet 3 (Problem 10) (Table 2). In one learning group the above mentioned basic knowledge on the conversion of cyclohexene and cyclohexane with an aqueous bromine solution was communicated prior to working with the worksheets. The final Problem 10 could be understood as an extention of Problem 7. The latter only required 15 min in most cases. The students quickly identified the first pathway to be the most resource efficient procedure. The question as to what extent inherently safe substances can be weighed up with technological solutions for problematic substances is an interesting subject for a discussion (compare “green chemistry paradox” (Ritter, 2010)). That is, Problems 7 and 10 prompted the students to weigh up different aspects and to evaluate them, what is the highest level of requirement among the problems listed in Table 3. In two classes, most aspects were mentioned in a written statement. Interestingly, the economic performance (see Fig. 4 and solution to Problem 9) and toxicology of substances seemed to have a crucial impact on the students' opinion. More than half of the students considered pathway 2 of Scheme 2 to be preferable. Thus, the instructor should pay intention that waste disposal costs (e.g., for sodium borate) and potential solvent recycling are included in the discussion as well as substrate costs which might become more relevant for more expensive alkenes. In another class, unnamed aspects were integrated into the discussion by providing the sample solution. This was considered helpful to expand the students' perspective.
There seemed to be a need for a certain prearrangement. Necessary information has to be gathered (Problems 4, 6 and 9) by application of distinctive procedures using corresponding metrics. According to the students who where asked for their opinion, Problem 4 to 6 (Table 3) contributed to an understanding of the calculation procedures. The time demand to use EATOS to reproduce the data shown in the worksheet – Table 1 and 2 was about 40 min (worksheet 2). In the students' opinion the software greatly facilitated the amount of work and they appreciated the indication of results in the worksheet for the purpose of control.
Going further back to the logic of the problem composition, Problems 2, 3 and 5 were considered appropriate to prepare and support the cognitive capability for Problems 4, 6 and 9. They delivered the knowledge of calculation procedures making transparent the generation of relevant data, e.g., concerning waste production. However, some students had problems in successfully calculating the by-product amount (Table 3, Problem 5). The discussion with the instructor was necessary to make clear how to understand the theoretical mass flow within a stoichiometric reaction equation.
After the lesson plan, a Likert-questionnaire (5-step) was administered to the students in two of the learning groups (N = 22). The questionnaire comprised of 15 questions on the students self-reflection of the learning process, their perception of the changed focus of the lesson plan, and the consideration of the teaching materials. Feedback was collected from 22 students.
Overall, the students gave very positive feedback. The questions on the sustainability criteria and assessment competencies concerning mass balances and economic, ecological and social aspects were answered most affirmatively. The students were felt to have got an understanding of assessing criteria in the green chemistry context. They expressed their feeling to be able to assess chemical processes in the foreground of sustainability issues. The triple result of 73% and 27% is coincidental, the composition of students in the different groups of answers is different for these questions. The same is true for the questions related to the mass index and environmental factor (Table 4). The students agreed to have predominantly received a different view on chemistry and that they got an insight into chemistry's efforts to support sustainability. The wider focus of the lessons based on these teaching materials was widely acknowledged and helped the students in understanding how chemical processes are assessed (Table 5). Also feedback on the teaching material was very positive. The teaching materials seem to be feasible and interesting to the students (Table 6).
