Student misinterpretations and misconceptions based on their explanations of two computer animations of varying complexity depicting the same oxidation–reduction reaction
Deborah P.
Rosenthal
a and
Michael J.
Sanger
*b
aWashington State Community College, 710 Colgate Drive, Marietta, OH 45750, USA. E-mail: drosenthal@wscc.edu; Tel: +1 (740) 374-2147
bDepartment of Chemistry, Middle Tennessee State University, P.O. Box 68, Murfreesboro, TN 37132, USA. E-mail: mjsanger@mtsu.edu; Fax: +1 (615) 898-5182; Tel: +1 (615) 904-8558
First published on 31st August 2012
Abstract
A group of 55 students were shown unnarrated versions of two different particulate-level computer animations of varying complexity depicting the oxidation–reduction reaction of aqueous silver nitrate and solid copper metal. These students were asked to explain their understanding of the chemical reaction based on their interpretations of these animations. This study describes the common errors made by these students in their explanations, and includes both student misinterpretations and misconceptions from the animations. These errors included confusing the depicted water molecules as nitrate ions, seeing neutral ion-pair “molecules” in the solution, predicting incorrect ion charges, viewing incorrect silver![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrate ion ratios and silver
nitrate ion ratios and silver![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) copper reacting ratios, not recognizing that a transfer of electrons changes the charges and sizes of the metal atoms or ions, misidentifying the source of the blue colour in solution, and conflating macroscopic and particulate properties in the reaction. This study also discusses possible sources of these errors, the limitations of this study, suggestions for animators, and future directions for research based on the results reported.
copper reacting ratios, not recognizing that a transfer of electrons changes the charges and sizes of the metal atoms or ions, misidentifying the source of the blue colour in solution, and conflating macroscopic and particulate properties in the reaction. This study also discusses possible sources of these errors, the limitations of this study, suggestions for animators, and future directions for research based on the results reported.
Introduction
Chemical education researchers have recognized that students often have difficulty learning chemistry concepts, and have proposed several suggestions as to the reasons for this difficulty, including frequent overloading of student working memory (Carlson et al., 2003; Johnstone, 2006; Tasker and Dalton, 2006; Johnstone, 2010); issues of imprecise or confusing language (Herron, 1996; Gabel, 1999; Johnstone, 2010); inability to think about the same chemical processes at the macroscopic, particulate, and symbolic levels (Gabel, 1999; Gilbert and Treagust, 2009; Johnstone, 2010; Talanquer, 2011); the format and order of material presented in chemistry textbooks (Gabel, 1999; Sanger and Greenbowe, 1999; Österlund et al., 2010); and the fact that prior experiences may be inconsistent and interfere with subsequent learning (Bodner, 1991; Herron and Nurrenbern, 1999; Mulford and Robinson, 2002; Treagust et al., 2011). These difficulties often lead to student misconceptions, which in this study are defined as student conceptual or propositional knowledge that is inconsistent with the commonly accepted scientific consensus (Cho et al., 1985). Several chemical education researchers have studied student misconceptions for a variety of chemistry topics including chemical reactions (Yarroch, 1985; Andersson, 1986; Boo, 1998; Ardac and Akaygun, 2004; Sanger, 2005), dissolving compounds in water (Butts and Smith, 1987; Ebenezer and Erickson, 1996; Liu and Lesniak, 2006; Kelly and Jones, 2007; Tien et al., 2007; Kelly and Jones, 2008; Naah and Sanger, 2012), oxidation–reduction reactions (Garnett and Treagust, 1992a; Schmidt and Volke, 2003; Österlund et al., 2010), chemical bonding (Butts and Smith, 1987; Taber, 1994, 1997; Boo, 1998; Coll and Treagust, 2003; Smith and Nakhleh, 2011), and electrochemistry (Garnett and Treagust, 1992b; Sanger and Greenbowe, 1997; Huddle et al., 2000; Özkaya; 2002).Most of the research on misconceptions in oxidation–reduction reactions has focused on students' difficulties in properly identifying oxidation–reduction reactions (Garnett and Treagust, 1992a; Schmidt and Volke, 2003; Österlund et al., 2010). One reason for this difficulty is that chemistry teachers and textbooks often use more than one definition for the processes of oxidation and reduction. These definitions include the electron method (oxidation occurs when a substance loses electrons and reduction occurs when a substance gains electrons), the oxidation number method (an increase in the oxidation number occurs in oxidation reactions and a decrease in the oxidation number occurs in reduction reactions), the oxygen method (gaining oxygen atoms represents an oxidation reaction while losing oxygen atoms represents a reduction reaction), the hydrogen method (losing hydrogen atoms represents an oxidation reaction while gaining hydrogen atoms represents a reduction reaction), and others. Österlund et al. (2010) analysed several Swedish and English chemistry textbooks and found that chapters on inorganic chemistry focused on the electron and oxidation number methods, chapter covering organic chemistry topics favoured the oxygen and hydrogen methods, and biochemistry chapters used the hydrogen method and other alternative methods. Unfortunately, few of these books explained how the multiple methods are related or how they are consistent with each other. These authors proposed that textbook authors use balanced oxidation–reduction half-reactions in the organic and biochemistry chapters to show the consistency with the electron method favoured by the inorganic chapters.
Garnett and Treagust (1992a) found that students who were successful at identifying oxidation–reduction reactions generally used the oxidation number method. However, they found some students had difficulty assigning correct oxidation numbers; student errors included making the assumption that atoms always have the same oxidation number as their monatomic ions (i.e., Mg always has an oxidation number of +2), or that a polyatomic molecule or ion could have an oxidation number and that it would be the same as its overall charge (i.e., CO2 has an oxidation number of 0 and CO32− has an oxidation number of −2). Schmidt and Volke (2003) found similar errors when grade 11 and 13 students from Germany were asked to identify whether chemical reactions contained acids and bases or oxidizing and reducing agents. Subsequent interviews showed that students were using the electron and oxygen methods, but that some of them were using these methods incorrectly because they were confusing oxidation number with overall charges and confusing oxygen atom transfers with oxide transfers. For example, in the conversion of CO32− to CO2 (by the reaction with H+ ions), some students classified this as an oxidation process because the overall charge changed from −2 to 0, while others classified this as a reduction process because the carbonate ion has lost an oxygen atom.
In the present study, students were shown the unnarrated versions of two different particulate-level animations of differing complexity that depicted the same oxidation–reduction reaction involving silver nitrate and copper metal. The goal of this study was to identify student errors as they attempted to interpret and explain the chemical processes depicted in the unnarrated animations.
Most research involving the use of computer animations of chemical reactions at the particulate level have focused on instructional interventions to improve students' conceptual understanding of these chemical processes (Williamson and Abraham, 1995; Sanger et al. 2000; Kelly and Jones, 2008; Gregorius, 2010a, 2010b). This study is one of a few that have used these animations as part of the assessment process (Sanger et al., 2007; Naah and Sanger, 2012). This study focused on students' interpretations of animations without narration or explanation of the objects and symbols used in the animations. since it is likely that students would encounter examples of these or other animations lacking visual or audio (narrated) descriptions of the objects depicted in the animations.
Two types of student errors were identified from this study—misinterpretations and misconceptions. Misinterpretations occurred when students incorrectly identified the objects or symbols depicted in an animation. These errors were classified as misconceptions when students attempted to apply their misinterpretations of the objects or symbols in an animation to the chemical behaviour of these substances in the oxidation–reduction reaction.
Theoretical framework
This research involving the use of qualitative interviews to identify students' misconceptions was informed by the constructivist theory of knowledge and situated cognition (Bodner, 1986; Solomon, 1987; Bodner et al., 2001; Ferguson, 2007; Orgill, 2007). Personal or Piagetian constructivism (Ferguson, 2007) assumes that learners actively construct knowledge based on their interactions with the learning environment. Learners activate pre-existing schema (components of the learners' internal cognitive structures) in the learning process. Assimilation occurs when the new learning can be integrated into existing schema. When the new learning and the existing schema do not fit, learners experience disequilibrium; accommodation occurs when learners can change their existing schema to incorporate the new information. Social constructivism (Solomon, 1987) focuses on the importance of social interactions (e.g., student discussions during learning) that can influence how knowledge is constructed by learners. Situated cognition (Orgill, 2007) focuses on how the context of an authentic learning environment affects how learners construct knowledge. The learning environment not only includes instructional tools (such as computer animations) but also the other participants involved in the learning process. Clancey (1994) and Roschelle and Clancey (1992) have performed research based on situated cognition involving the use of computer programs to evaluate students' conceptions of physics and mathematics concepts.The effectiveness of using computer animations of chemical processes at the particulate level is based on Mayer's cognitive theory of multimedia learning (Mayer, 2001), which was adapted from Paivio's dual-coding theory (Paivio, 1986) and Baddeley's model of working memory (Baddeley, 1986). Mayer's theory assumes that learners possess separate cognitive channels for processing visual (pictorial) and auditory (verbal) information, that learners have limited processing capabilities in each channel, and that learners engage in active learning by attending to relevant information, organizing this information into mental schema, and integrating this new knowledge with pre-existing knowledge.
