Teaching two basic nanotechnology concepts in secondary school by using a variety of teaching methods
Ron
Blonder
* and
Sohair
Sakhnini
Department of Science Teaching, Weizmann Institute of Science, Rehovot 76100, Israel. E-mail: ron.blonder@weizmann.ac.il
First published on 1st October 2012
Abstract
A nanotechnology module was developed for ninth grade students in the context of teaching chemistry. Two basic concepts in nanotechnology were chosen: (1) size and scale and (2) surface-area-to-volume ratio (SA/V). A wide spectrum of instructional methods (e.g., game-based learning, learning with multimedia, learning with models, project based learning, and storytelling and narratives) was implemented to support students' understanding. Students' interviews and the content of students' final projects were used and analyzed to learn how using a variety of teaching methods influenced students' understanding of basic concepts in nanotechnology. In addition, the study examined which methods enhance students' understanding and which do not, according to students' perceptions. Students felt that most of the teaching methods facilitated their learning, with the exception of two activities: the Hungarian cube, and nano effect simulation; these two activities were very abstract and confused the students. Finally, we formulated recommendations regarding methods for teaching nanotechnology in the future.
Introduction
Nanotechnology: what is it?
Nanotechnology forms an “entrance to a new world” (Ratner and Ratner, 2003). Nano, a little word with huge potential, has been rapidly establishing itself in the world's consciousness, almost in every aspect of science and engineering, with implications for ethics, economics, international relations, day-to-day life, and even humanity's conceptions of its place in the universe (Ratner and Ratner, 2003; Roco, 2003). Obviously, from our real-world perspective, at the macro scale, chemical and physical properties of materials are not size dependent, regardless of the material's identity. Upon reaching the nanoscale, however, many properties of matter will change. Therefore, nanotechnology brought about a big challenge to chemistry educators.Engineers are concerned with better understanding and utilizing nanoscale materials. Materials scientists and electrical, chemical, and mechanical engineers deal with the unique properties of nanostructures and with how those special properties can best be utilized in manufacturing entirely new materials that could provide new capabilities in medicine, industry, recreation, and the environment (Ratner and Ratner, 2003; Roco, 2001). From the point of view of fundamental science, a better understanding of nanoscale is important if one strives to understand how matter is constructed and how the properties of materials reflect their components, their atomic composition, their shapes, and their sizes. These concepts traditionally belong to chemistry educators. Roco (2003) described the importance of education for the future development of this field: “One of the ‘grand challenges’ for nanotechnology is education, which is looming as a bottleneck for the development of the field” (Roco, 2003, p. 1247). Trying to deal with this challenge produced many educational programs, curricula, and modules in the area of nanotechnology and nanochemistry. A sample of those programs will be described next.
Nanotechnology in K-12
In their study, Ambrogi et al. (2008) performed an instructional teaching activity based on nanotechnology and nanochemistry. The educational aims of the project were to promote both content knowledge and social skills (e.g., team work, communications, and problem solving) in secondary school students. To this end, many students displayed initiative, drive, empathy, and commitment in working in this field. For example, in teaching a class at a Scientific-Technological Lyceum (age 17), it was decided that students will produce a PowerPoint presentation in order to introduce lower-grade students to nanochemistry and nanotechnology, which are unusual topics that are not included in the Italian high-school curriculum. Nanotechnology is a new field that is not covered by school textbooks. Therefore, the students had to cooperate in order to locate materials, use ICT (Information Communication Technology) sources, and make decisions, such as selecting information and choosing slide layouts. Furthermore, a cooperative learning methodology was employed to solve the problem of setting up the presentation. The initiators of this project found that the students involved seemed to lack critical thinking: When stoichiometric problems on equilibrium, such as acid–base, solubility, or coordination compound stability were posed, students immediately applied any algorithm, and rushed through a numerical output without considering its congruence within the context. This kind of behavior reflects their limited capability to be critical and their disinclination to analyze their work. In an attempt to correct these deficiencies, the students were required to complete a final project whereby they had to find, interpret, and select information. This learning route enhanced the students' content knowledge of elementary nanochemistry and nanotechnology, as well as enhanced their social skills such as team work, communication, and conflict management. This project was largely based on using ICT, and on making the computer the main component in the learning environment. As a consequence, these students learned to effectively use ICT, which is not only a curricular subject—it is also a very useful information source in everyday life and in almost every work place. Furthermore, situated learning that involves well-known tools (ICT tools) could help in approaching this new topic with a positive feeling. To carry out the group's investigation, the students had to use knowledge, social skills, and technological tools similar to those encountered in the real world; therefore, this activity can be regarded as authentic learning (Stein, 1998). The activity created a pleasant class atmosphere and also led to retention of the content knowledge.Harmer and Columba (2010) explored factors that contributed to engaging middle school students during a problem-based inquiry in which nanoscale science was introduced and nanotechnology-based solutions were discussed. They applied a mix-research methodology and used quantitative tools to measure students' knowledge, and qualitative tools to learn about students' engagement. They found that students' knowledge of nanoparticle measurement, size, and applications and the use of electrons in the scanning electron microscope increased. Students were highly engaged in the subject as a result of the program. The evaluation of the project indicated that it increased students' understanding of basic concepts in nanotechnology, increased their understanding of core science concepts, and supported inquiry-based teaching and learning. However, the project did not increase students' interest and engagement in learning science. A different curriculum unit called “NanoLeap” was also developed and implemented in secondary schools (Greenberg, 2009). This unit was designed to include transitional concepts that link core science concepts, as found in the National Science Education Standards, and those that are defined in the Big Ideas in Nanoscience (Stevens et al., 2009).
In a different paper, the authors presented a nanotechnology module that was designed for high-school students (Blonder and Dinur, 2011). Here, advanced contents were uniquely adapted to the high-school students' level by using a constructivist pedagogy in which the students are at the center of the learning. Moving from a teacher-centered to a student-centered pedagogy switches the control of the learning environment from the teacher to the students. Students' interviews and a semantic differential (SD) questionnaire were used to reveal students' motivation and their perceptions. The study indicated that the students appreciated the topic of light emitting diode (LED) and found that it increased their motivation to learn more about LED, nanotechnology, and chemistry. The student-centered pedagogy that was chosen also contributed positively to students' continuing motivation to learn this topic.
The above-mentioned programs and those of others were aimed at introducing nanotechnology to school children. Each focused on a certain aspect of nanotechnology and was not necessarily based on a thorough analysis of the field of nanotechnology as taught to school children. An attempt to identify the main concepts that make up nanotechnology and that are relevant in incorporating nanotechnology into high school science is important for better understanding the field.
Big ideas for teaching nanotechnology
The expression “big ideas” here refers to the fundamental concepts (key concepts) that would enable students to explain phenomena within and across disciplines (Stevens et al., 2009). Every scientific domain is built upon a set of core concepts, the understanding of which is essential to learning the domain. These core concepts might be needed to explain phenomena relevant to a field, or they may contribute to a broader conceptual understanding that must begin with foundational ideas. Big ideas form the very core of a domain. They are critical for gaining a basic knowledge of the field because a deeper understanding depends on these basic ideas, which constitute the building blocks for a deeper, conceptual understanding of science in the future. Big ideas may be cross-disciplinary, that is, they may be thought of as “big ideas” in science in general, rather than more narrowly conceived of as “big ideas” in only chemistry or biology (Stevens et al., 2009). For example, the basic physics of atoms and molecules is the foundation of all science. Therefore, early emphasis on these concepts would most likely prove beneficial for students when they study biology, chemistry, physics, and earth science, in addition to other subjects (Stevens et al., 2009). Building a basic understanding in all of these disciplines from the atomic and molecular levels can facilitate the interdisciplinary connections that students need to better understand nanoscience and other emerging sciences. Stevens et al. (2009) described a conference that was held in November 2006, in which a group of nanoscientists and science educators met for two and a half days to define the “big ideas” of nanotechnology. They agreed upon nine Big Ideas of Nanoscience: (1) Size & Scale, (2) Structure of Matter, (3) Size-Dependent Properties, (4) Forces and Interactions, (5) Quantum Effects, (6) Self-Assembly, (7) Tools & Instrumentation, (8) Models & Simulations, and (9) Nanoscience Technology and Society.Concepts such as size and scale and “surface-area-to-volume ratio” are considered important by different science educators (Taylor and Jones, 2009; Jones et al., 2007) and underlie all of the big ideas that Stevens et al. (2009) defined. The surface-area-to-volume ratio is a potential threshold concept (Swarat et al., 2011) that explains many properties at the nanoscale. A sophisticated understanding of these two big ideas was regarded as a prerequisite to learning size-dependent properties and other advanced nanoscience concepts. In this paper we focused on two core concepts of nanotechnology: (1) size and scale and (2) the surface-area-to-volume ratio (SA/V).
Size and scale
The prefix “Nano” means one billionth (1 nm = 1 × 10−9 m). To get a sense of the nanoscale, a human hair measures 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nm, and a bacterial cell measures a few hundred nanometers across. The smallest things that humans are able to see with an unaided eye are 10
000 nm, and a bacterial cell measures a few hundred nanometers across. The smallest things that humans are able to see with an unaided eye are 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nanometers across. Nevertheless, size and scale are not about size alone; however, defining the words “size and scale” is very important. Size is defined in terms of the nanoscale itself, and scale is a critical concept because it defines the set of rules needed to explain the behavior of matter at that scale (Stevens et al., 2009), because not only the size of objects and systems changes with scale—also the way in which they function or behave.
