How does the science writing heuristic approach affect students' performances of different academic achievement levels? A case for high school chemistry†
Sevgi
Kingir
*a,
Omer
Geban
b and
Murat
Gunel
c
aSelcuk University, Graduate School of Natural and Applied Sciences, Konya, Turkey. Fax: 90 332 2410520; Tel: 90 332 2410525E-mail: kingirsevgi@gmail.com
bMiddle East Technical University, Department of Secondary Science & Mathematics Education, Ankara, Turkey. Fax: 90 312 2107971; Tel: 90 312 2104049E-mail: geban@metu.edu.tr
cTED University, Department of Primary Education, Ankara, Turkey. Fax: 90 312 4184148; Tel: 90 312 4174202 158E-mail: murat.gunel@tedu.edu.tr
First published on 20th July 2012
Abstract
This study investigates the effects of the Science Writing Heuristic (SWH), known as an argumentation-based science inquiry approach, on Grade 9 students' performance on a post-test in relation to their academic achievement levels. Four intact classes taught by 2 chemistry teachers from a Turkish public high school were selected for the study; one class from each teacher was assigned as the treatment group, and the other class from each teacher was assigned as the control group. Students in the treatment group were instructed using the SWH approach while those in the control group were instructed using traditional instruction. Students' achievement levels were determined based on their chemistry mean scores for the previous semester, which were used to determine the impact of the treatment on varying levels of achievement. A test measuring students' achievement in chemical change and mixture was administered as pre- and post-test for both groups. The data were analyzed by using Analysis of Covariance (ANCOVA). The findings indicate that the SWH approach contributed to students' test performances significantly better than the traditional approach. Student performance on the post-test differed with respect to their academic achievement levels significantly. Low achievers and middle achievers in the SWH group significantly outperformed those in the traditional group on the post-test.
Introduction
One of the goals of science teaching is the development of students' science literacy. Science literacy can be defined as “knowledge and understanding of scientific concepts and processes required for personal decision making, participation in civic and cultural affairs, and economic productivity” (National Research Council, 1996, p. 22). Many scholars have realized the importance of science literacy for future citizens and have expended considerable efforts to improve science literacy. For example, substantial changes have been made in the curricula of many countries, including Turkey. Curricula revision processes in Turkey began in 2004 and encompassed all the learning domains at the elementary and high school levels. Science literacy is defined in Turkish science curricula as the combination of scientific knowledge, attitudes, values, capabilities, and understanding necessary for life-long learning, maintenance of curiosity, and development of inquiry, problem-solving, critical thinking and decision-making skills of individuals (Ministry of National Education [MNE], 2004).In Turkey, the revised science curriculum was based on constructivism to achieve the goals of science literacy. Constructivism is a learning theory that is widely accepted in the science education community (Palmer, 2005). In constructivism, learning is defined as an active construction of meaning and involves a change in the learner's conceptions (Posner et al., 1982). For this reason, learners are not viewed as passive recipients of knowledge; rather, they are seen as responsible for their own learning. Students' constructions of meaning are also influenced by social contexts. The classroom environment acts as a complex social context for learning because students can negotiate meaning through interactions with their peers and teachers. As students engage in discussions about concepts and share their understanding, they can resolve the conflicts between new and prior conceptions. In this way, new ideas can be integrated into their existing cognitive structure (Driver, 1988). According to the constructivist view, the characteristics of individuals influence their learning as much as the teacher and school (Yager, 1991). This idea highlights the importance of students' prior knowledge for their future learning. Students' prior conceptions may facilitate or hinder their further learning.
Development of critical thinking skills and habits of mind, which tackle everyday problems with scientific reasoning, is a fundamental component of science literacy and the Turkish national curriculum. As Ford (2008) pointed out, students need to be active in knowledge generation and critiquing claims in order to understand how scientific knowledge is constructed and how scientists work. That is, students need to have the opportunity to practice disciplinary authority and accountability in practising and learning science. Inquiry and argumentation are two main streams of research areas that scaffold students' understanding of science. There are various instructional approaches that aim to center inquiry or argumentation in learning environments, which can include guided discovery (Carin et al., 2005), the learning cycle (Marek and Cavallo, 1997; Eisenkraft, 2003; Bybee et al., 2004), Toulmin's model of argumentation (Erduran et al., 2004; Simon et al., 2006), and role play or debate (Simonneaux, 2001; Walker and Zeidler, 2007). However, the majority of those approaches tend to focus on either inquiry for teaching science concepts or argumentation for teaching reasoning skills. On the other hand, there are approaches, e.g., argument-driven inquiry (Sampson and Gleim, 2009; Sampson et al., 2009), personally seeded discussions (Clark and Sampson, 2007), and the science writing heuristic (Keys et al., 1999), that blend inquiry and argumentation in science teaching.
Grounded within the constructivist philosophy, the Science Writing Heuristic (SWH) is perceived as an argument-based inquiry approach (Keys et al., 1999; Nam et al., 2010). The SWH consists of two parts: a teacher framework and a student framework. The teacher framework (Table 1) includes a series of suggested activities demonstrating what teachers need to do when using inquiry activities.
| Exploration of pre-instructional understanding (e.g. individual or group concept mapping). |
| Pre-instructional activities (e.g. informal writing, making observations, brainstorming, and posing questions). |
| Participation in science activity. |
| Negotiation phase I – writing personal meanings for science activity (e.g. writing journals). |
| Negotiation phase II – sharing and comparing data interpretations in small groups (e.g. making a group chart). |
| Negotiation phase III – comparing science ideas to textbooks or other printed resources (e.g. writing group notes in response to focus questions). |
| Negotiation phase IV – individual reflection and writing (e.g. creating a presentation such as a poster or report for a larger audience). |
| Exploration of post-instructional understanding through concept mapping. |
The second part of the SWH, the student framework (Table 2), includes a set of questions that prompts students to build scientific knowledge. The questions also provide a scaffold for students to construct science arguments. Students use the terms claims and evidence when they are explaining or defending their decisions after testing their questions.