Methodology
Sample size and selection
The sample consisted of 55 volunteer students (19 males and 36 females) from the same section of a second-semester introductory chemistry course taught by the same chemistry instructor that was intended for chemistry, biology, and health science majors. Each student was interviewed for 40–70 min after receiving classroom instruction on oxidation–reduction reactions.Identification and validation of the conceptual and propositional knowledge statements
Conceptual and propositional knowledge statements needed to fully understand simple oxidation–reduction reactions created for this study were derived by the researchers after reviewing several introductory college chemistry textbook chapters on oxidation–reduction reactions and electrochemistry. These statements, which appear in Table 1, were reviewed by two college chemistry professors, and their comments were used to revise the original list. These statements provided a list of scientifically accepted knowledge required to fully understand simple oxidation–reduction reactions, and were used as a framework for the development of the interview protocol and the subsequent data analysis.| 1. Oxidation–reduction reactions involve the transfer of electrons from one substance to another. The substance that gives up or loses the electrons is oxidized (undergoes oxidation); the substance that accepts or gains the electrons is reduced (undergoes reduction). |
| 2. In this reaction, copper metal atoms start out as a neutral solid, and are oxidized to 2+ ions after giving two electrons to the silver ions. The copper(II) ions produced in this reaction are smaller in size than the neutral copper atom reactants. The copper(II) ions become aqueous and dissolve in the solution. Aqueous solutions of compounds containing copper(II) ions are blue in colour. As the reaction proceeds and copper(II) ions are produced, the solution changes from colourless to pale blue to an increasingly darker blue. |
| 3. In this reaction, the silver ions start out as 1+ aqueous ions, and are reduced to neutral silver metal atoms after accepting one electron from the copper metal atoms. The silver metal atoms produced in this reaction are larger in size than the silver ion reactants. The silver metal atoms form solid silver on the copper surface and are no longer dissolved in the solution. As the reaction proceeds and silver metal atoms are produced, the smooth copper-coloured surface of the copper metal becomes covered in fuzzy silver dendrites. |
| 4. Water serves as a medium (solvent) for the aqueous silver, copper(II), and nitrate ions in this reaction; water also hydrates each of these ions. When the solid silver nitrate is added to the water, the solid dissolves and the silver ions and nitrate ions dissociate from each other and are no longer bonded together in the solution; the copper(II) ions produced in this reaction also remain unbonded with and disassociated from the nitrate ions. |
| 5. The nitrate ions in this reaction are not involved in the chemical reaction (chemical spectators). The nitrate ions are present in the reaction as charge-balancers for the silver ions and copper(II) ions involved in the reaction. When the reaction starts, there should be an equal number of silver ions (+1) and nitrate ions (−1); when the reaction occurs, there should be two nitrate ions (−1) for every copper(II) ion (2+) produced. |
| 6. The oxidation–reduction reaction occurs when the aqueous silver ions come in contact with the piece of solid copper. This reaction occurs only at the solution–surface interface, and not in the solution. Because each copper atom gives up two electrons and each silver ion accepts one electron, the reaction requires two silver ions to react for every copper atom. This reaction occurs because the silver ions have a higher affinity for the electrons (as evidenced by a more positive standard reduction potential) than the copper(II) ions. |
Interview protocol
Students' misinterpretations or misconceptions of the two animations were identified through the use of semi-structured interviews, which provided the flexibility for interviewers and participants to respond to emerging ideas and seek clarification (Borg and Gall, 1983). The interview protocol for the interviews appears in Fig. 1. Each interview consisted of four parts. For the first part, the participants were shown a demonstration in which solid silver nitrate was dissolved in water; after the solid dissolved, a piece of solid copper metal was added to the solution and allowed to react. After the demonstration, the participants were asked to explain what they believed was happening in this reaction. For the second part, the participants were shown one of the animations and asked to explain how their perceptions of this reaction changed based on viewing the animation. In the third part, the participants were shown the other animation and asked to explain how their perceptions of this reaction changed based on viewing this animation. The participants were allowed to watch each animation as many times as they wanted during the interview process, and were allowed to start and stop the animations to focus on a particular object or to see how two or more objects interacted with each other. Half of the students viewed the more complex animation first while the other half saw the more simplified animation first. After viewing and discussing both animations, the participants were asked to comment on the strengths and weaknesses of each animation in the fourth part of the interview. This study focuses on student misinterpretations and misconceptions of the animations that were identified from students’ responses to Parts II and III of the interview protocol. | ||
| Fig. 1 Interview protocol used for the semi-structured interviews involving the oxidation–reduction reaction of copper metal and silver ions. | ||
Computer animations
The more simplified animation of the silver-copper reaction was created by the second author (Fig. 2a). This program was animated as two-dimensional and when two objects approach each other, they were animated as colliding and bouncing off each other. The total viewing time for this animation is about 30 s. The animation started with several copper-coloured circles in an organized pattern (copper metal) placed against a blue background occupying the bottom three-fourths of the screen (water). Floating freely in the blue were several silver-coloured circles with a ‘+’ symbol on them (silver ions) and an equal number of atom clusters containing one blue circle with a ‘−’ symbol on it surrounded by three red circles (nitrate ions). As the reaction occurs, two silver circles approach one of the copper circles; when they contact, two red ‘e−’ (electrons) appear on the copper circle and each of them are transferred from the copper circle to one of the two silver circles. When these ‘e−’ are transferred, the copper circle becomes smaller and now has a ‘2+’ symbol on it and each silver circle becomes larger and loses its ‘+’ symbol (electron transfer). The two silver circles remain attached to the cluster of copper circles and the smaller copper circle with the ‘2+’ symbol floats freely into the blue background. Each of the four times this process occurs in the animation, the blue background becomes a darker blue colour. The blue/red clusters move throughout the blue background area and collide with other objects, but do not change during the animation.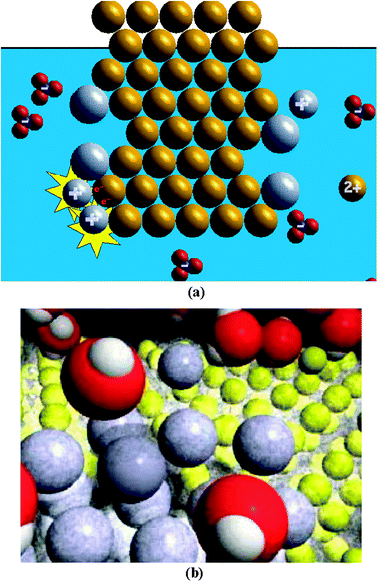 | ||
| Fig. 2 Screen shots for (a) the more simplified copper-silver animation created by Sanger and (b) the more complex copper–silver animation created by Tasker. | ||
The more complex animation of the silver-copper reaction (Fig. 2b) was created by Roy Tasker as part of the VisChem project (Tasker and Dalton, 2006) and was used in this study with his permission. This program was animated as three-dimensional and when two objects approach each other, they were animated as moving in front of or behind each other. The total viewing time for this animation is also about 30 s; although this animation has a narration associated with it, the narration was not used during the interviews. The animation started with several yellow spheres in an organized 3-D pattern (copper metal) placed against a black background occupying the screen. The yellow spheres (copper nucleus and core electrons) are surrounded by a transparent light grey sphere of “fuzziness” (valance electrons). Surrounding the 3-D pattern of yellow/light grey spheres is a large array of red spheres with two smaller white spheres embedded in them (water molecules). Mixed within the array of the many red/white shapes are a few grey spheres (silver ions). Occasionally, a single cluster of a blue sphere surrounded by three red spheres (nitrate ion) appears. As the reaction occurs, a grey sphere approaches the cluster of yellow/light grey spheres; when they contact, the transparent light grey sphere envelops the grey sphere (electron transfer), and the grey/light grey sphere remains attached to the yellow/light grey cluster. At a different time and spot on the cluster of yellow/light grey spheres, a yellow sphere will lose its light grey outer sphere (electron transfer) and leave the cluster of yellow/light grey spheres to mingle with the red/white shapes. An analysis of the entire animation shows that twice as many grey spheres become attached to the yellow/light grey cluster as yellow spheres leave the cluster. The red/white shapes surround the grey spheres (and later the yellow spheres) with their red spheres pointed toward these spheres; the red/white shapes also surround the blue/red cluster with their white spheres pointed toward this cluster. The red/white shapes appear to bring the grey sphere to the yellow/light grey cluster and then leave the grey sphere to re-enter the array of red/white shapes when the light grey sphere envelops the grey sphere. The red/white shapes also appear to pull a yellow sphere in the yellow/light grey cluster away from the cluster and away from its outer light grey sphere, and the yellow sphere (surrounded by red/white shapes) joins the array of red/white shapes. The blue/red clusters move among the red/white shapes, but do not change during the animation.