000 nanometers across. Nevertheless, size and scale are not about size alone; however, defining the words “size and scale” is very important. Size is defined in terms of the nanoscale itself, and scale is a critical concept because it defines the set of rules needed to explain the behavior of matter at that scale (Stevens et al., 2009), because not only the size of objects and systems changes with scale—also the way in which they function or behave.
Several research studies explored students' conceptualization of size and scale (Tretter et al., 2006). Accordingly, these authors tried to determine the cognitive frameworks concerning conceptualizations of size and scale; they detected potential variability as a function of age. They also sought to better understand the possible influence of formal educational and other experiences. Finally, they compared students' conceptualizations of scale with those of ‘experts’ in science. Two hundred and fifteen students were involved; they were divided into five groups: 5th, 7th, and 9th grade students from a single school district, 12th grade ‘gifted’ students, and a few doctoral students. Data analysis tended to reveal the conceptual boundaries of distinctly different sizes, among all students. It appeared that 12th graders and doctoral students (referred to as ‘experts’) distinguished more refined categories for small-sized objects. But it was difficult for all students to distinguish between nanometric and micrometric objects. The limit of visibility to the naked eye was at a scale where accuracy dropped precipitously for all groups. There were appreciable differences in nanoscale conceptions between experts and K-12 students, and research revealed that a primary reason for those differences was the extensive experiences the experts had acquired by interacting with nanoscale phenomena over the course of many years. Thus, the authors stressed the importance of past, direct, and indirect experience to enhance size and scale knowledge. They suggested that resorting to virtual reality tools could prove beneficial to those students needing direct experiences. These experiences (e.g., visualization) helped the students advance their knowledge about size and scale (Jones et al., 2011). Jones et al. (2007) found that use of a commonly used instructional tool targeting the concept of scale was very useful. Specifically, when the video the Powers of Ten (http://powersof10.com) was used for teaching this topic to middle school students, it positively increased their understanding of the powers of ten and scale. Jones et al. (2007) also reported that teachers who had used this video indicated that the zooming strategy used in moving slowly away from human scale in both small and large directions was effective in enhancing students' understanding of the different scales. An understanding of scale is becoming increasingly important due to the technological advancements that now allow us to explore new worlds and realms of science (Jones et al., 2011; Roco, 2001; Swarat et al., 2011).
Use of visualization and haptic tools offers a meaningful approach for dealing with the size and scale concepts and area-to-volume relationships (Jones et al., 2006). Using visualization helped students build a deeper understanding of the concepts and improved the students' attitudes towards science or even towards the idea of making science their profession. Thinking with images plays a central role in the students' scientific creativity. Moreover, the need for visual–spatial cognition is fundamental in the science classroom (Mathewson, 1999).
The term haptic encompasses the study of touch and human interactions with an external environment through touch. Jones et al. (2006) investigated the impact of haptic augmentation through virtual reality in a science inquiry program in which students learned about viruses and nanoscale science. The study assessed how adding different types of haptic feedback (e.g., active touch and kinesthetic feedback), combined with computer visualizations, influenced middle- and high-school students' experiences. The results indicated that adding haptic feedback provided a more immersive learning environment that not only made the instruction more engaging but may also influence the way that the students construct their understandings about abstract science concepts such as viruses and the microstructure of organelles within a cell. Some researchers believe that many of the science concepts that students are expected to understand are invisible and abstract, whereas the thinking of most school-age children relies heavily on sensory information (Wu et al., 2001).
Surface-area-to-volume ratio (SA/V)
If a property of matter is dependent on volume (e.g., heat capacity and mass), then it will change much faster than those properties dependent on area (e.g., cooling surface and absorptivity) for a given change in size (Stevens et al., 2009). Many of the special properties that matter exhibits on the nanoscale result from the effect of size on the surface-area-to-volume ratio. In chemistry, this relates to the number of atoms on the surface relative to that of the bulk material. Because the surface atoms/molecules interact with the environment, the surface-area-to-volume ratio (SA/V) significantly affects the chemical reactivity (Stevens et al., 2009). The smaller the particles are, the more the SA/V ratio will increase dramatically and the higher the SA/V and the reactivity of the material will be.Taylor and Jones (2009) tried to determine whether there is a correlation between proportional reasoning ability and understanding surface-area-to-volume relationships. This aspect plays a prominent role in explaining the behavior of some nano-phenomena and is indeed of central importance to understanding some particular properties of nanomaterials. The study involved 19 students (aged 11 to 13) who took part in a five-day summer camp. Students' proportional reasoning skills were assessed by means of an instrument previously developed, consisting of 10 open-ended questions. In addition, an instrument aimed at probing students' understanding of surface-area-to-volume relationships was developed. Each of these tests consisted of multiple choice questions, problem-solving items, diagrams to be filled in, and required the students to explain some answers. Interestingly, the students' proportional reasoning ability and their achievement in the test on surface-area-to-volume relationships were significantly correlated. In their discussion, the authors raised an important question regarding “other constructs (e.g., visual and spatial skills) that may be involved in understanding of scale applications such as limits to size and surface-area-to-volume relationships.” (Taylor and Jones, 2009, p. 1237). Jones et al. (2007) argue that in order for students to understand essential concepts of scale in science, proportional reasoning is required. Tobin (1981) suggested that students' difficulty in solving proportional reasoning problems is directly related to abstract thinking and that it has serious implications for science instruction. Taylor's (2008) study examined students' understandings of the relationship between surface-area-to-volume and its application in various situations. The results of this study indicated that proportional reasoning ability is not only correlated with students' ability to understand surface-area-to-volume relationships—it may be a possible predictor of how well a student can comprehend this scaling concept. As a result of their study, three questions emerged as being important for further study: (a) What developmental level is necessary for students to fully understand the relationship between surface area and volume? (b) What type of background knowledge is necessary for students to understand surface-area-to-volume relationships and how does it affect the sequence of science instruction? (c) What other constructs may be involved in understanding scale applications such as limits to size and surface-area-to-volume relationships?
According to Taylor (2008), different students may approach a proportional reasoning problem with different methods and perhaps get the same answer, but the thought processes may reflect different levels of reasoning. This variation in methods was seen in the results of the present study when students were asked to choose the greatest surface-area-to-volume ratio from different size cubes. Some students intuitively chose the largest cube which is actually an incorrect answer. Only students that possessed an understanding of how the two measurements (surface area and volume) related to each other proportionally were able to accurately apply the concept to a specific scientific context. Students at a transitional level of understanding may be able to calculate surface area and/or volume, but may or may not be able to apply and explain the concept to a scientific context. Higher cognitive skills are required for students to be able to take the mathematics concept of surface-area-to-volume ratio and fuse it with a scientific concept. Therefore, students must make the transition from concrete understanding of surface-area-to-volume to a more abstract understanding of the concept.
Other factors such as visual–spatial skills could affect an individual's ability to understand and apply surface-area-to-volume ratios. Thinking with images plays a central role in scientific creativity (e.g., graphics, metaphors, and unifying ideas/themes) and underscores the need for visual–spatial cognition in the science classroom (Mathewson, 1999). For example, students were asked which primate (pygmy monkey, gorilla, lemur, or spider monkey) would have a greater rate of heat loss if placed in a cold environment (Taylor, 2008). Height and weight information for each primate were provided and the students had to understand how the size of primates (greater surface area and less volume) contributes to a higher rate of heat loss owing to a greater surface area and lesser volume. The study concluded that proportional reasoning is positively correlated to students' understanding of surface-area-to-volume relationships and that instructional intervention promotes knowledge of surface-area-to-volume ratios in science contexts (Taylor, 2008).
To address the challenges of bringing emerging science into the classroom, we need to use suitable teaching methods in order to capture the students' interests in science, motivate them to learn new challenging topics in science, and support their understanding. In practice, it is often difficult to respond to each student's needs and learning style; however, much can be achieved if teachers employ a wide repertoire of instructional methods (Hofstein et al., 2006). This enables many students to study chemistry in ways that are more aligned with their own interests and learning styles (Young et al., 2003). Implementing a wide spectrum of instructional techniques and ways of measuring students' achievement and progress requires matching an appropriate assessment tool to each technique.
Next our working definition for each of the teaching methods that were used in this study will be described. In addition, a short literature review regarding the educational benefits and characterization will be described.