| Beginning Ideas – What are my questions? |
| Tests – What did I do? |
| Observations – What did I see? |
| Claims – What can I claim? |
| Evidence – How do I know? Why am I making these claims? |
| Reading – How do my ideas compare with others? |
| Reflection – How have my ideas changed? |
The students are highly active both mentally and physically throughout the activities provided in SWH classes. At the beginning of the learning sessions, students as a group ask testable questions and design the experiment. Then, they engage in investigations, collect data, and make observations. After the completion of the investigation task, the students negotiate the meaning of those data and observations via intra- and inter-group discussions. They construct knowledge by making claims and supporting those claims with evidence based on their experimentation. Finally, they attempt to integrate new knowledge with their existing knowledge through the reflection. As a facilitator, the teacher allows students to form their own groups; provides opportunities for students to discuss beginning questions; sets up the environment for student-centered learning; and makes students organize their data, generate knowledge claims and evidence (Keys et al., 1999; Burke et al., 2005).
Building on the writing-to-learn framework (Hand et al., 2004), the SWH approach suggests writing as a mode of thinking in classrooms. Writing promotes personal construction of meaning for verbal symbols, which further enhances the construction of scientific knowledge and development of science literacy (Keys et al., 1999; Hand et al., 2004). Writing improves the students' critical thinking and reasoning about the meaning of the data. In SWH classes, students write investigation reports based on the questions in the student template (Table 2). A science investigation report written in SWH format differs from the traditional format in several ways. Traditional reports tend to separate connections among investigation questions, methods, observations, data, evidence, claims, and hypotheses; the SWH format encourages writing about the connections between the above-mentioned sections (Keys et al., 1999). In traditional format, procedures are the same for each student, data are similar, claims match expected outcomes; all of these factors inhibit the development of scientific reasoning skills. However, in SWH format, students pose questions, propose methods to address these questions, conduct appropriate investigations, generate claims and support them with evidence, and analyze the relations between questions, claims, and evidence (Burke et al., 2005).
Comparison of the SWH approach with the traditional approach resulted in a better understanding of scientific concepts (Keys et al., 1999; Rudd et al., 2007; Schroeder and Greenbowe, 2008; Günel et al., 2010; Nam et al., 2010). For example, Keys et al. (1999) examined the influence of SWH activities on the students' meaning-making, conceptual, and reasoning abilities. As a result, students' written reports illustrated the presence of their science learning, metacognitive thinking, and reflection of self-understanding. Students reasoned about the meaning of data, and they interpreted the data to support their claims. Some of the reflections on self-understanding indicated conceptual change about the science concepts. The studies also demonstrated that the SWH approach facilitated the generation of arguments (Cavagnetto et al., 2010; Choi et al., 2010; Hand and Choi, 2010; Nam et al., 2010). For example, Choi et al. (2010) investigated the impact of the writing component of the SWH approach on 5th, 7th, and 10th grade students' construction of reasonable arguments. They tried to determine the components of arguments (questions, claims, questions-claims relationship, evidence, claims-evidence relationship, and reflection) that predict the quality of arguments. Their findings revealed that the relationship between claims and evidence was the most critical element in predicting the quality of arguments. It was also shown that the SWH approach was useful in promoting students' construction of reasonable arguments.
In another study (part of the first author's dissertation like this manuscript), the authors examined the effects of the SWH approach on students' misconceptions and understanding of chemical change and mixture concepts using a two-tier test (different than the one used in this study) and semi-structured interviews (Kingir et al., manuscript submitted for publication). The results indicated that students in the SWH group demonstrated better scientific understanding and fewer misconceptions than those in the control group did. Although several research studies have focused on the comparison of academic achievement between the SWH and traditional approaches, the effect of the SWH approach on students' academic performance in relation to their achievement level appears to be the next level of concern.
Achievement level
Achievement level was introduced by the National Assessment Governing Board in reporting results from the National Assessment of Educational Progress. The board identified three achievement levels with descriptors, cut-scores, and sample items for different grade levels and various content areas. These levels indicate students' knowledge and skills at the basic, proficient, and advanced levels (Loomis and Bourque, 2001). For grade 9, students performing at the basic level in science can carry out simple experiments; observe, measure, collect, and record data from investigations; read simple graphs, diagrams, and tables; and recognize cause-effect relationships to some extent. Students at the proficient level can interpret and make predictions from graphs, diagrams, and tables; design an experiment; and have an understanding of the models. Students at the advanced level can provide scientific explanations, design a controlled experiment, and establish cause-effect relationships. If these levels and their sub-components are compared, it can be inferred that students become more scientifically literate as they move from basic to advanced levels. For that reason, closing the gap among different levels of achievement is a serious issue that needs to be considered. However, the authors want to caution the reader about the complexity of achievement and measurement of achievement since the idea of achievement as discussed above includes several sub-domains such as content, skills, and performance (Martin et al., 2000). In this paper, we focused only on students' domain-specific academic achievement levels.In pursuit of academic achievement levels, some studies have investigated the effects of the SWH approach across different achievement levels. For example, Akkus et al. (2007) examined the effect of the SWH approach and achievement level on the conceptual understanding of middle school students; they found that the quality of implementation affected the students' performance, with high-quality implementation of the SWH approach closing the science achievement gap. As the implementation quality of the SWH approach improved, the benefit to low-achieving students gradually increased.