| agree | rather agree | undecided | rather do not agree | do not agree | |
|---|---|---|---|---|---|
| I can state criteria for the assessment of sustainability of chemical syntheses. | |||||
| 73% | 27% | 0% | 0% | 0% | |
| I can assess synthetic protocols regarding resource demand and waste production. | |||||
| 73% | 27% | 0% | 0% | 0% | |
| I understood what the mass index is. | |||||
| 50% | 32% | 18% | 0% | 0% | |
| I understood what the E-factor is. | |||||
| 50% | 36% | 9% | 0% | 5% | |
| I have learned to assess synthesis procedures regarding economy, ecology and social aspects. | |||||
| 73% | 27% | 0% | 0% | 0% | |
| agree | rather agree | undecided | rather do not agree | do not agree | |
|---|---|---|---|---|---|
| The teaching unit equipped me with a different view of chemistry. | |||||
| 32% | 45% | 9% | 14% | 0% | |
| The teaching unit delivered insight into the efforts of chemistry for sustainability. | |||||
| 41% | 41% | 18% | 0% | 0% | |
| It is important that impacts of chemistry on environment, economy and society will be reflected in education | |||||
| 55% | 36% | 5% | 5% | 0% | |
| I would have preferred to only devote myself to synthetic procedures and formulas. | |||||
| 0% | 5% | 18% | 36% | 41% | |
| The teaching unit vitalized my capabilities to better assess chemical processes. | |||||
| 36% | 50% | 14% | 0% | 0% | |
| agree | rather agree | undecided | rather do not agree | do not agree | |
|---|---|---|---|---|---|
| a This question was only answered by those thirteen persons who only recently worked with the computer tool. | |||||
| The information was understandable and reasonable. | |||||
| 41% | 41% | 18% | 0% | 0% | |
| I liked the application of the computer software.a | |||||
| 54% | 23% | 23% | 0% | 0% | |
| The tasks were too difficult. | |||||
| 0% | 0% | 36% | 41% | 23% | |
| I was able to cope well with the teaching material. | |||||
| 19% | 62% | 19% | 0% | 0% | |
| The problems were interesting and challenging. | |||||
| 9% | 59% | 23% | 9% | 0% | |
Conclusions and implications
The focus of this article is learning about evaluating efficient resource utilization and production in chemical synthesis and provides training opportunities (Hansmann et al., 2010) also for communication skills. Along with the developed tasks and worksheets, students dealt with aspects of safety, health and environment, using three bromination reactions as an example. Mass balances, raw material costs and qualitative properties (toxicology, flammability, etc.) were considered against the background of the concept sustainable chemistry. By these means, the material described in this article was designed to contribute to new curricula which are desired for a green chemistry culture (Tucker, 2010), and is considered to be appropriate by the students.From the experiences, the lesson plan proved to be feasible and motivating for students on upper secondary level. Its design and demands should also be suitable for students on undergraduate level, when it comes to integrating a quantitative approach into green chemistry efforts. For a quantitative look also at qualitative characteristics such as toxicology, see, e.g., Eissen and Metzger (2002b), Saling et al. (2005), Martins et al. (2007), or Saling (2008).
Of course, there are also alternative areas which are part of the concept of sustainable chemistry and are worthwhile to consider. Emissions could be a further subject, e.g., their distribution and spreading in the environment or strategies for their avoidance. The latter is, amongst others, the subject in the consideration of musk fragrances (Marks and Eilks, 2010) and pharmaceuticals (Eissen and Backhaus, 2011) in the environment. The herein described lesson plan can be expanded to these areas and the gained knowledge and competencies might be transferred to other areas for building up a more settled understanding of chemistry's contribution to sustainable development.
Notes and references
- Akerman C. O., Gaber Y., Ghani N. A., Lämsä M. and Hatti-Kaul R., (2011), Clean synthesis of biolubricants for low temperature applications using heterogeneous catalysts, J. Mol. Catal. B: Enzym., 72, 263–269.
- Aktoudianakis E., Chan E., Edward A. R., Jarosz I., Lee V., Mui L., Thatipamala S. S. and Dicks A. P., (2008), “Greening Up” the Suzuki reaction, J. Chem. Educ., 85, 555–557.
- Aktoudianakis E., Chan E., Edward A. R., Jarosz I., Lee V., Mui L., Thatipamala S. S. and Dicks A. P., (2009), Comparing the Traditional with the Modern: A Greener, Solvent-Free Dihydropyrimidone Synthesis, J. Chem. Educ., 86, 730–732.
- Anastas P. T. and Warner J. C., (1998), Green Chemistry: Theory and Practice, Vol. Oxford University Press, Oxford.
- Andraos J., (2005), Unification of Reaction Metrics for Green Chemistry: Applications to Reaction Analysis, Org. Process Res. Dev., 9, 149–163.
- Andraos J., (2006), On using tree analysis to quantify the material, input energy, and cost throughput efficiencies of simple and complex synthesis plans and networks: Towards a blueprint for quantitative total synthesis and green chemistry, Org. Process Res. Dev., 10, 212–240.
- Andraos J., (2009), Global Green Chemistry Metrics Analysis Algorithm and Spreadsheets: Evaluation of the Material Efficiency Performances of Synthesis Plans for Oseltamivir Phosphate (Tamiflu) as a Test Case, Org. Process Res. Dev., 13, 161–185.
- Andraos J. and Sayed M., (2007), On the use of “green” metrics in the undergraduate organic chemistry lecture and lab to assess the mass efficiency of organic reactions, J. Chem. Educ., 84, 1004–1010.