Data analysis
The participant–interviewer conversation for each interview was digitally recorded; student-generated balanced equations were written on the question sheets used during the interview. The conversations were transcribed verbatim by the first author. These transcriptions were analysed and a summary of misinterpretations and misconceptions was made for each participant that were confirmed or refuted by referring to the individual digital recordings. These summaries were combined to identify common student misconceptions and misinterpretations. The digital recordings were analysed by two chemical education researchers; any initial disagreements were discussed and resolved by these researchers.Results and discussion
The examples of students' misinterpretations of the objects or events depicted in the animations are summarized in Table 2. When the students attempt to apply or map these misinterpretations to the chemical behaviours of the substances in the system, these errors are categorized as misconceptions (Table 3). The misinterpretations and misconceptions are consecutively numbers in the text below; it should be noted that it is possible for the same error to be categorized as both a misinterpretation and a misconception, based on the students' comments.| 1. Outer valence electrons are not part of a metal atom and do not affect its size. |
| 3. The red/white shapes represent nitrate ions, so nitrogen atoms are drawn as red and oxygen atoms are drawn as white. |
| 4. The blue/red clusters represent water molecules. |
| 5. Cations and anions are attached or bonded together as ion pairs in water. |
| 6. There are more silver ions than nitrate ions before the reaction. |
| 7. There are more nitrate ions than silver ions before the reaction. |
| 9. Nitrate ions in this reaction have a −2 charge. |
12. The silver ions and copper atoms react in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. 1 ratio. |
13. The silver ions and copper atoms react in a ratio greater than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. |
| 15. An unspecified number of electrons are transferred from copper to silver. |
| 16. Gaining or losing electrons will not change the size of an atom or ion. |
| 17. Water molecules force the reaction to occur by bringing the silver ions to the surface to react and pulling the copper ions away from bulk metal. |
| 18. The behaviour of the nitrate ions drive this reaction—this reaction occurs because the nitrate ions are more attracted to the copper ions than the silver ions. |
| 19. The combination of copper and nitrate ions are causing the blue colour in the aqueous solution. |
| 20. The blue sphere in the blue/red cluster is causing the blue colour in the aqueous solution. |
| 1. Outer valence electrons are not part of a metal atom and do not affect its size. |
| 2. The valence electrons in a metal represent molecular forces or bonds. |
| 3. The red/white shapes represent nitrate ions, so nitrogen atoms are drawn as red and oxygen atoms are drawn as white. |
| 5. Cations and anions are attached or bonded together as ion pairs in water. |
| 6. There are more silver ions than nitrate ions before the reaction. |
| 7. There are more nitrate ions than silver ions before the reaction. |
| 8. Silver ions in this reaction have a +2 charge. |
| 9. Nitrate ions in this reaction have a −2 charge. |
| 10. Copper ions in this reaction have a +1 charge. |
| 11. The charge of a cation is determined by counting the number of nitrate ions around it. |
12. The silver ions and copper atoms react in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. 1 ratio. |
| 14. When electrons are transferred from copper to silver, the charges of these species do not change. |
| 18. The behaviour of the nitrate ions drives this reaction—this reaction occurs because the nitrate ions are more attracted to the copper ions than the silver ions. |
| 19. The combination of copper and nitrate ions are causing the blue colour in the aqueous solution. |
| 20. The blue sphere in the blue/red cluster is causing the blue colour in the aqueous solution. |
| 21. The particulate depictions in the animations are a direct representation of the macroscopic properties of the substances present. |
Identifying solid copper before the reaction occurs
All 55 students properly identified solid copper in the chemical demonstration and in the two computer animations. In the more complex animation, neutral copper atoms are depicted by the solid yellow sphere surrounded by a transparent light grey sphere of valence electrons. Six students had difficulty interpreting whether the size of the metal atoms should include the transparent light grey sphere (Misinterpretation 1). The misconception that the valence electrons are not part of the atom was seen in student comments that the copper atoms in the bulk copper metal were not touching each other (Misconception 1).Participant: Yellow [sphere] is copper.
Interviewer: What makes you think yellow is copper?
Participant: Really close together, and nothing else is attached to it.
Interviewer: Is that what you thought it would look like?
Participant: No, I thought it would be closer together—touching. (Misconception 1).
Several students correctly identified the transparent fuzzy light grey sphere around the yellow spheres as electrons, an electron field, or an electron cloud. However, 17 students attributed this depiction to more abstract and intangible concepts like molecular forces, bonding, or metallic bonding (Misconception 2).
Interviewer: What about this fuzzy stuff between the copper [atoms]?
Participant: Maybe that is supposed to represent the forces that make it a solid. (Misconception 2).
Identifying liquid water before the reaction occurs
During the interviews about the chemical demonstration, all 55 students properly identified water as the clear colourless liquid in the demonstration, and all were able to draw water molecules as ‘H2O’. The more complex animation is dominated by the sheer presence of the red/white shapes. While many students were able to correctly identify these shapes as water molecules, 28 of the 55 students incorrectly identified them as nitrate ions (Misinterpretation 3). Of these 28 students, seven changed their identification of these shapes from nitrate ions to water molecules during their interview. Some students still identified these red/white shapes as nitrate ions even though there are only two white spheres attached to each red sphere.Interviewer: What else do you see?
Participant: The nitrates.
Interviewer: What do they look like?
Participant: They are the two red with a white. It should be one red [nitrogen] with three little white oxygens. Oh, I can't see the whole thing. (Misinterpretation 3).
Although this represents a simple misinterpretation of the colour scheme used in the animations, it did lead to other student difficulties. For those students who assumed that the red sphere was nitrogen and the white sphere was oxygen, many had difficulty trying to identify what the blue/red cluster was (Misconception 3).
Participant: The big red one with three white pieces on it is nitrate. The red is nitrogen and the three white are oxygen. [Later in the interview, about the blue/red cluster] It doesn't look like the nitrate [red] has any oxygen [white] on it like the… Does the little white balls stand for oxygen? (Misconception 3).
In the more simplified animation, the water molecules are not shown and the presence of water is implied by the blue background. Most students were able to recognize that this animation did not depict water molecules, but four students believed that the blue/red cluster represented water molecules even though it had three red balls surrounding each blue ball (Misinterpretation 4). None of these students appeared to make any attempt to apply this misinterpretation in their subsequent explanations of the chemical system.
Interviewer: What is the thing with three red balls and blue in the middle?
Participant: Water molecule. (Misinterpretation 4).
Identifying aqueous silver nitrate before the reaction occurs
All of the students recognized that the solid silver nitrate in the chemical demonstration would dissolve in water and become an aqueous solution. Of the 55 students in the study, 39 stated that the silver and nitrate ions would dissociate in water, five students thought ion-pairs would exist, and 11 weren't sure.The misconception that ionic compounds dissolve into water as neutral ion-pairs is well documented (Butts and Smith, 1987; Smith and Metz, 1996; Boo, 1998; Liu and Lesniak, 2006; Kelly and Jones, 2007; Tien et al., 2007; Kelly and Jones, 2008; Smith and Nakhleh, 2011; Nyachwaya et al., 2011), and appears in this study as well. Two students viewing the more simplified animation and 33 students viewing the more complex animation included comments suggesting the presence of ion pairs (Misconception 5).