Similarly, Grimberg and Hand (2008) tested the effectiveness of the SWH approach to close the achievement gap. In their study, students were classified as low-achieving or high-achieving based on their previous achievement scores. All the students were engaged in three different inquiry activities based on the SWH approach. The nature of the initial questions varied across three laboratory activities, namely, decision-making, descriptive/speculative, and integration. The study demonstrated that the reasoning processes of students were not dependent on their achievement level but on the nature of inquiry. High-level reasoning processes were mainly used in decision-making inquiries and used less frequently in integration activities. Low-level reasoning processes were mostly used in descriptive/speculative inquiries and used less frequently in decision-making inquiry activities. This study supported the notion that the SWH approach encourages the development of students' scientific argumentation strategies and closes the gap between low- and high-achieving students. Through their involvement in the SWH classroom, students had an opportunity to negotiate the meaning of ideas via intra- and inter-group discussions and constructed knowledge by developing scientific arguments.
Similarly, there is an achievement gap amongst Turkish students. It has been repeatedly shown that Turkish students' achievement in science is low in international exams (e.g., Trends in International Mathematics and Science Study [TIMSS]) although some students performed significantly above the international average (Martin et al., 2000). A possible reason for this gap could be the highly competitive and examination-driven nature of the Turkish educational system. In Turkey, high school students who want to attend university must pass normative examinations that are given nationwide. In such a competitive environment, teachers mainly focus on high achievers and ignore low achievers. However, the aim of the educational system is not to prepare students for exams but to promote the construction of scientific knowledge and the development of science literacy. The activities provided in classrooms have an impact on students' achievement in science (Nolen, 2003; Sungur and Güngören, 2009) and have the potential to close the achievement gap. Implementation of argument-based inquiry approaches (e.g., the SWH approach) may be influential in closing the achievement gap by embedding writing and argumentation within science inquiry activities (Hand et al., 1999; Akkus et al., 2007; Grimberg and Hand, 2008; Cavagnetto, 2010). In SWH classes, linking new information to previous knowledge, reading, writing, and negotiation contributes to the construction of scientific knowledge and, in turn, the development of science literacy (Krajcik and Sutherland, 2010).
Student achievement in chemistry has been investigated extensively in national (e.g., Kan and Akbaş, 2006; Tüysüz, 2010) and international studies (e.g., Turner and Lindsay, 2003; Udo, 2010). However, we could find no studies investigating Turkish students' chemistry achievement in relation to achievement level although there are some at the international level (e.g., BouJaoude and Attieh, 2008). Using appropriate strategies in chemistry may be helpful in closing the achievement gap and help all students to become scientifically literate. In a study related to chemistry, it was shown that students who entered a chemistry course with a low-level of previous chemistry knowledge and who were taught with the SWH approach demonstrated better performance in the course than those in previous years, with similar previous knowledge and who were not taught with the SWH approach (Poock et al., 2007). Therefore, the present research aimed at investigating the effect of the SWH approach on different achievement levels in the high school chemistry context.
The research questions guiding this study are as follows:
Is there a significant mean difference between the groups exposed to the SWH approach and traditionally designed chemistry instruction with respect to students' knowledge of physical changes, chemical changes and mixtures?
Is there a significant mean difference among low-, medium-, and high-achieving students with respect to their knowledge of physical changes, chemical changes and mixtures?
Is there a significant interaction effect between treatment and achievement level with respect to students' knowledge of physical changes, chemical changes and mixtures?
Method
In this section, sample characteristics, research instrument, and the procedure followed in this study are described in detail.Participants
The participants in this study were 122 grade 9 students from four intact classes of two teachers in a Turkish public high school. There were 62 students (33 males and 29 females) in the treatment group (one section from each teacher) and 60 students (30 males and 30 females) in the control group (the other section from each teacher). Students' ages ranged from 15 to 17 years. Students were from middle-class families.The participating teachers had no experience implementing the SWH approach prior to the study. In fact, at the time of the investigation, there was a change in the grade 9 chemistry curriculum from teacher-centered into a more student-centered approach (MNE, 2007). The teachers had difficulty in comprehending how a student-centered approach could be implemented in their classrooms. Therefore, this study was highly encouraging for them, and they were eager to learn the SWH approach. Prior to the study, one of the researchers had several meetings with the teachers at the school in order to train them in the implementation of the SWH approach for the treatment group. In the first meeting, the teachers were introduced to the SWH approach and given SWH information notes. The researcher asked the teachers to teach the control group students in the same way they taught before and not to do things specified for the treatment group.
Data collection tool
Students' achievement in chemical change and mixture was measured via pre- and post-test, which are described more fully below.Chemical change and mixture achievement test (CCMAT)
The CCMAT instrument was used to assess students' achievement in chemical change and mixture. The researchers developed this instrument by taking into account the objectives related to the chemical change and mixture units determined by the national chemistry curriculum (MNE, 2007). In the question-development process, the researchers used textbooks, the University Student Selection Examination (OSS), some international studies like TIMSS (1999, 2003), and the literature (Mulford, 1996). CCMAT consisted of 22 multiple-choice questions: 9 about chemical change and 13 about mixture. The reason for preferring multiple-choice items is that it is relatively easy to administer, and it can be scored objectively. Each test item consisted of five alternatives: one correct answer and four distracters. Items in the test were related to physical change, chemical change, types of chemical reactions, classification of mixtures, solutions, solubility, factors affecting solubility, and separation of mixtures. See Fig. 1 for sample CCMAT items.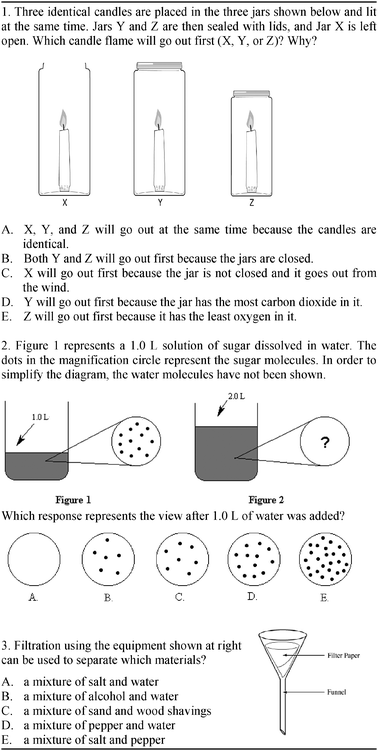 | ||
| Fig. 1 Sample CCMAT items. | ||
The test was examined by two professors, one assistant professor, and three research assistants in chemistry education to establish content validity, and by two chemistry teachers and two Turkish language teachers for the appropriateness of language and student level. Originally, there were 40 items in the test; after revisions, it was reduced to 22 items. A pilot test was conducted to evaluate reliability aspects of the test scores, and the Cronbach α reliability coefficient was computed as 0.75. In the scoring process, each correct response was scored as 1, and each incorrect response was scored as 0. Therefore, the total maximum score was 22 and the minimum was 0. The CCMAT was administered to all students as a pre- and post-test by the teachers during a regular class session and took 35 min.