Suitable teaching methods for nanotechnology concepts
Game-based learning
Games engage people, make learning fun, and motivate students and help them pay attention and stay focused on a subject. There is evidence that games allow students to focus and learn better (Barab et al., 2005). Lepper and Cordova (1992) found that rewriting a lesson with a story context, combined with a challenge for the student to overcome (in other words, making it into a game) significantly improves the learning performance of children. Computer games enhance learning through visualization, experimentation, and creativity of play (Betz, 1995). Visualization has tremendous value in computer games (Amory et al., 1999) because it plays an important role in discovery and problem solving (Rieber, 1995). According to Sekuler and Blake (1994), our sense of vision represents our most diverse source of information of the world around us. A growing body of research has investigated the efficiency of computer-generated visualizations in enhancing students' understanding of complex scientific topics such as molecular structure (Ferk, 2000; Ferk et al., 2003) or continuous symmetry (Tuvi-Arad and Blonder, 2010). Here visualization refers to anything that can help one visualize how a concept or a system works; this includes animations, simulations, images, and games. The following teaching method describes visualization in more detail.Learning with visualization and multimedia
Studies on cognition have shown that learners receive information through visual and auditory channels (Paivio, 1986, 1991; Mayer, 2001). It has been shown that the learner's ability to construct conceptual relationships or schema can be impaired if the information is received through only one of these channels; here the learner must store much of the received information in short-term, working memory (Sweller, 2002). Multimedia learning that uses both channels addresses limited cognitive capacity, supports students’ active processing, and results in deeper learning (Mayer, 2002). Kozma and Russell (2005) emphasized that visualizations can be used in chemical education to support the learning of difficult chemistry concepts and principles such as bonding, structure, reactivity, equilibrium, and acidity (Kozma and Russell, 2005). Instructional programs involving visualizations in a format that includes explicit learning objectives and structures resulted in the learner being actively engaged, and perhaps lead to higher motivation (Naps et al., 1996; Mayer, 1999). In our study we used images and two kinds of multimedia: movies and simulations.Learning with movies
Use of movies in science education has been successful in overcoming problems that cannot be eliminated with traditional teaching methods (e.g., understanding and conceptualization difficulties, misconceptions, and motivation) (Sanger and Greenbowe, 1997; Burke et al., 1998; Ebenezer, 2001; Kelly and Jones, 2007). Movies facilitate learning by allowing students to animate abstract chemical concepts in their minds (Williamson and Abraham, 1995; Cavanaugh and Cavanaugh, 1996; Goll and Woods, 1999; Sanger et al., 2000; Laroche et al., 2003; Yang et al., 2003; Marcano et al., 2004; Sanger et al., 2007) and make it easier for students to remember the important points of the subject matter (Kumar, 1991). It has been mentioned that movies have a positive impact on acquiring knowledge (Zahn et al., 2004; Michel et al., 2007) and contribute to the development of a student's cognitive capabilities, including interpreting, critical thinking, and problem-solving skills (Kumar et al., 1994; Hagen, 2002). The use of movies as teaching materials also has a positive impact on a student's motivation (Kumar, 1991; Hagen, 2002).Learning science with simulations
The terms “simulation” and “animation” refer to presentations of dynamic scientific processes or systems in order to drive or test possible explanations or theoretical models (Kozma and Russell, 2005). Computer simulations enhance student learning through visualization, experimentation, and creativity of play (Betz, 1995). They allow students to visually see the cause and effect of their actions on the whole system. In the context of phenomena that take place at the microscopic level or of scientific models, animations provide students with the opportunity to see things that cannot be directly perceived (Pekdag and Le Maréchal, 2010). This visual understanding has the potential for students to form intrinsic thinking skills in complex decision making situations (Betz, 1995). Furthermore, computer simulations (e.g., games) are fun and have quick playing times to hold students' interest (Betz, 1995). Animations can help students understand difficult concepts related to various chemical topics (Kozma and Russell, 2005). When students interact with information that is presented in both pictures and words, they are more likely to learn difficult concepts and principles as well as retain what they learn longer than if the information is presented in words alone (Kozma and Russell, 2005).Learning with models
A model in science is a representation of a phenomenon initially produced for a specific purpose (Gilbert and Boutler, 2000). The specific purpose for which any model is originally produced in science is to simplify the phenomenon to be used and to provide explanations of the natural world. “One of the major contributions of models to science education is that the formation of mental models and the public presentation of expressed models are central to the development of an understanding of any phenomenon” (Gilbert and Boutler, 2000). Our emphasis in this module is on expressed models, which are external representations used not only in communication but also in reasoning (Larkin, 1989; Larkin and Simon, 1987). Expressed models represent selected aspects of phenomena and of our mental models (Gilbert and Boutler, 2000). Model-based teaching makes use of expressed models to construct mental models through a recursive process of formation, use, revision, and elaboration. With mental models there are expectations about how the object, event, or system looks and behaves. Our mental models can also be used to understand and evaluate the expressed models produced by others. Direct experience with phenomena via video or simulations, or by interacting with many representations and expressed models in teaching can all contribute to model building (Gilbert and Boutler, 2000).Project based-learning (PBL)
Project based-learning (PBL) is a comprehensive perspective that focuses on teaching by engaging students in investigation. Project-based learning places students in realistic, contextualized problem-solving environments. Within this framework, students ask and refine questions, debate ideas, make decisions, design plans and/or experiments, collect data, draw conclusions, communicate their ideas and findings to others, ask new questions, and create artifacts (Blumenfeld et al., 1991). In addition, project-based learning is adaptable to different types of learners and learning situations (Blumenfeld et al., 1991). By using PBL, students gain a deeper understanding of the learned concepts. Projects also build vital workplace skills and lifelong habits of learning and allow students to address community issues, explore careers, interact with adult mentors, use technology, and present their work to audiences beyond the classroom. PBL can motivate students who might otherwise find school boring or meaningless (http://www.bie.org/about/what_is_pbl/).Storytelling and narratives
Storytelling can be described as a spoken narrative that is the outcome of a mental process that enables us to excise from our experience a meaningful sequence (Rosen, 1985). Teaching with storytelling or narratives gains the students' attention (Koenig and Zorn, 2002; Fairburn, 2002; Davis, 1993; Weimer, 2002), and enables the students to be exposed to scientific/ethical dilemma. In addition, it could be used as an alternative to the traditional academic essay, as an assessment tool to measure the level of learning that the student has attained (Vella, 2002), and it can be used as a research tool for critical reflection on practice (Koenig and Zorn, 2002). It also allows creative expression and an intuitive application of scientific knowledge (Leight, 2002).The study
Rationale and objectives
The goal of this work was to develop a nanotechnology teaching module that is based on choosing teaching methods that will support students' understanding of two basic concepts in nanotechnology: (1) Size and Scales, and (2) the Surface-Area-to-Volume Ratio (SA/V). In implementing a wide spectrum of instructional methods, one should evaluate the way students perceive the effectiveness of the different teaching techniques. Therefore, the following directions were explored: (1) determining how using a variety of teaching methods influenced students' understanding of basic concepts in nanotechnology and (2) determining which methods students prefer and support their learning. These two goals will provide an evaluation of the effectiveness of the nanotechnology module for ninth graders.Course setting and population
The nanotechnology lessons were given at the “Italian school”, Haifa, Israel, during the chemistry and technology lessons, for two classes of 9th grade students. The total number of students who participated was 60 (30 males/30 females). It was decided to implement the nanotechnology lessons in the framework of science and technology lessons because in those lessons the class is divided into two groups (four groups of 15 students). This division allows the students to be in a convenient learning setting within the computer classroom and supports the students' active learning. For example, the games were played by the students in pairs, the computer simulations were experienced by each student using a computer, and some of the activities were given as lectures (e.g., learning from narratives).Therefore, the student population was divided into four groups. Each group had one weekly lesson of 45 minutes for 12 consecutive weeks during the first trimester. The second author of this paper taught all the four groups. She is a chemistry and technology teacher with over 15 years of experience and previously taught these students chemistry and technology. The instruction was done in Arabic and all the materials that were originally in English were translated into Arabic by the second author (e.g., subtitles for the movies).
Module structure and content
The nanotechnology module consists of two main parts: The first part was devoted to teaching two basic concepts in nanotechnology and lasted 9 teaching hours. The second part was conducted at the end of the first trimester and included personal guidance of the students in a “final project” in nanotechnology. The projects were presented at a summarizing event that was held at the end of the course. In the summarizing event, each student presented a chosen article or a nanotechnology application, in the presence of his or her parents, the teaching staff, and a researcher from the Technion, the Israel Institute of Technology, who is an expert in nanotechnology applications.The first ten lessons focused on the following topics in nanotechnology:
• Introduction to nanotechnology
• Introduction to the nanoscale
• The significance of the nanotechnology
• Different types and shapes of nanomaterials
• Nanotubes, nanoparticles, and their applications
• The surface-area-to-volume ratio (SA/V)
• Applications of nanotechnology
In each of the lessons the teacher used diverse instructional methods that were adapted and suited to the contents and aims of the lessons, as will be described in Table 1. Table 1 presents the instructional activities and the goal of each of them, and indicates the corresponding teaching method, according to the teaching sequence, and the Web sources for the methods that required internet use. Some of the activities are taken from the students' world of entertainment and games.
| Instructional activity (Teaching methods) | Description and source | Learning goals |
|---|---|---|
|
1. Nano scraps
(Game-based learning) |
Lessons 1&2: Introduction to nanotechnology and the nanoscale | To identify students' initial knowledge for assessment purposes. |
| The students were asked to cut the smallest scrap of paper that they can, and write on it (without letting a student friend see what he/she wrote) the name of an object that, in his/her opinion, is on the nano-scale. | To make the students feel the difference between their regular “small world” size and the new “nano world” small size. | |
|
2. Size and scale (Learning with visualization)
|
The students saw and recognized the nanometric size and scale, and were able to categorize the size and scale for the different objects presented in the illustration and in the nano scrap activity. http://inl.int/what-is-nanotechnology-2 |
To revisit the nanometric scale and nano-size objects.