Interviewer: What is the function of water?
Participant: Just takes the copper [ions] and bonds it together with nitrate. (Misconception 5).
In the more complex animation, the idea that the silver or copper cations and the nitrate anions are associated as ion pairs (Misconception 5) seems to stem from the misinterpretation of the red/white shapes as nitrate ions (Misinterpretation 3). In fact, it seems that the misconception that silver or copper ions would be attached to the nitrate ions as ion pairs might have actually led several of these students to misidentify the red/white shapes as nitrate ions.
Participant: We saw that the silver from the nitrate [red/white shapes] is breaking apart, and the silver is connecting to the solid copper. And you can see that the yellow part is slowly being taken away from this, so it could be copper and nitrate… Copper started as solid and became aqueous when it connected to the nitrate. (Misinterpretation 5).
Participant: Silver came in with the reds and whites, so silver nitrate came in, and then it [red/white] goes to the copper… We see two things come together, so it has to be copper and nitrate. (Misinterpretation 5).
It is interesting to note that several students viewing the more simplified animation commented that this animation depicted the cations and anions as separate and that this depiction may have changed their initial belief that these ions would be associated together.
Interviewer: What are the products in the reaction, and has that changed based on what you saw?
Participant: It has, because according to the animation copper nitrate is not one of the products. It's silvers, copper ions, and the nitrates still floating by themselves… I knew the silver nitrate was in there to react, but that shows me that once it is in water the silver and nitrates don't associate with one another anymore, and really what is reacting is just the silver, and the nitrates are just spectating [sic]. (Correct conception).
Interviewer: So, copper nitrate in water is copper in water and nitrate in water, but not necessarily attached?
Participant: No, they are attached. I have to believe they are attached. But, according the diagram they are not. (Correct conception).
Identifying the ratio of aqueous silver and nitrate ions before the reaction occurs
While the more simplified animation shows an equal number of grey circles (silver ions) and blue/red clusters (nitrate ions) when the animation begins, the more complex animation shows many more light grey spheres compared to the blue/red clusters. Since the nitrate ions are spectators and are not involved in the chemical reaction, the animator may have chosen to leave out these spectator ions in the interest of making an already complex animation easier to understand. However, this decision appears to have serious repercussions. Fourteen students viewing the more complex animation noted that it was showing more silver ions than nitrate ions (Misinterpretation 6).Interviewer: What is the ratio of silver to nitrate?
Participant: There should be about the same.
Interviewer: What do you see?
Participant: There is too much water. I see mostly silver not much nitrate. (Misinterpretation 6).
While this is a correct interpretation of the animation as created, it does represent a misinterpretation of this chemical system. About half of these students recognized this discrepancy, and accepted the lack of nitrate ions an artistic liberty.
Interviewer: What does the animation show?
Participant: The silver [ions] and the copper [atoms] and every once in a while the nitrate. I think this is showing how unimportant the nitrate is. (Correct interpretation).
For some of these students, this misinterpretation led to a misconception as they tried to explain the larger concentration of silver ions compared to nitrate ions (Misconception 6).
Interviewer: What is [sic] silver and nitrate ions?
Participant: A-G-2-N-O-3, because so many more [silver spheres than blue/red clusters]. I think one for every two. (Misconception 6).
Ten of the students who misidentified the red/white shapes as nitrate ions in the more complex animation noted that there were more nitrate ions than silver ions in this animation (Misinterpretation 7).
Interviewer: So what about the ratio of silvers to nitrates?
Participant: They are totally different. Way more nitrates than silver. (MC, Misinterpretation 7).
This misinterpretation also led to a misconception for some students who questioned whether they had the correct formula for silver nitrate (Misconception 7). The fact that many students expected to see nitrate ions in the animation, and this animation shows very few of them that often went overlooked by the students, may have led to the incorrect interpretation of the red/white shapes as nitrate ions (Misinterpretation 3).
Participant: No, in fact there is so much nitrate [red/white shapes] floating around that is hard to see that any copper is breaking loose. It seems to me that the actual formula, if I were to write down what I see, that there would be a significantly greater amount of nitrate than there is the silver. There is, just seems to be a surplus of nitrate, which is not realistic in regards to the equation itself so it skews my perception. (Misconception 7).
Participant: I don't understand. If that is silver nitrate coming in, this [red/white shape] is nitrate, so now I am now like ‘Where is water?’ I am not sure what the red and white thing is or where the water is. I am waiting on you guys to tell me what is it. (Misinterpretation 3).
In order to determine the correct ratio of silver and nitrate ions in the aqueous solution, students need to know the charges of these ions. Although 36 of the 55 students started with the formula of AgNO3 for silver nitrate, many appeared to be guessing or were unsure of their formula. Ten of these students believed that silver forms Ag2+ ions (Misconception 8), and five believed that nitrate exists as NO32− ions (Misconception 9). Although these are labelled as misconceptions, many students who chose these charges, as well as those choosing the correct charges, appeared to be guessing or trying to remember memorized charges. So, these may be more appropriated labelled as a “lack of knowledge” rather than as misconceptions.
Participant: I was grasping ‘cause I could not remember nitrate's charge. (Misconception 9).
The more simplified animation has the charges labelled on the silver, copper, and nitrate ions while the more complex animation does not have any explicit labels for charges. Based on the students' descriptions of these animations and the fact that none of the students viewing the more simplified animation had incorrect ion charges, it appears that labels for the ion charges are very helpful for students.
One student used his incorrect assumption that there were twice as many silver ions (silver spheres) as nitrate ions (blue/red shapes) in the more complex animation (Misinterpretation 6) and his belief that the silver ion has a +1 charge to incorrectly determine the charge of the nitrate ion (Misinterpretation 9).
Interviewer: What is [sic] silver and nitrate ions?
Participant: A-G-2-N-O-3, because so many more [silver spheres than blue/red clusters]. I think one for every two.
Interviewer: Charge of nitrate?
Participant: It would be minus two… Silver nitrate, I thought was A-G-2-N-O-3—so silver +1, nitrate −2. (Misinterpretation 9).
Identifying the ratio of aqueous copper and nitrate ions after the reaction occurs
Determining the correct ratio of copper and nitrate ions in the aqueous solution after the reaction also requires students to know the charges of these ions. Before viewing the animations, only 16 of the 55 students started with the correct formula of Cu(NO3)2; all but two of the other students started with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for copper and nitrate (CuNO3). The responses from 32 of the 55 students showed a belief that copper forms Cu+ ions from this chemical reaction (Misconception 10), and five believed that nitrate exists as NO32− ions (Misconception 9).
1 ratio for copper and nitrate (CuNO3). The responses from 32 of the 55 students showed a belief that copper forms Cu+ ions from this chemical reaction (Misconception 10), and five believed that nitrate exists as NO32− ions (Misconception 9).
One student believed that the charge of a cation can be determined by counting the number of nitrate ions (misinterpreting the red/white shapes in the more complex animation as nitrate ions) around it (Misconception 11).
Interviewer: So what happens with the copper, the yellow is fuzzy and leaves without the fuzzy?
Participant: Well, I guess that it is losing an electron or how ever many it loses. It is not quite clear.
Interviewer: So can you tell charges from this?
Participant: Only if you can stop it to count the number of nitrates that are bonded to each, and you know the charge of the nitrates. You can only infer the charge. (Misconception 11).
Determining the reacting ratio of silver ions and copper atoms in the reaction
In the more simplified animation, the reaction of two silver ions and one copper atom were animated to happen at the same time and at the same spot on the copper surface. Because copper metal is an electrical conductor, this reaction does not have to occur at the same spot on the surface—i.e., two silver ions can attach anywhere on the metal surface, each gaining an electron from the bulk metal, and causing the release of a copper ion at another place on the surface. The more complex animation does attempt to show this more complex view of the oxidation–reduction process. However, this seemed to affect students’ ability to see the proper reacting ratio in the more complex animation. While many students recognized the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 silver
1 silver![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) copper reacting ratio (even if they didn't exactly see it), several other students said they couldn't see the reacting ratio for sure, but thought that they saw a 1
copper reacting ratio (even if they didn't exactly see it), several other students said they couldn't see the reacting ratio for sure, but thought that they saw a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Misinterpretation 12).