Procedure
A quasi-experimental design was used in this study, because it is unlikely to obtain administrative approval to randomly select and remove a few students from different classrooms for any study in a Turkish school. During the study, chemical change and mixture topics were covered as part of the regular chemistry curriculum. Four intact classes with two different teachers in a public high school participated in this study. Each teacher's one intact class was assigned as the treatment group and the other as the control group. The control group was instructed using the traditional approach while the treatment group was instructed using the SWH approach. Prior to the instruction, CCMAT was administered to both groups to determine whether there was a significant difference between the groups with respect to measures of this instrument. The implementation period started after the pre-test was administered. The instruction provided to both groups was during regular classroom hours and took place over a 10-week period. In both conditions, time on task was kept equal. At the end of the treatment, the same CCMAT was administered to both groups as a post-test.Implementation of the SWH approach
On the first day of class, students in the treatment group were taken to the chemistry laboratory where they were informed about safety rules, basic materials, and chemicals. The teachers asked students to form their own small groups and introduced the SWH approach to them, using a mystery death activity (Burke et al., 2005). Working in small groups (n = 5), students were asked to read a short story giving details of a potential crime scene with several possible outcomes. They were asked to play the role of a detective investigating the scene. Each group suggested a beginning question about the death, wrote a claim, and supported that claim with evidence. Each group, in turn, shared their claims and evidence with the rest of the class. The teacher encouraged other groups to ask clarifying questions during the presentation. Upon completion of all presentations, the teacher discussed with the students the aim of this non-chemistry activity. Throughout the discussion, the importance of generating questions, claims, and evidence in building scientific understanding was emphasized. The appropriateness of students' evidence for their claims was also discussed in class. This experience provided students a concrete example to understand the SWH, especially for differentiating between making claims and providing evidence. Toward the end of the class, the teacher tried to uncover what the students already knew about chemical change through questioning, “What is a chemical change?”, “What is a physical change?”, “What is the difference between physical and chemical change?”. For the next class meeting, the teacher asked students to write their own questions about chemical change and possible tests and procedures to explore those questions. While students articulated their own questions and planned their testing procedure, the teacher circulated through the classroom and facilitated students' thinking through questioning as follows, “What is your question?”, “What are you going to do to test your question?”, “What are you measuring?”, “How would you measure those variables?”. During the session, the teacher asked the groups to share their questions and procedures with the class.After the appropriateness of each group's questions and procedures were evaluated with a class discussion, each group tested their own questions experimentally by recording their observations. Students were eager to contribute to the whole process because they were investigating their own questions. In the collection of data, the teacher acted as a guide and asked the following questions, “What did you observe/find?”, “How would you organize your data?”. The teacher asked the students to work individually and write claims about what they thought happened, including evidence to justify their claims. The teacher circulated through the groups to serve as a resource person and asked questions as follows, “How do you interpret your findings?”, “How do you relate your findings with your research question?”, “Do you see any pattern or relationship between the concepts based on your data?”. Immediately afterward, students in small groups constructed group claims and evidence after a thorough discussion of their individual claims and evidence. Each group then shared them with the rest of the class.
For example, one group was interested in burning and wanted to understand the change when a candle burns. Their question was, “What type of change occurs if a candle burns?”. To test their question, they stated, “We are going to light a candle and observe it”. The students lit a candle and observed the changes on it. Based on their observations, they claimed, “Both physical and chemical changes occur when a candle burns”. They supported their claim by stating, “Only the wick burned; the wax melted”.
At the time of the presentation, the teacher and the remaining groups asked questions, which facilitated a class discussion. The teacher had the students evaluate their peers' work and discuss the quality of their work. In the example mentioned above, the group stated that only the wick burns in a candle. After that group's presentation, the teacher asked, “Do you agree with your friends?”. Some students agreed with the group's claim, but some stated that all burning processes are chemical. The teacher asked, “Why is wax used if only the wick is burning?” After this question, some students were dissatisfied with their answers. The teacher distributed a candle to each group and asked them to observe it carefully when it is burning. Some students stated, “Wax can be burning because we saw that some of the candle melted and then vaporized”. Some students agreed with their friends by stating, “When we extinguished the candle, we smelled candle odor. That means there is some candle vapor in the air”. Some students claimed, “The amount of wax after the burning process may not be the same as initially”. The teacher moderated the discussion by asking these questions, “Do you agree with your friend(s)?”, “How do you know that?”. Such a discussion facilitated students' construction of scientific knowledge about burning.