To correct the students' misconceptions about nanometric size and scale. |
|
3. Cutting it down to nano
(Game-based learning)
|
The students were invited to try to cut a strip of paper (dimensions 150 mm × 5 mm) in half until the piece of paper is just a nanometer wide, or until they cannot cut it anymore. Then they were told to cut the smallest piece of paper they have, 27 more times. The students tried, but of course, they could not do it. The students experienced a visual representation of the exponential decrease in the paper's size.
http://mrsec.wisc.edu/Edetc/EExpo/cutting/index.html |
To reinforce the idea that the nanoscale is much, much smaller than even the smallest visible objects.
To provide the students with the opportunity to “feel” how small the nano dimension is. To understand that one needs unique devices to cut and work with nanometric materials. |
|
4. “Nanotechnology: What is it?”
(Learning with movies)
|
The students watched two kids explaining what are nanotechnology, sizes and scales, and the significance of the area. The students zoom in on a human hair as a concrete model that can be touched by the students and which can demonstrate for them the nano scale and its magnitude.
http://www.youtube.com/watch?v=2iSE6XlFXhA |
To present to the students what is nanotechnology and what is its scale and magnitude in a friendly way mixed with humor.
To encourage the students to feel empathy toward the two kids in the video explaining nanotechnology. |
|
5. Nanoquest
(Game-based learning)
|
The students need to choose a character. Their character has to run around the crystal landscape collecting parts to build the nanocar.
The Nanoquest 3D computer game was both enjoyable and challenging for the students to play. http://www.nanoquest.ie/play.html |
To explain the nanometric scale to students in an attractive, interesting, and experiential way.
To expose the students to different shapes of nanomaterials (e.g., nanotubes, nanoparticles). |
|
6. “Oknano”
(Learning with movies)
|
Lessons 3&4: The significance of nanotechnology | To visually repeat the nanotechnology concepts learned to further motivate them. |
|
The students watched a short movie about nanotechnology: Dimensions, applications in industry and technology and its importance. The video repeats the three basic nanotechnology concepts that were explained before (the unique characteristics of nanometric materials, the surface-area-to-volume ratio, controlling the atomic structure of the matter and its arrangement). The students were amazed and fascinated by the applications presented in this video and were triggered to ask questions about the different applications they watched.
http://www.oknano.com/video/NanotechnologyforStudents.wmv |
To trigger a discussion about nanotechnology applications. | |
|
7. The nano effect
(Learning science with simulations) |
The simulation was used to provide an answer to a very important question in the field of nanotechnology:
Which characteristics of matter change in the nanometric scale? The students could understand that one of the answers to this question is that changes occur to the atoms found on the surface area with regard to the volume of the matter. The smaller the volume of the matter, the highest the number of atoms found on the surface. http://community.nsee.us/concepts_apps/tool_for_nano_thermodynamics/Computer-based_Learning_Tool.html |
To describe the mechanism underlying increasing the surface-area-to-volume at the nanoscale level.
To see how the SA/V ratio affects the characteristics of matter. To appreciate the impact of nanotechnology on our world. |
|
8.
a. Dices (Learning with models)
b. Hungarian cube (Learning with models)
c. Separated small cubes (Learning with models)
|
The students used concrete dices, cubes, and the Hungarian cube to explain the concept of the surface-area-to-volume ratio (SA/V), a concept that is hard for students to comprehend. | To explain the concept SA/V in a concrete way that can be felt (as opposed to simulation). |
|
9. Material polystyrene model
(Learning from models)
|
The students assembled a polystyrene ball cube, imitating the images shown. They used the material model they had created to explain the concept (SA/V):
Atoms found on the surface area of matter have characteristics different from the atoms of the same matter found inside. Atoms found on the surface area of matter are exposed to the outer environment. They are surrounded by fewer atoms, in comparison with the inside atoms of matter. The SA/V is connected to the change in the chemical and physical properties of matter (e.g., solubility, melting points, color, strength, the ability of matter to react). |
To connect visualization of nanoparticles to a concrete model.
To present the importance of the SA/V concept in nanotechnology science and applications. |
|
10. Analogical models from everyday life phenomena
(Learning with models)
|
When examples from students' everyday life were used, the students could use the scientific concept (SA/V) in examples they were familiar with for example: heat radiators, and how elephants succeed to cool themselves and keep their bodies at low temperatures when hot. |
To explain the concept SA/V.
To show connections from everyday life. |
|
11. The “Magic ball”
(Learning with models, & game-based learning)
|
The students arranged the carbon atoms, using the regular model of atoms, on the edges of the magic ball. They saw that when they arranged all the atoms on the surface of the ball, the atoms were not stable, and that because of their higher surface area energy, they tended to collapse and reduce their surface area energy by gathering together and returning to the micrometer scale (something that we are not interested in). |
To visually explain why nanomaterials have high surface area energy.
To give an explanation of the concept of the surface area energy of nanomaterials very simply. To emphasize that controlling the size of nanomaterials and preventing their re-crystallization is one of the challenges that the nanotechnology industry deals with. |
|
12. Models and the modified magic ball
(Learning with models, & game-based learning)
|
Lessons 4&5: Nanotechnology and its applications | To assemble different types and shapes of nanomaterial structures by using the regular atomic model. |
| The students used models to build fullerene and carbon nanotubes to demonstrate different nanoparticle shapes. For example, nanoparticles can be produced in different shapes (e.g., cube, bulk, and ellipse). It will be considered a nanoparticle if one of its dimensions is less than 100 nanometers. Nanotubes are nanomaterials in the form of a hollowed tube; their diameter is less than 100 nanometers, and their length can be thousands of nanometers. The students saw how carbon nanotubes (CNT) are formed. They referred to the covalent bonds formed between carbon atoms in the same layer and the van der Waals bonds formed between the layers and the differences between the strengths of these two kinds of bonds. | ||
|
13. Images of nanomaterials
(Learning with visualization)
|
Presenting different images of nanomaterials to the students such as carbon nanotubes (CNT), inorganic nanotubes (INT), single wall nanotubes (SWNT), and multi wall nanotubes (MWNT). | To expose the students to different types and shapes of nanomaterial structures, by using the regular atomic model for assembling the fullerene structure and carbon nanotubes. |
|
14. A story documented with photos about the teacher's experience working in a research nano-laboratory
(Storytelling and narratives) |
A story from the teacher's own experience about the time she spent working in a research nanolab at the Weizmann Institute of Science under the supervision of Prof. Reshef Tenne, the inventor of inorganic nanotubes (INT), and his group. The topic of the research was: How might the mechanical characteristics of the INT nanotubes be affected while nanoparticles are being inserted into them, and how can we insert the nanoparticles into the INT? |
To experience the scientific and the underlying real world through the teacher's experience.
To represent the real world of research work, its importance, the time and efforts devoted to this challenging work. To motivate students to learn about nanotechnology applications and their properties, a topic planned for the next lesson (lesson number 6&7). |
|
15. Papers & TEM images
(Teaching with models, & teaching with visualization)
|
Lessons 6&7: Nanotubes and their applications. | To understand TEM images of INT. |
|
The students arranged papers (size A4), one upon the other, to form a cylinder. The students were asked to count the number of layers that make up the cylinder. Then they understood that if they count the edges where the cylinders transect, they will know the number of layers that make up the cylinder. The activity was then compared to TEM images. The black lines indicate the number of nanotube layers.
Each layer appears as a line on the edges of both sides where the cylinder transects. |
||
|
16. Mechanical properties of the nanomaterials and their applications
(Learning with movies) |
The students watched videos showing the unique mechanical properties of the nanomaterials + examples of nanomaterial applications in several domains or fields. It was explained that a beam of light can pass through a CNT of 10–20 nm diameter. The CNT considered was a very good conductor of electricity. That is why they are used in touch screens. As opposed to the commonly used materials, carbon nanotubes are considered very strong, elastic, materials. They are not easily destroyed as a result of stretching or pressure (they are durable under stretching or pressure). CNTs have very strong mechanical properties (exactly as the student learned). Scientists believe that CNTs can be used as an electrode for a touch screen.
The first video showed the mechanical properties (e.g., pressure) of WS2 INT. https://www.weizmann.ac.il/materials/large_bending_deformation_0001.wmv The second video showed another mechanical property (stretching) of WS2 INT. https://www.weizmann.ac.il/materials/pulling2.wmv |
To emphasize the significance of the nanomaterials and their unique properties. |
|
17. “Sonol Nanolub” Oil additive
(Everyday life example)
|
The teacher brought the students the “Sonol Nanolub”oil additive bottle. This was the first time they saw a nanotechnology-based product. The advantages of “Sonol Nanolub” oil additive compared with normal oil additives are as follows:
The oil reduces friction, wear, and temperature; lubricants, especially at high loads, save energy and reduce pollution; green/environmentally friendly material, maintains the precision of machine parts. |
To present an existing application of nanomaterials, based on INT.
To show the students a common and practical nanotechnology product. To emphasize the importance of science in our everyday life. |
|
18.
Students' own technological gadgets, e.g. iPhone (Everyday life example)
|
Lessons 8&9: Nanotechnology applications |
To expose the students to different kinds and domains of nanotechnology applications.
To understand the technological applications resulting from the concepts students learned previously. To deal with new concepts that were not expressed in class. To present to the students how nanotechnology affects everyday life. To present the advantages of nanotechnology compared with regular technology. |
|
The applications were chosen by considering the boys' and girls' interests and connections to scientific concepts and the knowledge students gained during the previous lessons.