- Augé J., (2008), A new rationale of reaction metrics for green chemistry. Mathematical expression of the environmental impact factor of chemical processes, Green Chem., 10, 225–231.
- Augé J. and Scherrmann M.-C., (2012), Determination of the global material economy (GME) of synthesis sequences – a green chemistry metric to evaluate the greenness of products, New J. Chem., 36, 1091–1098.
- Bahadir M., Hopf H., Nijakowski A., Vogt R., Jastorff B., Störmann R., Ranke J., Doose C., Ondruschka B., Kreisel G., Nüchter M., Diehlmann A., Lenoir D., Parlar H., Wattenbach C., Metzger J. O., Biermann U., Eissen M., Ohmstede C., Reimer J., König B., Braig C. and Krelle B., Sustainability in the organic chemistry lab course (NOP, Nachhaltigkeit (sustainability), Organische Chemie (organic chemistry), Praktikum (lab course)). An Internet Laboratory Book (in Arabic, German, Greek, English, Indonesian, Italian, Russian, Turkish).
- Barhate N. B., Gajare A. S., Wakharkar R. D. and Bedekar A. V., (1999), Simple and Practical Halogenation of Arenes, Alkenes and Alkynes with Hydrohalic Acid/H202 (or TBHP), Tetrahedron, 55, 11127–11142.
- Blaser H.-U., Eissen M., Fauquex P. F., Hungerbühler K., Schmidt E., Sedelmeier G. and Studer M. (2003), Comparison of Four Technical Syntheses of Ethyl (R)-2-Hydroxy-4-Phenylbutyrate. In: Blaser H-U, Schmidt E (eds) Large-Scale Asymmetric Catalysis. Wiley-VCH, Weinheim, p 91–104.
- Böschen S., Lenoir D. and Scheringer M., (2003), Sustainable Chemistry: Starting points and Prospects, Naturwissenschaften, 90, 93–102.
- Burmeister M., Rauch F. and Eilks I., (2012), Education for Sustainable Development (ESD) and Chemistry Education, Chem. Educ. Res. Pract., 13(2) 10.1039/c1rp90060a.
- Calvo-Flores F. G., (2009), Sustainable Chemistry Metrics, ChemSusChem, 2, 905–919.
- Climent M. J., Corma A., Iborra S., Mifsud M. and Velty A., (2010), New one-pot multistep process with multifunctional catalysts: decreasing the E factor in the synthesis of fine chemicals, Green Chem., 12, 99–107.
- Committee on Industrial Environmental Performance Metrics, National Academy of Engineering, National Research Council, (1999), Industrial environmental performance metrics: challenges and opportunities, Vol. National Academy Press, Washington, D.C.
- Constable D. J. C., Curzons A. D. and Cunningham V. L., (2002), Metrics to green chemistry – which are the best?, Green Chem., 4, 521–527.
- Corradi A., Leonelli C., Rizzuti A., Rosa R., Veronesi P., Grandi R., Baldassari S. and Villa C., (2007), New “Green” Approaches to the Synthesis of Pyrazole Derivatives, Molecules, 12, 1482–1495.
- Curzons A. D., Constable D. J. C., Mortimer D. N. and Cunningham V. L., (2001), So you think your process is green, how do you know? - Using principles of sustainability to determine what is green – a corporate perspective, Green Chem., 3, 1–6.
- Curzons A. D., Jimenez-Gonzalez C., Duncan A. L., Constable D. J. C. and Cunningham V. L., (2007), Fast life cycle assessment of synthetic chemistry (FLASC (TM)) tool, Int. J. LCA, 12, 272–280.
- Dobmeier M., Herrmann J.M., Lenoir D. and König B., (2012), Reduction of benzylic alcohols and α-hydroxycarbonyl compounds by hydriodic acid in a biphasic reaction medium, Beilstein J. Org. Chem., 8, 330–336.
- Eissen M. and Backhaus D., (2011), Pharmaceuticals in the environment: an educational perspective, Environ. Sci. Pollut. Res., 18, 1555–1566.
- Eissen M., Brinkmann T., Klein M., Schwartze B. and Weiß M., (2011a), Einsatz von Kennzahlen in frühen Phasen der Syntheseentwicklung—Zwei Fallstudien; Application of Metrics in Early Stages of Process Development – Two Case Studies, Chem. Ing. Tech., 83, 1597–1608.