1 ratio (Misinterpretation 12).
Interviewer: Can you tell me anything about the ratio? Copper to silver?
Participant: It looks like 1 to 1 ratio in this. ‘Cause you can't tell, ‘cause it drops in and randomly come over and grab a copper… It does not depict the 2 to 1 ratio. It looked 1 to 1. (Misinterpretation 12).
For some students, the misinterpretation of an equal silver![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) copper reacting ratio led to a misconception when the time came to write the balanced chemical equation for the oxidation–reduction reaction (Misconception 12).
copper reacting ratio led to a misconception when the time came to write the balanced chemical equation for the oxidation–reduction reaction (Misconception 12).
Interviewer: So, in the reaction do we know how many coppers are reacting with how many silvers?
Participant: On a molecular level? Two copper with two silvers, but I don't know if I buy that. Certainly, we have… Well, I am thinking that the charge on… maybe my equation is wrong.
Interviewer: Are you seeing that from this animation?
Participant: They both seem like they are having a change. Like I said, when they [silver ions] hit the surface of the copper it gets the fuzziness around it.
Interviewer: Do you want to write an equation again?
Participant: Um… I don't know… In a theoretical world, I feel like what I wrote was right, but looking at the video I don't feel I am right. (Misconception 12).
For other students, the lack of knowledge regarding the charges of the copper, silver, and nitrate ions in solution affected their ability to write proper chemical formulas and to balance this oxidation–reduction reaction (Misconceptions 8–10).
Interviewer: Balanced equation? Does this animation make you change your answer?
Participant: I don't believe so. The only issue that remains is the charges. If I knew the charges of silver and copper, I would know charges of nitrate and that would change my subscripts [in the chemical formulas]. (Misconceptions 8–10).
Seven of the 55 students thought that they saw a much higher silver![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) copper reacting ratio of 3
copper reacting ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 5
1 or 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Misinterpretation 13).
1 (Misinterpretation 13).
Interviewer: What about the relative amounts? How many silvers are reacting and how many coppers are reacting?
Participant: It is like there are more silver, so like a 5 to 1 ratio. It is bigger than 2 to 1. (Misinterpretation 13).
Understanding the electron transfer process in the reaction
Before viewing the animations, all 55 students recognized this reaction as an oxidation–reduction reaction and that electrons would be transferred from one chemical species to another. The more simplified animation showed the electron transfer via the movement of red “e−” symbols from copper circles to silver circles, while the more complex animation showed the electron transfer as the loss of the transparent light grey sphere on the yellow spheres and the addition of the transparent light grey sphere on the dark grey spheres.One student who viewed the more complex animation recognized that the copper atoms lost electrons and that silver ions gained electrons, but did not believe that this transfer of electrons would change the charges of these objects (Misconception 14). It is not clear from her comments whether this misconception came from a misinterpretation of the more complex animation that did not explicitly show the charges of each atom and ion.
Interviewer: Is the charge of silver changing?
Participant: Mm, no… I don't think the charge is changing.
Interviewer: Are you seeing that from this animation?
Participant: They both seem like they are having a change. Like I said, when they [silver ions] hit the surface of the copper it gets the fuzziness around it.
Interviewer: So you think the silver is changing charge as the reaction occurs?
Participant: It is changing something, but I don't know if it is the charge. (Misconception 14).
While most students recognized that the loss or addition of the transparent light grey sphere in the more complex animation represented the loss or gain of electrons (respectively), eight of the 55 students expressed confusion regarding the number of electrons transferred as this reaction occurred (Misinterpretation 15).
Participant: It [silver ion] comes in contact with the copper solid, and then you can tell there is a transfer… or at least, the silver gets some electrons.
Interviewer: What about copper? In terms of the electrons?
Participant: It does not have any around it anymore. No white fuzzy clouds.
Interviewer: Can you tell it gave up two?
Participant: Not how many, but that it did. (Misinterpretation 15).
Understanding how the size of the metal atoms and ions change after the reaction
In the more simplified animation, the silver circle gets larger when the silver ion gains an electron and the copper circle gets smaller when the copper atom loses two electrons. In the more complex animation, the sizes of the yellow and dark grey spheres (representing the “core” electrons in the Cu2+ and Ag+ ions, respectively) stay the same; the metal atoms in their neutral form are depicted by the solid sphere (yellow or dark grey) surrounded by a transparent light grey sphere of valence electrons. Six students couldn't decide whether the transparent light grey sphere should be considered when determining the atom size (Misinterpretation 1). This misinterpretation was mentioned by 39 of the 55 students viewing the more complex animation as they tried to describe what would happen to the sizes of the copper atom and the silver ion after they reacted. Of these 39 students, 34 stated that even though silver ions gain electrons and copper atoms lose electrons, they would stay the same size (Misinterpretation 16).Interviewer: What do you think about the relative sizes? Did it look like they changed at all?
Participant: Uh huh. It looked like the actual solid structure stayed the same, but the electron cloud was being added or taken away.
Interviewer: Does that change size—having the electron cloud there or not?
Participant: It makes the two of them together that much larger, but I don't know if it changes… I think it keeps relatively the same size, it just has that electron cloud over it. (Misinterpretation 16).
Three students noted that even though they do not see any size changes in the silver ions and copper atoms in the more complex animation, their sizes should have changed.
Interviewer: What happened to the size [of silver] as it reacted?
Participant: It should be bigger, but it doesn't look bigger.
Interviewer: The copper as it is being pulled away? Charge and size?
Participant: Same size. [It's] losing electrons so [it] should be smaller, but [it] looks same. (Misinterpretation 16, correct conception).
Identifying the driving force for the reaction
The following student explanation shows a solid understanding of the role of water in the chemical process. It recognizes that water's role is more than simply acting as a “container” for the reaction, but that water is not forcing the reaction to occur.Interviewer: What do you think the role of water is in all of this?
Participant: To carry ions. I know it is polar. I know the ions are charged, so the ions are attracted to one of the poles and the water molecules are just floating around. They can carry them around.
Interviewer: Before you said the role of water was to dissolve, but now you are saying it is actively helping?
Participant: Yes, by letting the silver nitrate dissolve, dissociate. It dissociates because the ions can stick to the water. And since the water is surrounding copper ions, it has ions attached and it is smacking the ions into the copper.
Interviewer: Do you think it is in charge or just letting it happen?
Participant: Reaction would not happen unless all these compounds were present, so I think the water allows it to happen but I would not say causes it to happen. (Correct conception).
During the interviews about the chemical demonstration, 48 of the 55 students stated that water's only role was to serve as the solvent or a reaction vessel. Some of these students also stated that water's role was to dissolve and dissociate the silver and nitrate ions. While these views are simplistic, they were not identified as a misinterpretation or misconception in this study. However, 35 students viewing the more complex animation stated that the actions of the water molecules were the driving force for this reaction (Misinterpretation 17). They viewed water molecules as taking an active role by pushing the silver ions to the metal surface to react and by pulling the copper ions out from the bulk metal into the solution. Tasker and Dalton (2006) have previously identified this misinterpretation associated with the more complex animation used in their study.
Interviewer: What is the driving force, why [does] this reaction occurs?
Participant: The water.
Interviewer: What is the water doing?
Participant: It is pushing the silver into the copper.
Interviewer: Is it also helping with the copper when it leaves?
Participant: Yeah, it is like pulling the copper. (Misinterpretation 17).
Participant: It looks more like water is taking a very active role.
Interviewer: Do you think that is reality?
Participant: I don't know if it happens exactly like that, but since water is polar—partial positive, partial negative—that could be possible, but it almost makes it look like the water's specific job is to carry the silver to the copper and then release it, more than it is attracted to charge. (Misinterpretation 17).
Thirteen of the 35 students suggesting that the red/white shapes were forcing the reaction to occur had misinterpreted these red/white shapes as nitrate ions (Misinterpretation 3), and therefore talked about how the nitrate ions were forcing this reaction to occur (Misinterpretation 18).
Interviewer: Does this animation help you figure out why this reaction is occurring?
Participant: Not particularly. No. I mean, it looks like the nitrates are forcing silver in and pulling copper out, which in part is a little bit of motivation to the nitrates, but it does not tell you why the nitrates want this to happen. It looks like they are a lot more active… but it does not tell you why. (Misinterpretation 18).