The technology applications introduced new concepts. For example, increasing the available power from a battery and decreasing the time required to recharge a battery. These benefits are achieved by coating the surface of an electrode with nanoparticles. This increases the surface area of the electrode, thereby allowing more current to flow between the electrode and the chemicals inside the battery. This concept is connected to the SA/V that was one of the core concepts used to construct the module. |
||
|
19. Self-cleaning materials
(Learning with movies)
|
The students watched videos and saw that nowadays it is impossible to clean the house, its windows, car windows, wall stones, floors, etc., without much effort. On the other hand, nanotechnology introduces new materials that have self-cleaning properties for developing nanotechnology-based products. Students wanted to know how nanotechnology helps here and asked many questions.
Videos: http://www.youtube.com/results?search_query=never+wett&oq=never+wett&gs_l=youtube.3...1362.3296.0.3555.10.8.0.2.2.0.319.1612.2j1j4j1.8.0...0.0.z5ghn_ZMx4I |
To expose the students to other nanotechnology applications.
To trigger students' curiosity regarding the self-cleaning products. |
|
20. Sub-micron explanation of self-cleaning materials
(Learning with movies)
|
The students watched the images and video and saw the sub-micron explanation and the mechanism of self-cleaning materials. The activity provided the students with the scientific explanation of everyday applications.
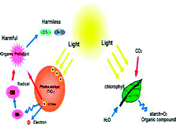
Illustration: http://www.mchnanosolutions.com/whatis.html Video: http://www.youtube.com/watch?v=N2qzFCO96xo&feature=related |
To explain the scientific mechanism underlying self-cleaning materials.
To insert new scientific concepts, such as a radical photocatalyst like TiO2 . To link between the science lessons and students' everyday life to arouse students' awareness of the importance of science in their everyday life. |
|
21. Nano-X-ray
(Learning with movies) |
The short video showed better cancer treatment based on nanotechnology. When nanoparticles are used, the X-ray treatment that is given to cancer tumors has fewer side-effects and therefore works better than the standard radiotherapy that is used today.
http://www.nanobiotix.com/ |
To introduce another example for nanotechnology applications. |
Lessons 10–12 final projects (Project-based learning-PBL)
The last three weeks were dedicated to personally guiding the students in their final projects (presentations and posters). The students were asked to select one application from a list of several nanotechnology applications that were given by the teacher, and are presented in Appendix 1, and to present it in front of their parents, teachers, the school principal, and a nanotechnology research professor from the Technion, the Israel Institute of Technology. The final projects were designed for understanding performances (Perkins, 1992). Students could express their understanding of the learned concepts in nanotechnology, while self-learning and preparing a presentation of a chosen nanotechnology application. Our aim was to expand the students' knowledge of nanochemistry, nanotechnology, and to expose them to different applications of this inter-disciplinary field (Fig. 1). | ||
| Fig. 1 A visual presentation of students' final projects dealing with various nanotechnology applications. (1) Nano food, (2) Nokia Morph nanotechnology home, (3) Nano-car, (4) Plastic and nanoparticles, (5) Osteoarthritis nanotherapy, (6) Nano self-cleaning materials, (7) Nano-nose cancer detection, (8) Nano fire-protection materials, (9) Space elevator, (10) Nano-keratein, (11) Nano-membrane, (12) Vitiligo disease and nanotechnology, (13) Graphene, (14) Nano iPhone batteries, (15) Nano brush for cleaning fresco, (16) Nano X-ray cancer therapy. | ||
Assessment instruments
Nano scraps
The students were asked to cut the smallest scrap of a paper that they could, and to write on it (without letting their fellow students see what they wrote) the name of an object that, in their opinion, represents the nano-scale. This activity, presented in Table 1, was designed to identify the students' initial knowledge regarding size and scale (pre). The nano scraps were also used to learn about the students' knowledge after they had learned the nanotechnology module (post intervention).Students' interviews
Eleven students (18%) were interviewed after the module was implemented (eight girls/three boys). We decided to interview students from different learning levels based on the students' level of ability in chemistry. The second author of the paper taught the class and therefore was able to choose students by categorizing them into the following three levels: four students below average—grades in chemistry below 70 (out of 100), three average students—grades between 70 and 84, and four excellent students—grades between 85 and 100. We used a structured interview to learn about students' perceptions regarding the nanotechnology module and regarding the different teaching methods that were included. The first part of the interview included general questions concerning the nanotechnology module. In the second part of the interview the students were shown a list of all the names of the activities along with an image representing each of them (similar to the figures in Table 1) to help them recall the activities that were used when they learned the nanotechnology module. Questions that were included in the interview are presented in Appendix 2.Final projects—content analysis
Thirty-six posters were prepared by the students for the final project. The students worked in pairs or prepared individual posters. The project included preparing posters that focused on nanotechnology applications that included an explanation in the sub-micro domain (Johnstone, 1991) and an oral presentation of the poster during the summarizing event. The posters' content was analyzed according to the following three categories: (1) the nano-dimension (size and scale), (2) the nanotechnology application presented, and (3) a sub-micro explanation based on the surface-area-to-volume ratio (SA/V) concept. These categories were based on the content that was learned in the nanotechnology module and based on the experience of Akaygun (2011). She found that pre-service teachers who participated in the nanotechnology workshop presented different levels of nanotechnology literacy. The basic level dealt with the size and scale concept, the second level included nanotechnology applications, and the highest level of nano-literacy was the ability to explain the molecular mechanism underlying the nanotechnology application.Results
Nano scraps
The students had no previous knowledge about the size and scale of the nano dimension. In the pre results they used small things that they were familiar with from their own experience in daily life or in school. Surprisingly, not even one student could give an example of an object in the nano dimension. An evaluation of the post nano scraps revealed that 86% of the students were able to write a correct example. Table 2 presents examples of students' ideas in the pre and post nano scraps.Students' interviews
Eleven students having different learning achievements were interviewed after the final event. All students interviewed stated that before beginning the nanotechnology module they were afraid of learning an advanced topic like nanotechnology. With the progression of the module, however, they felt that nanotechnology was an interesting and challenging topic, as presented in Table 3. The reason for this shift in the students' attitudes toward learning nanotechnology, based on the interview, was the use of a variety of teaching methods. The use of different methods helped the students to understand the nanotechnology concepts and stimulated interest in nanotechnology.| Students’ level | How did you feel? | What did you especially like? | What things didn't you like? |
|---|---|---|---|
| Excellent students | Sandra (F): “At the beginning the topic was difficult for me, but very slowly I began to understand and I felt that I am studying a new subject that I had never heard about before.” | “Everything was unusual: The lessons took place not in the regular classroom, and we learned using different methods. I learned the topic in an interesting way that allowed me to explain nanotechnology to others” | “I didn't find anything that I didn't like! I even explained to my mother about nanotechnology.” |
| Jasmine (F): “First, it was unclear, not understood, and complex. It was difficult to understand how small nano is. We said that a cell is a nano scale, and the teacher said it is much smaller!!! The nano scale is larger than the cell scale. How small can it be? After each lesson I went home and searched in the internet, the movies, and games that the teacher used. After watching them at home, I started to better understand and even explained to my parents about nanotechnology.” |
“I liked all the activities that the teacher did, because they helped me understand the subject…
I liked the fact that the subject is connected to everyday life and especially to medicine because it would help humankind… I came to realize that things that I thought were impossible when I was a young child, like self-cleaning materials and stuff like this, exist now thanks to nanotechnology.” |
“I liked everything about the nanotechnology lessons. I even think that I want to learn about nanotechnology at the Technnion when I'll finish high school.” | |
| Average students | Joe (M): “In the first two lessons I felt that this subject is too hard for me, but the use of many teaching methods and the integration of everyday life nanotechnology applications, especially in medicine, really helped me in understanding and loving the subject.” |
“I like the way in which we learned. Using games and movies for learning is fun and also helped to understand.”
”I liked that even when I really didn't understand, you didn't explain again in the same manner; instead you used a different way to explain it. You had movies, and games and more…” |
“The idea of Surface-Area-to-Volume Ratio was really difficult for me and also the nanoscale, but movies and the simulations really helped me understand these two concepts.” |
| Below average | Charlie (M): “At the beginning I didn't understand anything. I thought that the teacher teaches something that is not reasonable and strange.” | “I liked the way the teacher taught nanotechnology.” | “The subject seemed to me to be unreasonable and very difficult.” |
| Natalie (F): “I felt that nanotechnology is difficult, but that it is challenging and interesting to learn nanotechnology” | “Each time the teacher used a different method to teach the same concept and each time I felt that I understand the concept better and better.” | “There is nothing that I didn't understand. The teaching methods helped me understand all the subjects!” |
The excellent students did not mention anything negative regarding the nanotechnology lesson, as Sandra said: “I didn't find anything that I didn't like!”. The average students said that at the beginning that they had difficulties in understanding the concepts of size and scale and SA/V: They did not like the abstract concepts, as Joe explained: “The idea of Surface-Area-to-Volume Ratio was really difficult for me and also the nanoscale, but movies and the simulations really helped me understand these two concepts”. Students below average were very afraid at the beginning of the nanotechnology project; they found it difficult and unreasonable, as reflected from Joe's statement: “The subject seemed to me to be unreasonable and very difficult”. Nevertheless, the average and the below average students spontaneously mentioned the use of the diversity of teaching methods as the factor that helped them overcome these difficulties, even before they were asked about the variety of teaching methods in the interview, as Natalie said: “Each time the teacher used a different method to teach the same concept and each time I felt that I understand the concept better and better”. In the second part of the interview the students were asked to give their opinion of the different activities and teaching methods that were presented in Table 1. Table 4 presents samples of students' responses to each teaching method and their evaluation during the interview.
| Activity name (Teaching methods) | Sample of students' explanations | Positive/Negative (How many students perceived the teaching method to be positive) |
|---|---|---|
|
1. Nano scraps
(Game-based learning) |
Anna (F): “I liked the concrete activities like the nano scraps in addition to the movies and simulations.”