- Eissen M., Geisler G., Bühler B., Fischer C., Hungerbühler K., Schmid A. and Carreira E. M., (2008), Mass balances and life cycle assessment. In: Lapkin A, Constable DJC (eds) Green Chemistry Metrics, Measuring and Monitoring Sustainable Processes. John Wiley & Sons Ltd., Oxford, p 200–227.
- Eissen M., Hungerbühler K., Dirks S. and Metzger J. O., (2003a), Mass efficiency as metric for the effectiveness of catalysts, Green Chem., 5, G25–G27.
- Eissen M., Hungerbuhler K., Metzger J. O., Schmidt E. and Schneidewind U., (2004a), Sustainable Development and Chemistry. In: Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc.
- Eissen M., Kaling S., Strudthoff M., Backhaus S., Eismann C., Oetken G. and Lenoir D., (2011b), Oxidation numbers, Oxidants, and Redox Reactions: Variants of the Electrophilic Bromination of Alkenes and Variants of the Application of Oxone, J. Chem. Educ., 88, 284–291.
- Eissen M. and Lenoir D., (2008), Electrophilic Bromination of Alkenes: Environmental, Health and Safety Aspects of New Alternative Methods, Chem.–Eur. J., 14, 9830–9841.
- Eissen M., Mazur R., Quebbemann H.-G. and Pennemann K.-H., (2004b), Atom Economy and Yield of Synthesis Sequences, Helv. Chim. Acta, 87, 524–535.
- Eissen M. and Metzger J. O., (2002a), EATOS, Environmental Assessment Tool for Organic Syntheses.
- Eissen M. and Metzger J. O., (2002b), Environmental Performance Metrics for Daily Use in Synthetic Chemistry, Chem.–Eur. J., 8, 3580–3585.
- Eissen M., Metzger J. O., Schmidt E. and Schneidewind U., (2002), 10 Years after Rio – Concepts on the Contribution of Chemistry to a Sustainable Development, Angew. Chem., Int. Ed., 41, 414–436.
- Eissen M., Weiß M., Brinkmann T. and Steinigeweg S., (2010), Comparison of two alternative routes to an enantiomerically pure β-amino acid, Chem. Eng. Technol., 33, 629–637.
- Eissen M., Zogg A. and Hungerbühler K., (2003b), The runaway scenario in thermal safety assessment: Simple experimental access by the catalytic decomposition of H2O2, J. Loss Prev. Process Ind., 16, 289–96.
- Enquete Kommission “Schutz des Menschen und der Umwelt” des Deutschen Bundestages, (1994), Die Industriegesellschaft gestalten – Perspektiven für einen nachhaltigen Umgang mit Stoff und Materialströmen (Shaping of the industrial society – perspectives for a sustainable commerce of substance and material flows), Vol. Economica Verlag, Bonn.
- Fringuelli F., Lanari D., Pizzo F. and Vaccaro L., (2010), An E-factor minimized protocol for the preparation of methyl [small beta]-hydroxy esters, Green Chem., 12, 1301–1305.
- Gaber Y., Tornvall U., Orellana-Coca C., Ali Amin M. and Hatti-Kaul R., (2010), Enzymatic synthesis of N-alkanoyl-N-methylglucamide surfactants: solvent-free production and environmental assessment, Green Chem., 12, 1817–1825.
- Hansmann R., Mieg H. A. and Frischknecht P. M., (2010), Qualifications for Contributing to Sustainable Development A Survey of Environmental Sciences Graduates, Gaia-Ecological Perspectives for Science and Society, 19, 278–286.
- Ho S. V., McLaughlin J. M., Cue B. W. and Dunn P. J., (2010), Environmental considerations in biologics manufacturing, Green Chem., 12, 755–766.
- Ho T. T. T., Jacobs T. and Meier M. A. R., (2009), A Design-of-Experiments Approach for the Optimization and Understanding of the Cross-Metathesis Reaction of Methyl Ricinoleate with Methyl Acrylate, ChemSusChem, 2, 749–754.
- Jimenez-Gonzalez C., Ponder C. S., Broxterman Q. B. and Manley J. B., (2011), Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry To Drive More Sustainable Processes, Org. Process Res. Dev., 15, 912–917.
- Jödicke G., Zenklusen O., Weidenhaupt A. and Hungerbühler K., (1999), Developing environmentally-sound processes in the chemical industry: a case study on pharmaceutical intermediates, J. Clean. Prod., 7, 159–166.
- Kabalka G. W., Yang K., Reddy N. K. and Narayana C., (1998), Bromination of Alkenes using a Mixture of Sodium Bromide and Sodium Perborate, Synth. Commun., 28, 925–929.