The other four groups presented their arguments in the same way. At the end of the class session, the teacher asked students to write an investigation report in SWH format (Table 2). The teacher explained her expectations for each part of the report because the students were not familiar with the format of the SWH. When explaining the questions, claims, and evidence part of the SWH report, the teacher mostly referred to the mystery death activity because the aim of that activity was for students to become familiar with those concepts. Students were asked to use any relevant resources for their writing, including small group discussion notes, personal notes, readings, and classroom discussions. Students' writings were not used in data analysis for this study. The students engaged in further investigations about types of chemical reactions, classification of mixtures, and separation of mixtures in the following chemistry classes. For each investigation, the students followed the same approach.
Implementation of the traditional approach
Students in the control group were taught chemical change and mixture concepts mainly based on lecture and discussion methods. The chemistry textbook was the main source of knowledge in the control group and the students were required to read the related topic before the class hour. The teacher announced the goals of the lesson in advance and was highly active throughout the instruction. The teacher wrote the key terms on the board, defined and explained the concepts by giving examples, then asked questions about the concepts. The questions were in the form of, “What is …?”, “Give an example of …”. The aim of the questioning process was to ensure that all students understood the concepts in the same way. In this way, a discussion environment occurred through teacher-directed questions.After discussion, the teacher summarized what they did and asked students to take notes. Toward the end of the class session, the teacher wrote some algorithmic problems on the board and asked students to solve those problems individually. Then, the teacher asked one student to come to the board and solve a problem. The teacher helped that student during the problem-solving process. The problems that were not solved during the class session were given as homework. The students were also engaged in investigations for the aim of verifying what they learned in the classroom. Students were cautioned about safety concerns, especially when handling acids and bases. Students conducted the experiments in their textbook as a group under the direct control of the teacher. They were given the purpose of the experiment and asked to follow the step-by-step instructions in the textbook for the laboratory activities. During the experimentation process, the students were requested to answer questions related to the experiment provided in the textbook. At the end of the laboratory activity, students were asked to write a laboratory report in a traditional format, including purpose, procedure, observations and data, results, and discussion. The teacher helped students during the experimentation and asked questions to make students connect the laboratory activity with what they learned.
For example, at the beginning of the instruction of chemical change, the teacher stated, “Today, we are going to learn what chemical change is and give examples of it”. Then, the teacher defined the concept chemical change, stated the differences between physical and chemical change, and gave examples of chemical change from daily life. Immediately afterward, the teacher asked students to reiterate her explanations through questioning as follows, “What is a chemical change?”, “What are the differences between physical and chemical change?”, “Could you give examples of chemical change?”. The aim of this questioning was to ensure that all the students listened to the teacher, received and replicated knowledge given to them by the teacher. The teacher took students to the laboratory to see an example of chemical change through an experiment stated in their textbook. In advance of the laboratory activity, the teacher explained its purpose as, “Now, we are going to see the decomposition of water into its components using an electrical current”. The students worked in groups and conducted the experiment following step-by-step instructions provided in the textbook. The teacher helped students during experimentation and asked them to observe the changes on the tubes during electrolysis. Upon completion of the experiment, the teacher asked students to answer the questions in their textbook, “What did you observe when you brought the glowing end of a match into the test tube connected to the (+) side of a battery?”, “Which gases could be collected into the test tubes?”. When students failed to answer those questions, the teacher responded to them rather than giving hints to the students. At the end of the activity, the teacher asked students to write a report in traditional format.
Results
In this section, major findings of the study were reported through the quantitative analyses of data obtained from pre- and post-tests. The mean differences were interpreted as significant at the 0.05 level. The significance levels for pairwise comparisons among the achievement levels were adjusted using a Bonferroni correction (0.05/3 = 0.017). In this study, Cohen's d index was reported as a measure of effect size. An effect size is interpreted as small when Cohen's d is between 0.2 and 0.5 standard deviations, medium when it is between 0.5 and 0.8 standard deviations, and large when it is greater than 0.8 standard deviation (Cohen, 1992).Identifying students' chemistry achievement levels
Prior to the initial statistical analyses, students' achievement levels were determined using their chemistry mean scores in the previous semester. The mean of students' previous chemistry scores was 47.6, while the standard deviation was 24.6. The students who scored a half standard deviation (−0.5 to 0.5) around the mean were in the medium-achievement level, students who scored a half standard deviation below the mean were in the low-achievement level (−0.5 and down), and students who scored a half standard deviation above the mean were in the high-achievement level (0.5 and up) (Akkus et al., 2007). The number of the students in low-, medium-, and high-achievement levels were 27, 13, and 20 in the treatment group and 28, 12, and 20 in the control group, respectively. The achievement level was used as an independent variable along with the group (control and treatment) variable.Statistical analyses of pre-test scores
ANOVA results indicated that there was a statistically significant mean difference between the treatment group (M = 9.18, SD = 2.79) and the control group (M = 7.73, SD = 3.69) with respect to pre-CCMAT scores (F (1, 114) = 6.69, p = 0.011). There were also significant differences between low-achieving (M = 7.61, SD = 2.98) and high-achieving (M = 9.75, SD = 3.30) students on pre-CCMAT scores (MD = −2.13, SE = 0.66, p = 0.005). No significant interaction effect between group and achievement level was found. That is, there were significant mean differences in pre-CCMAT between low- and high-achieving students in both treatment and control groups prior to the study.Statistical analyses of post-test scores
ANCOVA was used in order to control students' prior achievement differences measured by pre-CCMAT on the post-CCMAT scores. Students' pre-CCMAT scores contributed significantly to their post-CCMAT scores (F (1, 112) = 10.78, p < 0.001), by explaining 9% of the variation on the post-CCMAT.Main effect