Natalie (F): “This activity was the best for explaining nano dimension.” |
Positive (11/11) |
| 2. Size and scale (Learning with visualization) | Meirele (F): “This illustration helped me understand that the nano-dimensions are much smaller than any other thing I had known, even smaller than bacteria.” | Positive (11/11) |
|
3. Cutting it down to nano
(Game-based learning) |
Joe (M): “This activity helped me feel and understand the nano-dimensions.” | Positive (10/11) |
|
4. “Nanotechnology: What is it?”
(Learning with movies) |
Natalie (F): “It helped me to understand nano-dimensions. I keep in my brain the way the movie presents nanotechnology.” | Positive (11/11) |
|
5. Nanoquest
(Game-based learning) |
Lora (F): “Only after playing this game did I really understand what is the nano-dimension, even the activity of cutting the paper was not enough.”
Joe (M): “The game was a good teaching method because kids like to play games.” |
Positive (11/11) |
|
6. “Oknano”
(Learning with movies) |
Lora (F): “It helped me understand because it connects between the picture and your [the teacher] explanations.” | Positive (10/11) |
|
7. The nano effect
(Learning science with simulations) |
Jasmine (F): “This method is not concrete. I understood the concept of SA/V better when you used the dices.” | Negative (2/11) |
|
8. b. Hungarian cube
(Learning with models) |
Anna (F): “It did not help me understand the concept of SA/V.”
Dania (F): “I didn't understand the connection between the Hungarian cube and nanotechnology.” Joe (M): “It didn't help because I couldn't break it into its pieces, although you showed us the picture of the cube, which is decomposed into pieces.” |
Negative (3/11) |
|
8. c. Separated small cubes.
(Learning with models) |
Meirele (F): “The small cubes helped me to understand the most difficult concept we learned—SA/V.”
Jasmine (F): “It is much better than the simulation or a movie because it is concrete.” |
Positive (10/11) |
|
9. Material polystyrene model
(learning from models) |
Dania (F): “It helped me realize that you cannot control the atoms that are inside the particle.” | Positive (10/11) |
|
10. Analogical models from everyday life phenomena
(Learning with models) |
Mario (M): “The images helped me understand the concept of SA/V and to recognize its importance in real examples that I am familiar with from my life.” | Positive (11/11) |
|
11. The “Magic ball”
(Learning with models, & game-based learning) |
Meirele (F): “It helped me see that the surface energy of nanomaterials is very high and therefore they aim at merging each other.” | Positive (9/11) |
|
12. Models and the Magic ball
(Learning with models, & game-based learning) |
Charlie (M): “I wouldn't skip this method. It helped me to understand and to see the way graphite is built, and it was easier for me to use it than to draw the structure on the board.” | Positive (10/11) |
| 13. Images of nanomaterials (Learning with visualization) | Mario (M): “It made me familiar with the variety of kinds and shapes of nanomaterials.” | Positive (11/11) |
|
14. A story documented with photos about the teacher's experience working in a research nano-laboratory
(Storytelling and narratives) |
Anna (F): “Wow, it was nice for the teacher to be there with all the scientists and to perform experiments in their labs. It is so interesting. Can I join this lab and work there too?” | Positive (11/11) |
|
15. Papers & TEM images
(Teaching with models, & teaching with visualization) |
Sandra (F): “The papers helped me realize what I see in the TEM images.”
Charlie (M): “Using the pages and the TEM images, I was able to understand that the TEM images don't show the real structure of the CNT and the WS2 nanotube; they show a cross section of them.” |
Positive (10/11) |
|
16. Mechanical properties of the nanomaterials and their applications
(Learning with movies) |
Lora (F): “It is amazing to see a tube that is so much thinner than one hair. It is unbelievable.” | Positive (9/11) |
|
17. “Sonol Nanolub”
(Everyday life example) |
Charlie (M): “Do they already sell the Sonol Nanolub oil? I plan to tell my father to buy it. This material is very interesting.” | Positive (11/11) |
|
18. Students' own technological gadgets, e.g. iPhone
(Everyday life example) |
Dania (F): “Can I choose an iPhone for my final project? I saw in the internet that its battery is based on nanotechnology.” | Positive (11/11) |
|
19. Self-cleaning materials
(Learning with movies) |
Jasmine (F): “In this part of the lesson I felt that I was dreaming. Self-cleaning materials was something I thought exists only in my dreams.” | Positive (11/11) |
|
20. Sub-micron explanation of self-cleaning materials
(Learning with movies) |
Jasmine (F): “These videos helped me understand how these materials work. The explanation is very clear.” | Positive (10/11) |
|
21. Nano-X-ray
(Learning with movies) |
Lora (F): “I felt that the nano X-ray subject is very important. After the lesson I invited friends from my neighborhood and explained to them about this cancer treatment, and I used this video.” | Positive (10/11) |
Most of the teaching methods used were found to be beneficial to students' learning. However, some of the activities were found to be confusing. The nano effect simulation (instructional activity 7) and the Hungarian cube game (instructional activity 8b), both dealing with SA/V ratio concept, were described by the students as unclear and abstract, as reflected from students statements: “I didn't understand the connection between the Hungarian cube and nanotechnology” (Dania, F), “This method [the Nano effect] is not concrete. I understood the concept of SA/V better when you used the dices” (Jasmine, F). These two activities deal with the SA/V concept. In the nano effect simulation the students watched the simulation passively and were not actively engaged in the learning process. With the Hungarian cube game the students were asked to imagine the difference between the SA/V ratio for the whole cube and for the sum of its parts. Although games usually involve students in active learning, in this case the Hungarian cube game was not used as a game; rather, it was used as an object that presents an abstract concept. Therefore, the game-based activity did not fulfill its potential as an engagement medium that can support learning. If teachers decide to use these two activities, they should take into consideration that their efficiency as a stand-alone activity is limited. Another important result that emerged from students' interviews is their recommendation to use a variety of teaching methods. They recognized that using a wide spectrum of teaching methods greatly improved their understanding of this subject.
Final projects—content analysis
Thirty-six posters were prepared in the final project and different nanotechnology applications were presented. Students chose a nanotechnology application from a list (appendix 1) or searched for an application independently. In all the chosen applications, they were asked to present and explain the application using the concept they learned (e.g., size & scale, SA/V). We analyzed the posters' content according to the following three categories: (1) the presentation of the nanotechnology application (2), explaining the nano-dimension (size and scale) in the chosen application, and (3) the sub-micro explanation based on the SA/V concept. These three categories are based on a qualitative analysis that was done in Turkey (Akaygun, 2011). Most of the posters included all three categories. Students chose to present different applications (33/36) and based their explanations on the concept of size and scale (33/36) and SA/V (29/36). According to Perkins and Blythe (2003), the final projects were designed to be “understanding performances” because the students used concepts that were learned in class to explain nanotechnology applications that were not previously learned. In addition to exploring students' projects to learn more about their understanding of their projects, we also examined their perceptions using a variety of teaching methods throughout the project. All groups decided to use more than two teaching methods when they presented their final projects in the final event. This result supported their statements during the interview that using a variety of teaching methods helped them to understand the difficult subject of nanotechnology.Concluding remarks and recommendations
In this study we explored two topics to support the development of the nanotechnology module within the chemistry class. In the first we evaluated the use of a variety of methods for teaching students basic concepts in nanotechnology. In the second we evaluated students' perceptions regarding the effectiveness of the methods in supporting students' understanding. We showed that all the students appreciated using a variety of teaching methods and even chose to use several methods themselves when they presented their final projects. The combination of using different teaching methods was shown to be effective in teaching students having different learning levels. One can appreciate the importance of using a wide range of teaching methods by examining the different words that the students chose to use in describing the different methods. They used the following verbs: see, feel, understand, play, help, keep in my mind, and realize (Table 4). The variety of verbs that were used to describe their understanding represents the variety and the wide-range teaching methods and different learning channels that were applied (Kozma and Russell, 2005). The needs of students were met in the presented nanotechnology module regardless of the students' learning achievements.In developing this module, we chose 22 activities and 7 different teaching methods. Nevertheless, it is important to note that there are many other different activities on the Web that can be used to explain basic concepts in nanotechnology. Appropriate activities also appear in the newer textbooks, which often recommend activities suitable for high-school students (e.g., Jones et al., 2007). When you plan your own nanotechnology course for school students, you can choose your own set of activities applying different teaching methods; just keep in mind to use a variety of methods that differ in their nature and therefore meet the needs of the various students in your class.