- Kuzemko M. A., Van Arnum S. D. and Niemczyk H. J., (2007), A green chemistry comparative analysis of the syntheses of (E)-4-cyclobutyl-2- 2-(3-nitrophenyl)ethenyl thiazole, Ro 24-5904, Org. Process Res. Dev., 11, 470–476.
- Lenoir D., (2006), Selective Oxidation of Organic Compounds – Sustainable Catalytic Reactions with Oxygen and without Transition Metals?, Angew. Chem., Int. Ed., 45, 3206–3210.
- Lombardo M., Gianotti K., Licciulli S. and Trombini C., (2004), An environmentally friendly a-hydroxyallylation reaction of the Garner aldehyde: a comparative assessment of alternative Barbier conditions, Tetrahedron, 60, 11725–11732.
- Marks R. and Eilks I., (2010), Research-based development of a lesson plan on shower gels and musk fragrances following a socio-critical and problem-oriented approach to chemistry teaching, Chem. Educ. Res. Pract., 11, 129–141.
- Martins A. A., Mata T. M., Costa C. A. V. and Sikdar S. K., (2007), Framework for Sustainability Metrics, Ind. Eng. Chem. Res., 46, 2962–2973.
- McKenzie L. C., Huffman L. M. and Hutchison J. E., (2005), The Evolution of a Green Chemistry Laboratory Experiment: Greener Brominations of Stilbene, J. Chem. Educ., 82, 306–310.
- McKeown R., (2006), Education for sustainable development toolkit. http://unesdoc.unesco.org/images/0015/001524/152453eo.pdf, accessed 27/12/2011.
- Piang-Siong W., de Caro P., Lacaze-Dufaure C., Shum Cheong Sing A. and Hoareau W., (2012), Effect of catalytic conditions on the synthesis of new aconitate esters, Ind. Crops Prod., 35, 203–210.
- Podgoršek A., Eissen M., Fleckenstein J., Stavber S., Zupan M. and Iskra J., (2009a), Selective Aerobic Oxidative Dibromination of Alkenes with Aqueous HBr and Sodium Nitrite as a Catalyst, Green Chem., 11, 120–126.
- Podgoršek A., Zupan M. and Iskra J., (2009b), Oxidative Halogenation with “Green” Oxidants: Oxygen and Hydrogen Peroxide, Angew. Chem., Int. Ed., 48, 8424–8450.
- Protti S., Dondi D., Fagnoni M. and Albini A., (2007), Photochemistry in synthesis: Where, when, and why, Pure Appl. Chem., 79, 1929–1938.
- Protti S., Dondi D., Fagnoni M. and Albini A., (2009a), Assessing photochemistry as a green synthetic method. Carbon–carbon bond forming reactions, Green Chem., 11, 239–249.
- Protti S., Ravelli D., Fagnoni M. and Albini A., (2009b), Solar light-driven photocatalyzed alkylations. Chemistry on the window ledge, Chem. Commun., 7351–7353.
- Ranke J., Bahadir M., Eissen M. and König B., (2008), Developing and Disseminating NOP: An Online, Open-Access, Organic Chemistry Teaching Resource To Integrate Sustainability Concepts in the Laboratory, J. Chem. Educ., 85, 1000–1005.
- Ravelli D., Dondi D., Fagnoni M. and Albini A., (2010), Titanium dioxide photocatalysis: An assessment of the environmental compatibility for the case of the functionalization of heterocyclics, Appl. Catal., B, 99, 442–447.
- Ravelli D., Protti S., Neri P., Fagnoni M. and Albini A., (2011), Photochemical technologies assessed: the case of rose oxide, Green Chem., 13, 1876–1884.
- Ribeiro M. G. T. C. and Machado A. A. S. C., (2011), Metal-Acetylacetonate Synthesis Experiments: Which Is Greener?, J. Chem. Educ., 88, 947–953.
- Ritter S. K., (2010), Greening Up Process Chemistry, Chem. Eng. News, 88, 45–47.
- Rosini G., Borzatta V., Paolucci C. and Righi P., (2008), Comparative assessment of an alternative route to (5-benzylfuran-3-yl)methanol (Elliott's alcohol), a key intermediate for the industrial production of resmethrins, Green Chem., 10, 1146–1151.