The findings indicated that there was a significant mean difference between the groups with respect to post-CCMAT scores when the effects of pre-CCMAT mean scores were controlled (F (1, 112) = 70.97, p < 0.001). Students in the treatment group (M = 13.75, SD = 2.23) had higher mean scores on post-CCMAT than those in the control group (M = 8.98, SD = 3.65). The size of the mean difference between the groups was large (Cohen's d = 1.6). That means, the difference detected between the groups arises from the treatment effect and this difference has practical importance. Moreover, significant differences were detected between low-achieving and high-achieving students (MD = −2.85, SE = 0.58, p < 0.001) with respect to post-CCMAT scores.Interaction effect
The results revealed that there was a significant interaction effect between group and achievement level with respect to post-CCMAT scores, F (2, 112) = 19.90, p < 0.001, indicating a large effect size (Cohen's d = 1.2). According to Fig. 2, low, medium, and high achievers performed closer to each other on post-CCMAT in the treatment group; but in the control group, there were differences among low, medium, and high achievers.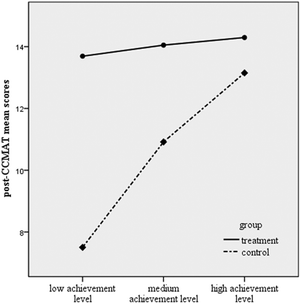 | ||
| Fig. 2 Interaction between group and achievement level. | ||
Further ANOVA results revealed that there were no significant differences among low-, medium, and high-achieving students in the treatment group but there were significant differences between high- and low-achieving students (F (1, 46) = 54.74, p < 0.001), and medium- and low-achieving students (F (1, 38) = 15.18, p < 0.001) in the control group. In addition, low-achieving students in the treatment group significantly outperformed low-achieving students in the control group with respect to post-CCMAT, F (1, 51) = 106.34, p < 0.001. Similarly, medium-achieving students in the treatment group scored significantly higher than medium-achieving students in the control group, F (1, 21) = 10.48, p = 0.004. The size of the effects of the SWH approach opposed to the traditional approach for low-achieving students (Cohen's d = 2.6) was larger than that for medium-achieving students (Cohen's d = 1.4). However, the difference between high-achieving students in the treatment and control groups was not significant.
Discussion
The findings obtained from this study are consistent with the findings of other national and international studies in terms of supporting the idea that the SWH approach leads to greater conceptual understanding (Keys et al., 1999; Akkus et al., 2007). In addition, low- and middle-achieving students in the SWH treatment group outperformed low- and middle-achieving students in the control group on the post-test. The achievement gap between low- and high-achieving students in the treatment group disappeared at the end of the study. However, the gap between achievement levels in the control group was still significant at the end of the study. This finding implies that implementation of the SWH approach was effective in closing the gap among the achievement levels for grade 9 students in learning chemistry.Implementation of the SWH approach helped low-achieving students to develop conceptual understanding of chemistry concepts. The reason why low achievers benefited from the SWH approach could be explained by emphasizing the nature of the learning environment provided in the treatment class. The SWH approach encouraged all the students to think and reason in a deeper manner. Students were actively involved in the learning process, and they constructed their own knowledge both personally and as a group (Krajcik and Sutherland, 2010). The students were engaged in investigations through which they sought answers for their own questions. Seeking solutions to their own questions was meaningful for the students, which naturally stimulated them to learn. They were aware of what they were doing and why they were doing it. Students posed claims and provided justifying evidence to make sense from experimental results. When students stated a claim, they demonstrated a generalization, a pattern, a relationship or provided an explanation that they have uncovered by their experimentation. By stating a knowledge claim and justifying it with evidence, students displayed scientific argumentation skills. When students construct arguments in an inquiry-based learning environment, it becomes apparent that they have learned the concepts under investigation and are not just providing rote information (Cavagnetto, 2010). The students were also engaged in small-group and whole-class negotiations when they were sharing their claims and evidence. Discussion of the concepts in a social context facilitated their understanding of the concepts. Sharing ideas through student–student and teacher–student interactions influenced the students' association of the results of their investigations with their current understandings (Fellows, 1994; Burke et al., 2005).
Students were also engaged in writing activities through the report writing in SWH format. The students were involved in writing activities before, during, and after the instruction. Before the instruction, students wrote their beginning ideas, their own questions, and the procedure for their investigations. During the instruction, they wrote data and observations based on their experimentation and wrote claims and evidence based on their data and observations. At the end of the instruction, students read from other sources, compared their interpretations with that of other sources and peers, and then wrote them in the report. They also wrote their reflections throughout the learning process. The reflection part of the investigation report format helped students compare their beginning ideas with the ideas learned through the classroom activities. The act of writing facilitates the linkage of new information with prior conceptions and, thereby, it has the potential for promoting deeper thinking and understanding of chemical change and mixture concepts (Driver, 1988; Fellows, 1994).
Moreover, the pedagogy adopted by the teacher in SWH classes may be encouraging for low-achieving students to learn better and catch up to the high-achieving students. The teachers in SWH classes attempted to elicit students' prior understandings through a pre-class discussion. However, in traditional classes teachers did not consider students' prior conceptions. There is substantial evidence that students' prior knowledge contributed to their achievement of chemical change and mixture significantly for this study. Prior knowledge is a well-known cognitive characteristic influencing student achievement in science (Chandran et al., 1987; Reynolds and Walberg, 1992). Because the SWH approach considers students' prior learning, it was found effective on the academic performance of students with low prior chemistry knowledge (Poock et al., 2007). In addition, the teachers in SWH classes allowed students work in groups, provided opportunities for students to examine their own questions, and encouraged students to generate knowledge claims and evidence based on their investigations (Burke et al., 2005). Questions, claims, and evidence used in SWH classes are the components of argumentation (Keys et al., 1999). Students' participation in the argumentation process is essential for meaning making and the advancement of science literacy since it develops their reasoning abilities and cognitive, metacognitive, communication, and critical thinking skills (Jimenez-Aleixandre and Erduran, 2007; Cavagnetto, 2010).