While most of these comments about the red/white “nitrate” driving this reaction appeared to be the result of misinterpreting the actions of these red/white shapes during the animation, 4 of these 13 students believed that the driving force for this reaction was a competition between the two metals for the nitrate ions (Misconception 18). These students believed that the copper ions were more attracted to the nitrate ions than the silver ions were, and this is why the reaction occurs.
Interviewer: Are the nitrates reacting?
Participant: Yes.
Interviewer: What is the overall reason this reaction occurs? The driving force?
Participant: Copper must drive the silver away from the nitrate in order to bond with it.
Interviewer: Who starts with the nitrate and who ends with the nitrate?
Participant: Silver starts with nitrate and copper ends with nitrate.
Interviewer: So who wants nitrate more?
Participant: Copper. (Misconception 18).
Explaining the source of the blue colour in solution after the reaction
Before viewing any animation, 19 students told the interviewers that the aqueous solution turns blue because of the formation of Cu2+(aq) ions. After viewing the animations, all 55 students watching the more simplified animation and 8 watching the more complex animation explained that the blue colour was caused by aqueous copper ions.Interviewer: What is causing the blue colour?
Participant: I am not sure. Either the copper solution or copper nitrate solution. I see it getting darker. I see the nitrate is not doing much but bouncing off copper solid, and copper ions also. But every time two silvers crash into the copper solid, causes a copper ion to be released, the blue gets a shade darker. So, at this point I would say the blue in the reaction is caused by copper ions in solution rather than copper nitrate in solution. (Correct conception).
As part of the chemical demonstration interviews, 19 students believed that the combination of copper and nitrate ions produced the blue colour (Misconception 19). Eight of the 55 students watching the more complex animation explained that the blue colour was caused by the combination of copper and nitrate ions.
Interviewer: [Is there] anything to explain the blue colour?
Participant: The copper released into solution and makes an ion with the nitrate and makes blue. (Misconception 19).
Three of the eight students stating that the blue colour comes from the combination of copper and nitrate ions had misidentified the red/white shapes in the more complex animation as nitrate ions (Misinterpretation 3). This misinterpretation may have led them to believe that the blue colour was caused by the combination of the yellow sphere and the red/white shapes (copper and nitrate ions).
Participant: I think the nitrate and the copper bond[ing] together is causing the blue colour to form, ‘cause if you look at silver and nitrate, nothing [no colour] is formed until we switch it and you have copper and nitrate. (Misinterpretation 19).
While viewing the more complex animation, 20 of the 55 students stated that the blue sphere in the blue/red cluster was responsible for the colour change (Misinterpretation 20). It is coincidental (and unfortunate) that the symbol for nitrogen is blue and that the solution turns blue, and this ultimately led to some confusion among the students in this study. This represents a confusion of the macroscopic view of the world and the particulate representation used in this animation.
Nine of these 20 students stated that the blue sphere represented the colour of the solution, without any explanation of what kind of atom the blue sphere represents.
Interviewer: Any idea what is causing the blue colour?
Participant: We can see it in the beaker. Can we see it in the computer? I don't see any blue. I saw a little blue thing going across the screen, but I don't know if that is the blue you are talking about.
Interviewer: Let's find that.
Participant: The thing that is carrying the blue… is distributing the colour around.
Interviewer: So, the blue thing is depicting the colour?
Participant: Yeah, that is what I believe. I am OK with that. (Misinterpretation 20).
Seven of these students recognized that the blue/red cluster represents the nitrate ion, but their misinterpretation of the blue sphere as the source of the blue colour now caused them to believe that the nitrate ion (or the blue nitrogen atom within the nitrate ion) was the source of the blue colour in solution. (Misconception 20).
Interviewer: What is the blue?
Participant: Three hydrogen and some blue. Wait a minute, now I am confused. No, three oxygens… I keep calling them hydrogen. It is a nitrate.
Interviewer: What is the blue thing?
Participant: Nitrogen. So the nitrogen in the water is turning it blue. I thought the copper was turning it blue. (Misconception 20).
Four of these students believed that the blue sphere now represented a copper ion since they (correctly) believed that the copper ions are responsible for the blue colour in solution.
Interviewer: What is that [pointing to a blue/red cluster]?
Participant: The blue attached to water [red atoms]. It is the copper aqueous solution. It is the bond between the copper and water that makes it blue. (Misinterpretation 20).
Conflating the particulate drawings and the macroscopic properties of the substances present in the reaction
Conflation occurs when two distinct concepts that share some characteristics are collapsed into a single concept, resulting in a loss of the differences between the two concepts (Haught, 1995). The belief that the blue sphere in the particulate view of the animation must be responsible for the blue colour of the solution (a macroscopic view) and the assignment of the blue sphere in the blue/red cluster as a copper atom because the copper ions cause the blue colour in the aqueous solution are perfect examples of students conflating the macroscopic and particulate descriptions of this reaction (Misconception 21). The conflation of the macroscopic properties of chemical substances and the particulate symbols used to depict them in computer animations has been previously reported (Andersson, 1986; Ben-Zvi et al., 1986; Sanger et al., 2001; Kelly and Jones, 2008).Several other conflations of these two views were noticed during these interviews. These conflations include participants who believed that: (1) water molecules should be depicted as being blue because water is blue, (2) the fuzzy light grey transparent spheres surrounding the yellow spheres in the more complex animation represented the fuzzy silver dendrites formed on the copper surface, and (3) the yellow spheres in the more complex animation becoming smaller as they left the bulk metal occurred because the piece of copper metal was slowly being eaten away by the silver nitrate solution.
Interviewer: What are the red and white things?
Participant: Water.
Interviewer: Is that what you think water would look like?
Participant: No.
Interviewer: What would water look like?
Participant: Blue. (Misconception 20).
Interviewer: [What is the] white fuzziness between them [yellow spheres in the bulk copper metal]?
Participant: Silver nitrate starting to react with copper. (Misconception 20).
Interviewer: What about the yellow ones [spheres]? What is happening to their sizes?
Participant: Smaller, I guess.
Interviewer: Why are they getting smaller?
Participant: Silver nitrate is eating it away.
Interviewer: So, the copper wire is getting smaller. What about the copper atoms?
Participant: It is smaller.
Interviewer: Why does it get smaller?
Participant: It is breaking down into solution. Dissolving, I guess. (Misconception 20).
Conclusions
This study identified several difficulties that students had in interpreting two different computer animations of differing complexity regarding the same oxidation–reduction reaction. Some of these difficulties represent simple student misinterpretations of the symbol systems used by the computer animations, while others represent student misconceptions that do not match the scientifically accepted explanations given by chemists. These misconceptions include the ideas that ions in solution will form neutral ion pairs/molecules, that adding or losing valence electrons will not affect the overall size or charge of the object, that water drives the oxidation–reduction reaction, and that the nitrate ion causes the blue colour of the solution. Some of these student difficulties represent deficiencies in the students' propositional knowledge—writing incorrect charges for the silver, copper(II), and nitrate ions; not recognizing the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for silver and nitrate ions before the reaction occurs; not recognizing the 1
1 ratio for silver and nitrate ions before the reaction occurs; not recognizing the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 reacting ratio of the copper atoms and silver ions; etc. This study also showed that several students confused or conflated the particulate properties depicted in the computer animations with the macroscopic properties they observed as part of the chemical demonstration. This confusion of the macroscopic and particulate representational levels in chemistry has been reported previously (Andersson, 1986; Ben-Zvi et al., 1986; Sanger et al., 2001; Kelly and Jones, 2008) and demonstrates a lack of representational competence (Kozma and Russell, 1997; Madden et al., 2011; Naah and Sanger, 2012) that would allow students to move seamlessly between the representational levels.
2 reacting ratio of the copper atoms and silver ions; etc. This study also showed that several students confused or conflated the particulate properties depicted in the computer animations with the macroscopic properties they observed as part of the chemical demonstration. This confusion of the macroscopic and particulate representational levels in chemistry has been reported previously (Andersson, 1986; Ben-Zvi et al., 1986; Sanger et al., 2001; Kelly and Jones, 2008) and demonstrates a lack of representational competence (Kozma and Russell, 1997; Madden et al., 2011; Naah and Sanger, 2012) that would allow students to move seamlessly between the representational levels.