As was mentioned here, teaching nanotechnology poses new challenges for science educators. However, it also represents an opportunity to teach students a subject that is interesting, significant, and relevant, if presented using a variety of teaching methods. It was shown that teachers who learned about AFM using a teaching model (Blonder, 2010) had positive attitudes toward teaching nanotechnology and AFM. Since high-school teachers are not usually familiar with the field of nanotechnology, they must learn about nanotechnology in order to be able to teach it effectively. Using a variety of teaching methods in the teachers' course will be beneficial to the teachers when they teach nanotechnology in their classes. In this way, teachers will gain the PCK together with the content of nanotechnology.
Lastly, introducing nanotechnology into the chemistry curriculum has the advantage that teachers must learn a new subject before they teach it in their class. When they begin to teach a new subject they had never taught, they are more likely and willing to adopt new pedagogical approaches. Using simulations instead of traditional teaching (Gregorius et al., 2010) and a variety of teaching methods in the nanotechnology lessons may lead to a positive change in their chemistry lessons as well.
Appendix 1
Internet resources dealing with different applications of nanotechnology, as a base for students' final project:| Project | Web address | |
|---|---|---|
| 1. | Food and nanotechnology | http://www.nano2life.org/ |
| http://www.hayadan.org.il/could-nanotechnology-make-an-average-donut-into-health-food-1603091/ | ||
| http://www.sciencemuseum.org.uk/antenna/nano/lifestyle/141.asp | ||
| 2. | Nokia Morph nanotechnology phone | http://www.livemint.com/2009/06/02182604/Nokia-Morph--Nanotechnology.html |
| http://gizmodo.com/360260/nokia-morph-cellphone-rolls-up-stretches-cleans-itself | ||
| 3. | Nanocar | http://www.nanoprotect.co.uk/nano-for-car.html |
| http://nanoauto.eu/index.php?option=com_content&task=blogsection&id=6&Itemid=59 | ||
| 4. | Plastic and nanoparticles | http://www.hayadan.org.il/plastics-and-nanoparticles-%E2%80%93-the-perfect-combination-0311104/ |
| http://www.hayadan.org.il/paper-strips-can-quickly-detect-toxin-in-drinking-water-1401102/ | ||
| http://www.hayadan.org.il/nanoparticle-protects-oil-in-foods-from-oxidation-spoilage-1812091/ | ||
| 5. | Osteoarthritis nanotherapy | http://www.hayadan.org.il/nano-particles-against-austroatreitis-0906093/ |
| 6. | Nano self-cleaning materials | http://www.mchnanosolutions.com/self-cleaning.html |
| http://www.mchnanosolutions.com/whatis.html | ||
| http://www.nanoprotect.co.uk/nano-for-household.html | ||
| 7. | Nanonose cancer detection | http://rbni.technion.ac.il/?cmd=news.0&act=read&id=226 |
| http://www.mfa.gov.il/MFA/InnovativeIsrael/Sniffing_disease-May_2011.htm?DisplayMode=print | ||
| 8. | Nano fire-protection materials | http://www.nanonext.net/sitecmj/index.php?option=com_content&view=article&id=121&Itemid=263 |
| http://www.azonano.com/news.aspx?newsID=23321 | ||
| 9. | Space elevator | http://www.nanooze.org/english/pdfs/nanoozeissue_09.pdf |
| http://spaceelevatorconference.org/AbouttheSpaceElevator.aspx | ||
| http://crnano.typepad.com/crnblog/2009/01/space-elevator-tethers-coming-closer.html | ||
| http://www.understandingnano.com/nanotubes-space-elevator.html | ||
| 10. | Nano keratin | http://www.nanokeratin.co.il/site/index.asp?depart_id=88317&lat=en |
| 11. | Nano membrane | http://www.siemens.com/innovation/en/publikationen/publications_pof/pof_fall_2007/materials_for_the_environment/trends.htm |
| 12. | Vitiligo disease and nanotechnology | http://www.biotecharticles.com/Healthcare-Article/Vitiligo-Skin-Disease-Causes-Symptoms-and-Treatment-364.html |
| http://www.cidpusa.org/VITILIGO1.html | ||
| 13. | Graphene | http://en.wikipedia.org/wiki/Graphene |
| http://www.youtube.com/watch?v=-YbS-YyvCl4 | ||
| http://www.zdnet.co.uk/news/emerging-tech/2011/06/10/the-10-strangest-facts-about-graphene-40093050/ | ||
| 14. | Nano iPhone batteries | http://www.understandingnano.com/nanotech-applications.html |
| http://www.understandingnano.com/batteries.html | ||
| 15. | Nano brush for fresco cleaning | http://stwww.weizmann.ac.il/chemcenter/img/news/19.pdf |
| http://stwww.weizmann.ac.il/chemcenter/img/news/20.pdf | ||
| http://www.sciencedaily.com/releases/2007/05/070514100150.htm | ||
| 16. | Nano X-ray cancer therapy | http://www.nanobiotix.com/ |
Appendix 2: Students’ interviews
First part
• How did you feel during the nanotechnology lessons?• What did you especially like in these lessons? Give details.
• What things didn't you like? Give details.
Second part
• Which of the activities and teaching methods did you really like?• How did they help you to better understand the material?
• Which of the activities and teaching methods do you recommend be used next time we teach the nanotechnology module (to other students)?
• Which teaching methods were not useful for you? Try to explain why?
• Which teaching methods did you use in presenting your final project during the summarizing event?
Acknowledgements
We would like to thank Prof. Reshef Tenne and his graduate student Ronen Kreizman from the Weizmann Institute of Science for their scientific support and for opening the doors of their research labs to chemistry teachers, and to Prof. Hossam Haick from the Technion, the Israel Institute of Technology, for his willingness to support nanoeducation and for his participation in the final event of the project. This study was part of the Rothschild-Weizmann program for Excellence in Science Teaching that is supported by the Rothschild-Caesarea Foundation.References
- Akaygun S., (2011, September), Developing literacy and awareness of nanoscience in pre-service teachers. Paper presented at the European Science Education Research Association conference, Lyon, France.
- Ambrogi P., Caselli M., Montalti M. and Venturi M., (2008), Make sense of nanochemistry and nanotechnology, Chem. Educ. Res. Prac., 9, 5–10.
- Amory A., Naicker K., Vincent J. and Adams C., (1999), The use of computer games as an educational tool: Identification of appropriate game types and game elements, Brit. J. Educ. Technol., 30, 311–321.
- Barab S., Thomas M., Dodge T., Carteaux R. and Tuzun H., (2005), Making learning fun: Quest Atlantis, a game without guns, ETR&D–Educ. Technol. Res., 53, 86–107.
- Betz J. A., (1995), Computer games: Increase learning in an interactive multidisciplinary environment, J. Educ. Technol. Syst., 24, 195–205.
- Blonder R., (2010), The influence of a teaching model in nanotechnology on chemistry teachers' knowledge and their teaching attitudes, Journal of Nano Education, 2, 67–75.
- Blonder R. and Dinur M., (2011), Teaching nanotechnology using student-centered pedagogy for increasing students' continuing motivation, J. Nano Educ., 3(1–2), 51–61.
- Blumenfeld P. C., Soloway E., Marx R. W., Krajcik J. S., Guzdial M. and Palincsar A., (1991), Motivating project-based learning: Sustaining the doing, supporting the learning, Educational Psychologist, 26, 369–398.
- Burke K. A., Greenbowe T. J. and Windschitl M. A., (1998), Developing and using conceptual computer animations for chemistry instruction, J. Chem. Educ., 75, 1658–1660.
- Cavanaugh T. and Cavanaugh C., (1996), Learning science with science fiction films. Paper presented at the meeting of Florida Association of Science Teachers, Key West, FL, Retrieved from the ERIC database (ED411157).
- Davis B. G., (1993), Tools for Teaching. San Francisco, CA: Jossey-Bass.
- Ebenezer J. V., (2001), A hypermedia environment to explore and negotiate students' conceptions: Animation of the solution process of table salt, J. Sci. Educ. Technol., 10, 73–92.
- Fairburn G. J., (2002), Ethics, empathy and storytelling in professional development, Learn Health Soc. Care, 1, 22–32.
- Ferk V., (2000), The impact of different molecular structure representations on the processes of perception, rotation and reflection (Master's thesis). (Ljubljana: University of Ljubljana, Faculty of Natural Sciences and Engineering, Department of Chemical Education and Informatics).
- Ferk V., Vrtacnik M., Blejec A. and Gril A., (2003), Students' understanding of molecular structure representations, Int. J. Sci. Educ., 25, 1227–1245.
- Gilbert J. K., Boutler C. J., (2000), Developing models in science education, Dordrecht, The Netherland: Kluwer.
- Goll J. G. and Woods B. J., (1999), Teaching chemistry using the movie Apollo 13, J. Chem. Educ., 76, 506–508.
- Greenberg A., (2009), Integrating nanoscience into the classroom: Perspectives on nanoscience education projects, ACSNano, 3, 762–769.
- Gregorius R. M., Santos R., Dano J. B. and Gutierrez J. J., (2010), Can animations effectively substitute for traditional teaching methods? Part I: Preparation and testing of materials, Chem. Educ. Res. Prac., 11, 253–261.
- Hagen B. J., (2002), Lights, camera, interaction: Presentation programs and the interactive visual experience. Paper presented at the Society for Information Technology and Teacher Education international conference, Nashville, TN. Retrieved from the ERIC database.