Consequently, this study demonstrated the value of the SWH approach in closing the achievement gap (Akkus et al., 2007; Grimberg and Hand, 2008). Low-achieving students were involved in the same cognitive and metacognitive processes as high-achieving students in SWH classes. Both low- and high-achieving students worked in cooperative groups and engaged in the construction of arguments and writing activities throughout the inquiry-based activities, which further facilitated their acquisition of scientific knowledge and development of science literacy (Hand et al., 1999; Cavagnetto, 2010; Krajcik and Sutherland, 2010). However, active student engagement in the learning process was not emphasized in the traditional class. Low-achieving students were not prompted to use the same cognitive and metacognitive processes used by the high-achieving students. This resulted in a further continuing achievement gap in the traditional class.
The findings of this research study have important implications for policy makers, stakeholders and practitioners. Helping low-achievers to be successful in highly competitive educational systems is always at the center of the interest through legislations and reform movements (e.g., No Child Left Behind in USA, Every Student Can Achieve in Turkey). However, political manoeuvres are mostly based upon foreseen needs of the society and conjecture. In order to make reform movements effective and sustainable, there is need for a substantial amount of empirical research studies that investigates ways of boosting up low-achieving students in the systems. Further, findings of such empirical studies can also help the stakeholders and practitioners in a variety of aspects. Data driven reforms can guide the stakeholders to effectively plan and implement reforms to tackle school failure. Moreover, they can help to shift practitioner beliefs and consequently implementations toward inquiry, science literacy and argumentation-based learning environments.
Non-traditional approaches are often considered to be time-consuming attempts, since their effects on achievement levels and standardized tests are not evident or not persuasive enough to create paradigm shifts. It becomes crucial to portray the results of classroom applications of non-traditional approaches such as the SWH and their effects on students from all achievement levels in order to create a systematical change in the horizon.
References
- Akkus R., Günel M. and Hand B., (2007), Comparing an inquiry-based approach known as the science writing heuristic to traditional science teaching practices: Are there differences? Int. J. Sci. Educ., 14, 1745–1765.
- BouJaoude S. and Attieh M., (2008), The effect of using concept maps as study tools on achievement in chemistry, Eurasia Journal of Mathematics, Science & Technology Education, 4(3), 233–246.
- Burke K. A., Greenbowe T. J. and Hand B. M., (2005), Excerpts from the process of using inquiry and the science writing heuristic. Retrieved from the World Wide Web, December 05, 2011, at http://avogadro.chem.iastate.edu/SWH/Resources.htm.
- Bybee R. W., Trowbridge L. W. and Powell J. C., (2004), Teaching secondary school science: strategies for developing scientific literacy (8th edn), Upper Saddle River, NJ: Pearson Merrill Prentice Hall.
- Carin A. A., Bass J. E. and Contant T. L., (2005), Methods for teaching science as inquiry (9th edn), Upper Saddle River, NJ: Pearson/Merrill Prentice Hall.
- Cavagnetto A. R., (2010), Argument to foster scientific literacy: A review of argument interventions in K-12 science contexts, Rev. Educ. Res., 80(3), 336–371.
- Cavagnetto A., Hand B. and Norton-Meier L., (2010), The nature of elementary student science discourse in the context of the science writing heuristic approach, Int. J. Sci. Educ., 32(4), 427–449.
- Chandran S., Treagust D. F. and Tobin K., (1987), The role of cognitive factors in chemistry achievement, J. Res. Sci. Teach., 24(2), 145–160.
- Choi A., Notebaert A., Diaz J. and Hand B., (2010), Examining arguments generated by year 5, 7, and 10 students in science classrooms, Res. Sci. Educ., 40, 149–169.
- Clark D. B. and Sampson V. D., (2007), Personally-seeded discussions to scaffold online argumentation, Int. J. Sci. Educ., 29, 253–277.
- Cohen J., (1992), A power primer, Psychological Bulletin, 112(1), 155–159.
- Driver R., (1988), Changing conceptions, Tijdschift voor Didactiek der β-wetenschappen, 6(3), 161–198.
- Eisenkraft A., (2003), Expanding the 5E model, The Science Teacher, 70(6), 56–59.
- Erduran S., Simon S. and Osborne J., (2004), TAPping into argumentation: Developments in the application of Toulmin's argument pattern for studying science discourse, Sci. Educ., 88, 915–933.
- Fellows N. J., (1994, April), Dynamics of conceptual change in small group science interactions. Paper presented at the American Educational Research Association Annual Meeting, New Orleans, LA.
- Ford M., (2008), Disciplinary authority and accountability in scientific practice and learning, Sci. Educ., 92(3), 404–423.
- Grimberg B. I. and Hand B., (2009), Cognitive pathways: analysis of students' written texts for science understanding, Int. J. Sci. Educ., 31(4), 503–521.
- Günel M., Kabataş-Memiş E. and Büyükkasap E., (2010), Effects of the science writing heuristic approach on primary school students’ science achievement and attitude toward science course, Educ.Sci., 35(155), 49–62..
- Hand B. and Choi A., (2010), Examining the impact of student use of multiple modal representations in constructing arguments in organic chemistry laboratory classes, Res. Sci. Educ., 40, 29–44.
- Hand B., Prain V. and Yore L. D., (1999), A writing in science framework designed to enhance science literacy, Int. J. Sci. Educ., 21(10), 1021–1035.
- Hand B., Wallace C. W. and Yang E., (2004), Using a science writing heuristic to enhance learning outcomes from laboratory activities in seventh-grade science: quantitative and qualitative aspects, Int. J. Sci. Educ., 26(2), 131–149.