- Rybak A. and Meier M. A. R., (2008), Cross-Metathesis of Oleyl Alcohol with Methyl Acrylate: Optimization of reaction conditions and comparison of their environmental impact, Green Chem., 10, 1099–1104.
- Saha A., Kumar R. and Devakumar C., (2010), Development and assessment of green synthesis of hydrazides, Indian J. Chem., Sect B, 49, 526–531.
- Saling P., (2008), Die Ökoeffizienz-Analyse als Instrument zur Bewertung nachhaltigerer Chemie, Die Aktuelle Wochenschau der GDCh, Woche 2.
- Saling P., Maisch R., Silvani M. and Konig N., (2005), Assessing the environmental-hazard potential for life cycle assessment, eco-efficiency and SEEbalance (R), Int. J. LCA, 10, 364–371.
- Selva M. and Perosa A., (2008), Green chemistry metrics: a comparative evaluation of dimethyl carbonate, methyl iodide, dimethyl sulfate and methanol as methylating agents, Green Chem., 10, 457–464.
- Sheldon R. A., (1994), Consider the environmental quotient, CHEMTECH, 3, 38–47.
- Snyder H. R. and Brooks L. A., (1943), 1,2-Dibromocyclohexane, Org. Syn., 2, 171.
- Stark A., Ott D., Kralisch D., Kreisel G. and Ondruschka B., (2010), Ionic Liquids and Green Chemistry: A Lab Experiment, J. Chem. Educ., 87, 196–201.
- Trost B. M., (1991), The atom economy – a search for synthetic efficiency, Science, 251, 1471–1477.
- Tucker J. L., (2010), Green Chemistry: Cresting a Summit toward Sustainability, Org. Proc. Res. Dev., 14, 328–331.
- Umweltbundesamt, (2009), Sustainable Chemistry – Positions and Criteria of the Federal Environment Agency, Dessau, http://www.umweltdaten.de/publikationen/fpdf-l/3798.pdf, accessed 08/07/2011.
- United Nations, (1987), Our common future (Brundtland Report) http://www.worldinbalance.net/pdf/1987-brundtland.pdf; http://www.un-documents.net/wced-ocf.htm, accessed 08/07/2011.
- United Nations, (1992), (a) Rio Declaration on Environment and Development; (b) Agenda 21, United Nations Conference on Environment and Development, Rio de Janeiro, http://www.un.org/esa/sustdev, accessed 10/07/2008.
- Van Arnum S. D., (2005), An approach towards teaching green chemistry fundamentals, J. Chem. Educ., 82, 1689–1692.
- Villa C., Rosa R., Corradi A. and Leonelli C., (2011), Microwaves-Mediated Preparation of Organoclays as Organic-/Bio-Inorganic Hybrid Materials, Curr. Org. Chem., 15, 284–295.
- Weiß M., Brinkmann T. and Gröger H., (2010), Towards a greener synthesis of (S)-3-aminobutanoic acid: process development and environmental assessment, Green Chem., 12, 1580–1588.
- Wheeler K. A., (2000), Sustainability from five perspectives. In: Wheeler KA, Bijur AP (eds) Education for a sustainable future. Kluwer, New York:, p 2–6.
- Wiesner J., Christ C., Führer W., Behre H., Stockburger D., Schmidhammer L., Lohrengel G., Kerker L., Rothe U., Jordan V., Glarner T., Stolzenberg K., Van Os C., Higman C., De Piaggi R., Miyachi M. and Schumacher G., (1995), Production-Integrated Environmental Protection. In: Ullmann's Encyclopedia of Industrial Chemistry, Vol B8. VCH publisher, Weinheim, p 213–309.
- Winterton N., (2001), Twelve more green chemistry principles, Green Chem., 3, G73–G75.
- Young D. M., Welker J. J. C. and Doxsee K. M., (2010), Green Synthesis of a Fluorescent Natural Product, J. Chem. Educ., 88, 319–321.
- Zvagulis A., Bonollo S., Lanari D., Pizzo F. and Vaccaro L., (2010), 2-tert-Butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphospho rine Supported on Polystyrene (PS-BEMP) as an Efficient Recoverable and Reusable Catalyst for the Phenolysis of Epoxides under Solvent-Free Conditions, Adv. Synth. Catal., 352, 2489–2496.
Footnotes |
| † This article is part of a themed issue on sustainable development and green chemistry in chemistry education. |
| ‡ Electronic supplementary information (ESI) available: Worksheet. See DOI: 10.1039/c2rp90002e |
| This journal is © The Royal Society of Chemistry 2012 |