- Harmer A. J. and Columba L., (2010), Engaging middle school students in nanoscale science, nanotechnology, and electron microscopy, J. Nano Educ., 2, 91–101.
- Hofstein A., Mamlok R. and Rosenberg O., (2006), Varying instructional methods and students assessment methods in high school chemistry. In M. McMahon, P. Simmons, R. Sommers, D. De Baets and F. Crawley (Eds.), Assessment in Science (pp. 139–148). Arlington, VA: NSTA Press.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7, 75–83.
- Jones M. G., Falvo M. R., Taylor A. R. and Broadwell B. P., (2007), Nanoscale science: Activities for Grades 6–12., NSTA Press.
- Jones M. G., Gardner G., Taylor A., Wiebe E. and Forrester J., (2011), Conceptualizing magnification and scale: The roles of spatial visualization and logical thinking, Res. Sci. Educ., 41, 357–368.
- Jones M. G., Minogue J., Tretter T., Negishi A. and Taylor R., (2006), Haptic augmentation of science instruction: Does touch matter? Sci. Educ., 90, 111–123. Retrieved from http://www.tcnj.edu/~minogue/Research/Publications/Science%20Ed_Puppy_Paper.pdf.
- Jones M. G., Taylor A., Minogue J., Broadwell B., Wiebe E. and Carter G., (2007), Understanding scale: Powers of ten, J. Sci. Educ. Technol., 16, 191–202.
- Kelly R. M. and Jones L. L., (2007), Exploring how different features of animations of sodium chloride dissolution affect students' explanations, J. Sci. Educ. Technol., 16, 413–429.
- Koenig J. M., Zorn C. R., (2002), Using storytelling as an approach to teaching and learning with diverse students, J. Nurs., 41, 393–399.
- Kozma R. and Russell J., (2005), Multimedia learning of chemistry. In R. Mayer (Ed.), Cambridge handbook of multimedia learning, New York, NY: Cambridge University Press.
- Kumar D. D., (1991), Hypermedia: A tool for STS education? Bull. Sci. Technol. Soc., 11, 331–332.
- Kumar D. D., Smith P. J., Helgeson S. L. and White A. L., (1994), Advanced technologies as educational tools in science: Concepts, applications, and issues, Columbus, OH: National Center for Science Teaching and Learning.
- Larkin J. H., (1989), Display-based problem solving in complex information processing, Hillsdale, NJ: Erlbaum.
- Larkin J. H., Simon H. A., (1987), Why a diagram is (sometimes) worth ten thousand words, Cog. Sci., 11, 65–99.
- Laroche L. H., Wulfsberg G. and Young B., (2003), Discovery videos: A safe, tested, time-efficient way to incorporate discovery-laboratory experiments into the classroom, J. Chem. Educ., 80, 962–966.
- Leight S. B., (2002), Starry night: Using stories to inform aesthetic knowing in women's health, Nurs. J. Adv. Nurs., 37, 108–14.
- Lepper M. R. and Cordova D. I., (1992), A desire to be taught: Instructional consequences of intrinsic motivation, Motiv. Emotion, 16, 187–208.
- Marcano A. V., Williamson V. M., Ashkenazi G., Tasker R. and Williamson K. C., (2004), The use of video demonstrations and particulate animation in general chemistry, J. Sci. Educ. Technol., 13, 315–323.
- Mathewson J., (1999), Visual–spatial thinking: An aspect of science overlooked by educators, Sci. Educ., 83, 33–54.
- Mathewson J., (1999), Visual-spatial thinking: An aspect of science overlooked by educators, Sci. Educ., 83, 33–54.
- Mayer R., (2001), Multi–media learning, New York, NY: Cambridge University Press.
- Mayer R., (2002), Cognitive theory and the design of multimedia instruction: An example of the two–way street between cognition and instruction, New Directions Teach. Learn., 2002(89), 55–71.
- Mayer R. E., (1999), Designing instruction for constructivist learning. In C. M. Reigeluth (Ed.), Instructional design theories and models (vol. II, pp. 141–159), Norwood, NJ: Erlbaum.
- Michel E., Roebers C. M. and Schneider W., (2007), Educational films in the classroom: Increasing the benefit, Learn. Instr., 17, 172–183.
- Naps T. L., Chair, Working Group on Visualization., (June 1996), An overview of visualization: Its use and design. In Proceedings of the Conference on Integrating Technology into Computer Science Education, Barcelona, Spain (pp. 192–200).
- Paivio A., (1986), Mental representations: A dual coding approach, New York, NY: Oxford University Press.
- Paivio A., (1991), Dual coding theory: Retrospect and current status, Can. J. Psychol., 45, 255–287.
- Pekdag B. and Le Maréchal J.-F., (2010), An explanatory framework for chemistry education: The two-world model, Educ. Sci., 35, 84–99.
- Perkins D. N., (1992), Smart schools: From training memories to educating minds, New York, NY: Free Press.
- Perkins D. and Blythe T., (2003), Putting understanding up front, Educ. Leadership, Association for Supervision and Curriculum Development. Used with permission.
- Ratner M. A. and Ratner D., (2003), Nanotechnology: A gentle introduction to the next big idea, Upper Saddle River, NJ: Pearson Education.
- Rieber L. P., (1995), A historical review of visualization in human cognition, Educ. Technol. Res. Dev., 43, 45–56.
- Roco M. C., (2001), From vision to the implementation of the U.S. national nanotechnology initiative, J. Nanopart. Res., 3, 5–11.
- Roco M. C., (2003), Converging science and technology at the nanoscale: Opportunities for education and training, Nat. Biotech., 21, 1247–1249.
- Rosen H., (1985), Stories and meanings, Sheffield: National Association for the Teaching of English.
- Sanger M. J. and Greenbowe T. J., (1997), Students' misconceptions in electrochemistry: Current flow in electrolyte solutions and salt bridge, J. Chem. Educ., 74, 819–823.
- Sanger M. J., Campbell E., Fekler J. and Spencer C., (2007), Concept learning versus problem solving: Does particle motion have an effect? J. Chem. Educ., 84, 875.
- Sanger M. J., Phelps A. J. and Fienhold J., (2000), Using a computer animation to improve students' conceptual understanding of a can-crushing demonstration, J. Chem. Educ., 77, 1517–1520.
- Sekuler R., Blake R., (1994), Perception (3rd ed.), New York. NY: Knopf.
- Stein D., (1998), Situated learning in adult education, ERIC Digest. Retrieved from http://www.ericdigests.org/1998-3/adult-education.html.
- Stevens S., Sutherland L. M. and Krajcik J. S., (2009), The big ideas of nanoscale science and engineering: A guidebook for secondary teachers, Arlington, VA: NSTA Press.
- Swarat S., Light G., Park E. J. and Drane D., (2011), A typology of undergraduate students' conceptions of size and scale: Identifying and characterizing conceptual variation, J. Res. Sci. Teach., 48, 512–533.
- Sweller J., (2002), Visualization and instructional design. Retrieved from http://www.iwm-kmrc.de/workshops/visualization/sweller.pdf.
- Taylor A. R., (2008), Students' and teachers' conceptions of surface area to volume in science contexts: What factors influence the understanding of the concept of scale? (Doctoral dissertation), Retrieved from http://repository.lib.ncsu.edu/ir/bitstream/1840.16/5212/1/etd.pdf.
- Taylor A. and Jones M. G., (2009), Proportional reasoning ability and concepts of scale: Surface area to volume relationships in science, Int. J. Sci. Educ., 31, 1231–1247.
- Tobin K., (1981), Patterns of reasoning: Probability, Res. Sci. Educ., 12, 42–49.
- Tuvi-Arad I. and Blonder R., (2010), Continuous symmetry & chemistry teachers: Learning advanced chemistry content through novel visualization tools, Chem. Educ. Res. Prac., 11, 48–58.
- Vella J. K., (2002), Learning to listen, learning to teach: The power of dialogue in educating adults, San Francisco, CA: Jossey-Bass.
- Weimer M., (2002), Learner-Centred Teaching: Five key changes to practice, San Francisco, CA: Wiley.
- Williamson V. M. and Abraham M. R., (1995), The effects of computer animation on the particulate mental models of college chemistry students, J. Res. Sci. Teach., 32, 521–534.
- Wu H., Krajcik J. S. and Soloway E., (2001), Promoting understanding of chemical representations: Students' use of a visualization tool in the classroom, J. Res. Sci. Teach., 38, 821–842.
- Yang E., Andre T., Greenbowe T. J. and Tibell L., (2003), Spatial ability and the impact of visualization/animation on learning electrochemistry, Int. J. Sci. Educ., 25, 329–349.
- Young M. R., Klemz B. R. and Murphy J. W., (2003), Enhancing learning outcomes: The effects of instructional technology, learning styles, instructional methods, and student behavior, J. Mark. Educ., 25, 130–142.
- Zahn C., Barquero B. and Schwan S., (2004), Learning with hyperlinked videos: Design criteria and efficient strategies for using audiovisual hypermedia, Learn. Instr., 14, 275–291.
| This journal is © The Royal Society of Chemistry 2012 |















 TEM image of a CNT
TEM image of a CNT TEM image of a WS2Nanotube
TEM image of a WS2Nanotube