- Jimenez-Aleixandre M. P. and Erduran S., (2007), Argumentation in science education: An overview, In S. Erduran and M. P. Jimenez-Aleixandre (ed.), Argumentation in science education: Perspectives from classroom-based research (pp. 3–28). Dordrecht, Netherlands: Springer.
- Kan A. and Akbaş A., (2006), Affective factors that influence chemistry achievement (attitude and self efficacy) and the power of these factors to predict chemistry achievement-I, Journal of Turkish Science Education, 3(1), 76–85.
- Keys C. W., Hand B., Prain V. and Collins S., (1999), Using the science writing heuristic as a tool learning from laboratory investigations in secondary science, J. Res. Sci. Teach., 36(10), 1065–1084.
- Kingir S., Geban O. and Günel M., Using the science writing heuristic approach to enhance student understanding in chemistry (manuscript submitted for publication)..
- Krajcik J. S. and Sutherland L. M., (2010), Supporting students in developing literacy in science, Science, 328, 456–459.
- Loomis S. C. and Bourque M. L., (2001), National Assessment of Educational Progress achievement levels, 1992–1998 for science, Washington, DC: National Assessment Governing Board. Retrieved from the World Wide Web, December 09, 2011, at http://www.nagb.org/publications/sciencebook.pdf.
- Marek E. A. and Cavallo A. M. L., (1997), The learning cycle: elementary school science and beyond (rev. edn), Portsmouth, NH: Heinemann.
- Martin M. O., Mullis I. V. S., Gonzalez E. J., Gregory K. D., Smith T. A., Chrostowski S. J., Garden R. A. and O'Connor K. M., (2000), TIMSS 1999 International Science Report: Findings from IEA's repeat of the Third International Mathematics and Science Study at the eighth grade, Chestnut Hill, MA: International Study Center, Boston College.
- Ministry of National Education, (2004), Elementary science and technology curriculum, Ankara, Turkey: Author.
- Ministry of National Education, (2007), 9th grade high school chemistry curriculum, Ankara, Turkey: Author.
- Mulford D. R., (1996), An inventory for measuring college students' level of misconceptions in first semester chemistry, Unpublished Master Thesis, Purdue University.
- Nam J., Choi A. and Hand B., (2010), Implementation of the science writing heuristic (SWH) approach in 8th grade science classrooms, Int. J. Sci. Math. Educ., 9(5), 1111–1133.
- National Research Council, (1996), National science education standards, Washington, DC: National Academies Press, Retrieved from the World Wide Web, October 20, 2011, at http://www.nap.edu/books/0309053269/html/index.html.
- Nolen S. B., (2003), Learning environment, motivation, and achievement in high school science, J. Res. Sci. Teach., 40(4), 347–368.
- Palmer D., (2005), A motivational view of constructivist-informed teaching, Int. J. Sci. Educ., 27(15), 1853–1881.
- Poock J. R., Burke K. A., Greenbowe T. J. and Hand B. M., (2007), Using the science writing heuristic in the general chemistry laboratory to improve students' academic performance, J. Chem. Educ., 84(8), 1371–1379.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982), Accommodation of a scientific conception: toward a theory of conceptual change, Sci. Educ., 66(2), 211–227.
- Reynolds A. J. and Walberg H. J., (1992), A structural model of science achievement and attitude: an extension to high school, J. Educ. Psychol., 84(3), 371–382.
- Rudd J. A., Greenbowe T. J. and Hand B. M., (2007), Using the science writing heuristic to improve students' understanding of general equilibrium, J. Chem. Educ., 84(12), 2007–2011.
- Schroeder J. D. and Greenbowe T. J., (2008), Implementing POGIL in the lecture and the science writing heuristic in the laboratory – student perceptions and performance in undergraduate organic chemistry, Chem. Educ. Res. Pract., 9, 149–156.
- Simon S., Erduran S. and Osborne J., (2006), Learning to teach argumentation: Research and development in the science classroom, Int. J. Sci. Educ., 28, 235–260.
- Simonneaux L., (2001), Role-play or debate to promote students' argumentation and justification on an issue in animal transgenesis, Int. J. Sci. Educ., 23(9), 903–927.
- Sampson V. and Gleim L., (2009), Argument-driven inquiry to promote the understanding of important concepts & practices in biology, The Am. Biol. Teach., 71(8), 465–472.
- Sampson V., Grooms J. and Walker J., (2009), Argument-driven inquiry: A way to promote learning during laboratory activities, The Sci. Teac., 76(7), 42–47.
- Sungur S. and Güngören S., (2009), The role of classroom environment perceptions in self-regulated learning and science achievement, Elementary Education Online, 8(3), 883–900.
- Turner R. C. and Lindsay H. A., (2003), Gender differences in cognitive and noncognitive factors related to achievement in organic chemistry, J. Chem. Educ., 80(5), 563–568.
- Tüysüz C., (2010), The effect of the virtual laboratory on students' achievement and attitude in chemistry, International Online Journal of Educational Sciences, 2(1), 37–53.
- Udo M. E., (2010), Effect of guided-discovery, student-centered demonstration and the expository instructional strategies on students' performance in chemistry, Afr. Res. Rev., 4(4), 289–398.
- Walker K. A. and Zeidler D. L., (2007), Promoting discourse about socioscientific issues through scaffolded inquiry, Int. J. Sci. Educ., 29, 1387–1410.
- Yager R. E., (1991), The constructivist learning model: Towards real reform in science education, The Sci. Teach., 58(6), 53–57.
Footnote |
| † This study is a part of the first author's doctoral dissertation. |
| This journal is © The Royal Society of Chemistry 2012 |