Students had more difficulty correctly interpreting the more complex animation. This is consistent with Mayer's coherence principle (2001), which states that students learn better when extraneous material is excluded rather than included. It appears that at least some of the additional information presented in the more complex animation may not have been necessary for students to understand some of the concepts addressed in this study and may have distracted students’ attention away from the more relevant details.
Many of the deficiencies identified in this study could be the result of students' misinterpretations of the information depicted by the computer animations. For example, many students tried to determine the ratio of silver to nitrate ions by counting the number of each ion depicted by the animations. Some students had similar difficulties interpreting the reacting ratio of the copper atoms and silver ions or the total number of electrons accepted/lost by these substances based on the animations. Other students didn't understand the extent to which the water molecules “drove” the oxidation–reduction reaction (Tasker and Dalton, 2006) or that the sizes of the neutral atoms and cations of each metal would have different sizes because they were unsure how to interpret the information depicted in these animations.
One of the most pervasive misinterpretations identified in this study was students' incorrectly identifying the red/white shapes in the more complex animation as nitrate ions instead of water molecules (Misinterpretation 3). Based on the students' responses during the interviews, there seems to be at least two major reasons for this misinterpretation. The first reason is that the more complex animation does not show very many nitrate ions (blue/red clusters) and many students expected to see the nitrate ions prominently depicted, so they assumed that the red/white shapes were the nitrate ions. The second reason is that many of these students expected to see silver-nitrate or copper-nitrate “molecules” (ion pairs) and the red/white shapes were attached to the grey spheres (silver ions) and the yellow spheres (copper ions) so they assumed that the red/white shapes were nitrate ions. Another unfortunate coincidence exacerbated this confusion. During the interviews, we had expected that when students saw the blue/red cluster, they would recognize that it must be the nitrate ion and therefore that the red/white shapes must be something else and would come to the realization that the red/white shapes were water molecules. Many students did come to the conclusions that we had expected. However, other students assumed that the blue/red cluster represented the blue colour that appears in the aqueous solution as the reaction occurs and therefore did not change their assignment of the red/white shapes. If the solution had changed to a colour other than blue or if the colour scheme for nitrogen was not blue, it is likely that more of these students would have changed their assignments of the red/white shapes.
Unfortunately, this incorrect assignment of the red/white shapes as nitrate ions also affected some of the students' other conceptions of the reaction. In particular, students who misinterpreted the red/white shapes were also likely to assume that there were more nitrate ions present compared to the silver ions, which ultimately affected their beliefs of the formula/ratio for silver nitrate and the charges of these ions (e.g., twice as many nitrates versus silver ions means a formula of Ag(NO3)2, and ion charges of Ag2+ and NO3−). The incorrect assignment of the red/white shapes as nitrate ions also led many students to believe that it was the combination of copper and nitrate ions that causes the blue colour in the solution instead of the combination of copper ions and water molecules.
Limitations of this study and suggestions for animators
The major limitation of this study is that the results are based on an interview protocol that used the two computer animations in a way that was not intended by their animators. Both animators recommend explaining the visual systems used in the computer animations and viewing the computer animations with narration; however, we were interested to see if the students could interpret the animations without narration or further explanation of the objects depicted in the animations. This information would be valuable since students could watch these animations on a computer without sound or without viewing previous multimedia lessons that explain the objects depicted in these animations. As a result, many of the student misconceptions and misinterpretations identified in this study may not have existed if the students had seen the previous lessons or heard the narrations associated with these animations. Mayer (2001), in describing his multimedia principle, states that students learn better from words (narrations or text) and pictures than from words alone. This is based on his cognitive theory of multimedia learning, which assumes that learners process information through a dual-coding capability involving an auditory/verbal channel and a visual/pictorial channel (Paivio, 1986; Mayer, 2001). The dual-coding capability of processing information asserts that students would learn better with words (narrations or text) and pictures than from pictures alone (Mayer and Anderson, 1991).Readers should also be cautioned against assuming that simply because a misconception was identified using these computer animations, that the computer animation was responsible for the misconception. It is very likely that students arrived at the interviews with pre-conceived notions that did not match the scientifically accepted view of chemists before viewing these animations. In these cases, the computer animation may simply have allowed the researchers to identify existing student misconceptions without necessarily being the cause of or reinforcing the misconception.
This study showed that many students had difficulty properly interpreting the two computer animations that were shown without their accompanying narration. These results show the importance of quality narration to explain what is being depicted in a computer animation, and animators and designers need to carefully plan and then evaluate the effectiveness of the narration accompanying any animation. In the more complex animation used in this study, we believe that the number of the nitrate ions (blue/red clusters) depicted should be increased and made more prominent in the animation. We would also suggest labelling the ions (silver, copper(II), and nitrate) in this animation with the proper charges. This is especially important given the fact that a study on student misconceptions in writing balanced equations for dissolving ionic compounds in water (Naah and Sanger, 2012) recently reported that several students believe that these ionic compounds dissolve in water as neutral atoms or molecules—for example, solid lithium chloride dissolving as Li(aq) and Cl(aq) and solid calcium carbonate dissolving as Ca and CO3(aq).
Future studies
Since the two animations used in this study describe the same chemical reaction, future research could compare the effectiveness of the more simplistic and the more complex animations on students' conceptual understanding of the oxidation–reduction process occurring in this reaction. We are currently working on a manuscript describing this study; similar studies have been performed using two different animations depicting the process of dissolving solid sodium chloride in water (Kelly and Jones, 2007; Kelly and Jones, 2008). These comparisons would allow researchers to determine whether the additional information provided in the more complex animation is required to better understand the chemical processes or if this information is extraneous and distracts from learning (Mayer, 2001). We are also interested in comparing students' conceptions of this oxidation–reduction process after viewing both animations, and whether viewing the more simplified or the more complex animation first will have an effect. A similar study was performed to determine whether the order of viewing a particulate animation and a videotaped demonstration of the same reaction affects students' conceptual understanding (Velázquez-Marcano et al., 2004). They found that students' conceptions improved after viewing each visualization, but found no preference regarding the visualization order. Since this study identified several student misconceptions and misinterpretations of these animations when narration was excluded, a study comparing students' conceptions based on viewing an animation with or without the accompanying narration would allow us to further test Mayer's multimedia principle (Mayer, 2001) with respect to viewing pictures alone versus viewing pictures and narrated text.Notes and references
- Andersson B., (1986), Pupils' explanations of some aspects of chemical reactions, Sci. Educ., 70, 549–563.
- Ardac D. and Akaygun S., (2004), Effectiveness of multimedia-based instruction that emphasizes molecular representations on students' understanding of chemical change, J. Res. Sci. Teach., 41, 317–337.
- Baddeley A. D., (1986), Working memory, Oxford: Oxford University Press.
- Ben-Zvi R., Eylon B.-S. and Silberstein J., (1986), Is an atom of copper malleable? J. Chem. Educ., 63, 64–66.
- Bodner G. M., (1986), Constructivism: A theory of knowledge, J. Chem. Educ., 63, 873–878.
- Bodner G. M., (1991), I have found you an argument, J. Chem. Educ., 68, 385–388.
- Bodner G., Klobuchar M. and Geelan D., (2001), The many forms of constructivism, J. Chem. Educ., 78, 1107.
- Boo H. K., (1998), Students' understandings of chemical bonds and the energetics of chemical reactions, J. Res. Sci. Teach., 35, 569–581.
- Borg W. R. and Gall M. D., (1983), Educational Research, (4th edn, pp. 441–443), New York: Longman.
- Butts B. and Smith R., (1987), HSC chemistry students' understanding of the structure and properties of molecular and ionic compounds, Res. Sci. Educ., 17, 192–201.
- Carlson R., Chandler P. and Sweller J., (2003), Learning and understanding science instructional material, J. Educ. Psych., 95, 629–640.
- Cho H., Kahle J. B. and Nordland F. H., (1985), An investigation of high school biology textbooks as sources of misconceptions and difficulties in genetics and some suggestions for teaching genetics, Sci. Educ., 69, 707–719.
- Clancey W. J., (1994), Situated cognition: How representations are created and given meaning, in Lewis R. and Mendelsohn P. (ed.), Lessons from learning (pp. 231–242). Amsterdam: North-Holland.
- Coll R. K. and Treagust D. F., (2003), Learner's mental models of metallic bonding: A cross-age study, Sci. Educ., 87, 685–707.
- Ebenezer J. V. and Erickson G. L., (1996), Chemistry students' conceptions of solubility: A phenomenography, Sci. Educ., 80, 181–201.
- Ferguson R. L., (2007), Constructivism and social constructivism, in Bodner G. M. and Orgill M. (ed.), Theoretical frameworks for research in chemistry/science education (pp. 28–49). Upper Saddle River, NJ: Pearson.
- Gabel D., (1999), Improving teaching and learning through chemistry education research: A look to the future, J. Chem. Educ., 76, 548–554.
- Garnett P. J. and Treagust D. F., (1992a), Conceptual difficulties experienced by senior high school students of electrochemistry: Electric circuits and oxidation–reduction equations, J. Res. Sci. Teach., 29, 121–142.
- Garnett P. J. and Treagust D. F., (1992b), Conceptual difficulties experienced by senior high school students of electrochemistry: Electrochemical (galvanic) and electrolytic cells, J. Res. Sci. Teach., 29, 1079–1099.
- Gilbert J. K. and Treagust D. (ed.), (2009), Multiple representations in chemical education, Dordrecht: Springer-Verlag.
- Gregorius R. Ma., Santos R., Dano J. B. and Gutierrez J. J., (2010a), Can animations effectively substitute for traditional teaching methods? Part I: Preparation and testing of materials, Chem. Educ. Res. Pract., 11, 253–261.
- Gregorius R. Ma., Santos R., Dano J. B. and Gutierrez J. J., (2010b), Can animations effectively substitute for traditional teaching methods? Part II: Potential for differentiated learning, Chem. Educ. Res. Pract., 11, 262–266.
- Haught J. F., (1995), Science & religion: Formulas for successful teaching (pp. 161–182). Washington, DC: American Chemical Society.
- Herron J. D., (1996), The chemistry classroom: From conflict to conversation (p. 13). Mahwah, NJ: Paulist Press.
- Herron J. D. and Nurrenbern S. C., (1999), Chemical education research: Improving chemistry learning, J. Chem. Educ., 76, 1353–1361.
- Huddle P. A., White M. D. and Rogers F., (2000), Using a teaching model to correct known misconceptions in electrochemistry, J. Chem. Educ., 77, 104–110.
- Johnstone A. H., (2006), Chemical education research in Glasgow in perspective, Chem. Educ. Res. Pract., 7, 49–63.
- Johnstone A. H., (2010), You can't get there from here, J. Chem. Educ., 87, 22–29.
- Kelly R. M. and Jones L. L., (2007), Exploring how different features of animations of sodium chloride dissolution affect students' explanations, J. Sci. Educ. Technol., 16, 413–429.
- Kelly R. M. and Jones L. L., (2008), Investigating students' ability to transfer ideas learned from molecular animations to the dissolution process, J. Chem. Educ., 85, 303–309.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: Expert and novice responses to different representations of the same chemical phenomena, J. Res. Sci. Teach., 34, 949–968.
- Liu X. and Lesniak K., (2006), Progression in children's understanding of the matter concept from elementary to high school, J. Res. Sci. Teach., 43, 320–347.
- Madden S. P., Jones L. L. and Rahm J., (2011), The role of multiple representations in the understanding of ideal gas problems, Chem. Educ. Res. Pract., 12, 283–293.
- Mayer R. E., (2001), Multimedia Learning, New York: Cambridge University Press.
- Mayer R. E. and Anderson R. B., (1991), Animations need narrations: An experimental test of a dual-coding hypothesis, J. Educ. Psych., 83, 484–490.
- Mulford D. R. and Robinson W. R., (2002), An inventory for alternative conceptions among first-semester general chemistry students, J. Chem. Educ., 79, 739–744.
- Naah B. M. and Sanger M. J., (2012), Student misconceptions in writing balanced equations for dissolving ionic compounds in water, Chem. Educ. Res. Pract., 13, 186–194.
- Nyachwaya J. M., Mohamed A.-R., Roehrig G. H., Wood N. B., Kern A. L. and Schneider J. L., (2011), The development of an open-ended drawing tool: An alternative diagnostic tool for assessing students' understanding of the particulate nature of matter, Chem. Educ. Res. Pract., 12, 121–132.
- Orgill M., (2007), Situated cognition, in Bodner G. M. and Orgill M. (ed.), Theoretical frameworks for research in chemistry/science education (pp. 187–203). Upper Saddle River, NJ: Pearson.
- Österlund L.-L., Berg A. and Ekborg M., (2010), Redox models in chemistry textbooks for the upper secondary school: Friend or foe? Chem. Educ. Res. Pract., 11, 182-192.
- Özkaya A. R., (2002), Conceptual difficulties experienced by prospective teachers in electochemistry: Half-cell potentials, cell potential, and chemical and electrochemical equilibrium in galvanic cells, J. Chem. Educ., 79, 735–738.
- Paivio A., (1986), Mental representations: A dual coding approach, New York: Oxford University Press.
- Roschelle J. and Clancey W. J., (1992), Learning as social and neural, Educ. Psychol., 27, 435–453.
- Sanger M. J., (2005), Evaluating students' conceptual understanding of balanced equations and stoichiometric ratios using a particulate drawing, J. Chem. Educ., 82, 131–134.
- Sanger M. J., Brecheisen D. M. and Hynek B. M., (2001), Can computer animations affect college biology students' conceptions about diffusion and osmosis? Am. Biol. Teach., 63, 104–109.
- Sanger M. J., Campbell E., Felker J. and Spencer C., (2007), Concept learning versus problem solving: Does particle motion have an effect? J. Chem. Educ., 84, 875–879.
- Sanger M. J. and Greenbowe T. J., (1997), Common student misconceptions in electrochemistry: Galvanic, electrolytic, and concentration cells, J. Res. Sci. Teach., 34, 377–398.
- Sanger M. J. and Greenbowe T. J., (1999), An analysis of college chemistry textbooks as sources of misconceptions and errors in electrochemistry, J. Chem. Educ., 76, 853–860.
- Sanger M. J., Phelps A. J. and Fienhold J., (2000), Using a computer animation to improve students' conceptual understanding of a can-crushing demonstration, J. Chem. Educ., 77, 1517–1520.
- Schmidt H.-J. and Volke D., (2003), Shift of meaning and students' alternative concepts, Int. J. Sci. Educ., 25, 1409–1424.
- Smith K. J. and Metz P. A., (1996), Evaluating student understanding of solution chemistry through microscopic representations, J. Chem. Educ., 73, 233–235.
- Smith K. C. and Nakhleh M. B., (2011), University students' conceptions of bonding and melting and dissolving phenomena, Chem. Educ. Res. Pract., 12, 398–408.
- Solomon J., (1987), Social influences on the construction of pupil's understanding of science, Studies Sci. Educ., 14, 63–82.
- Taber K. S., (1994), Misunderstanding the ionic bond, Educ. Chem., 31, 100–103.
- Taber K. S., (1997), Student understanding of ionic bonding: Molecular versus electrostatic framework? Sch. Sci. Rev., 78, 85–95.
- Talanquer V., (2011), Macro, submicro, and symbolic: The many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33, 179–195.
- Tasker R. and Dalton R., (2006), Research into practice: Visualisation of the molecular world using animations, Chem. Educ. Res. Pract., 7, 141–159.
- Tien T. L., Teichert A. M. and Rickey D., (2007), Effectiveness of a MORE laboratory module in prompting students to revise their molecular-level ideas about solutions, J. Chem. Educ., 84, 175–181.
- Treagust D. F., Chandrasegaran A. L., Zain A. N. M., Ong E. T., Karpudewan, M. and Halim L., (2011), Evaluation of an intervention instructional program to facilitate understanding of basic particle concepts among students enrolled in several levels of study, Chem. Educ. Res. Pract., 12, 251–261.
- Velázquez-Marcano A., Williamson V. M., Ashkenazi G., Tasker R. and Williamson K. C., (2004), The use of video demonstration and particulate animation in general chemistry, J. Sci. Educ. Technol., 13, 315–323.
- Williamson V. M. and Abraham M. R., (1995), The effects of computer animation on the particulate mental models of college chemistry students, J. Res. Sci. Teach., 32, 521–534.
- Yarroch W. L., (1985), Student understanding of chemical equation balancing, J. Res. Sci. Teach., 22, 449–459.
| This journal is © The Royal Society of Chemistry 2012 |
