Designing undergraduate-level organic chemistry instructional problems: Seven ideas from a problem-solving study of practicing synthetic organic chemists
Jeffrey R.
Raker
a and
Marcy H.
Towns
*b
aDepartment of Chemistry, Iowa State University, Ames, IA, USA
bDepartment of Chemistry, Purdue University, West Lafayette, IN, USA. E-mail: mtowns@purdue.edu
First published on 11th April 2012
Abstract
The development of curricular problems based on the practice of synthetic organic chemistry has not been explored in the literature. Such problems have broadly been hypothesized to promote student persistence and interest in STEM fields. This study reports seven ideas about how practice-based problems can be developed for sophomore-level organic chemistry courses; these ideas are the result of an investigation of the problem-solving experiences of eight practicing synthetic organic chemists.
Purpose
A key goal of science education reform has been to make “science learning better resemble science practice” (Edelson, 1998, p. 317). The Society Committee on Education of the American Chemistry Society (2003) has stated that chemistry education “needs to convey the nature of chemistry” (p. 2). Garratt (1997) has called for chemistry education to have more “emphasis on learning how to be a chemist” (p. 27). The argument is, and confirming research demonstrates that “engaging in authentic scientific practices” (Nersessian, 1995, p. 203) within classroom instruction indicate a connection to “later participation in scientific research” (Lindsay and McIntosh, 2000, p. 1174). K-12 educators, with little to no experience with science practice, struggle to include ‘real’ scientific experiences into their curriculum. College faculty members, on the other hand, at the forefront of scientific research have only tangentially explored the inclusion of nature of science concepts in their curriculum.The purpose of this manuscript is to outline seven ideas for the development of practice-based curricular materials from a problem-solving study of practicing synthetic organic chemists. While the ideas of synthetic organic chemists are not the sole focus of all organic chemistry research, synthetic organic chemistry provides a framework from which ideas of physical organic chemistry, for example, could be built. These ideas provide an initial framework for sophomore-level organic chemistry faculty to incorporate the practice of science into their courses. Data from a curricular assessment, interviews with eight practicing synthetic organic chemists, and interviews with eight undergraduate students provide an evidence-based foundation for the seven ideas. As well, references from the science education and organic chemistry literature provide further confirmation of the utility of the seven ideas for achieving the reform goal set by Edelson (1998) and others.
Methodology
An instructional design model (IDM) described by Jonassen and Hernandez-Serrano (2002) served as an overarching framework for participant selection, data collection, and data analysis. Jonassen and Hernandez-Serrano's model is based on transforming the problem-solving experiences of skilled practitioners into instructional problems for novice learners. The IDM describes the selection of curriculum to be revised, interviews with skilled practitioners, and revision of the curriculum to be more in line with practice.Selection of curriculum for revision
The study began with a curricular assessment of 792 instructional problems from four sophomore-level organic chemistry courses. Detailed results of this assessment can be found in this journal (Raker and Towns, 2010). This curricular assessment coupled with insights into the practice of organic chemistry from the literature (e.g., Bhattacharyya, 2004) and the first author's (JRR) academic training led to the development of five problems to be revised using the IDM methodology.Development of the five skilled practitioner problems originated in identifying broad problem types in synthetic organic chemistry research. These broad types included spectral identification of compounds, development of synthetic pathways, and implementation of reactions methodologies. Specific details of the problems emerged from doctoral dissertations from a highly selective university with a highly reputable doctoral program in synthetic organic chemistry. Dissertations from 2007 and 2008 were reviewed; these two years directly preceded initial data collection for this study, thus representing current synthetic organic chemistry research. Doctoral dissertations were chosen as problem sources given the abundance of experimental data and negative findings often reported in such documents.
The five skilled practitioner problems included two spectroscopic identification of a reaction product problems, one total synthesis of a natural product problem, one revision of synthetic pathways to a given target problem, and one application of a reaction methodology to an analogous series of compounds problem (see Raker, 2011, for the complete set of developed problems). A synthetic organic chemist, a non-participant in the study, provided consultation on the skill-level appropriateness and authenticity to the practice of organic chemistry research for each skilled practitioner problem; this consultation was used as a measure of reliability and validity in developing the skilled practitioner problems.
Skilled practitioner participants
Eight skilled practitioners participated in this study. Jonassen and Hernandez-Serrano (2002) discouraged the use of experts in reviewing curriculum with their IDM; in their opinion, it is difficult for experts to effectively articulate domain knowledge. Based on this recommendation, eight synthetic organic chemists were recruited for the study from a large academic research institution. The authors (JRR and MHT) acknowledge that industrial synthetic chemists were not sampled and thus findings from this study should be interpreted with this limitation in mind. Of the eight skilled practitioners, six were doctoral graduate students (third-year and above) and two postdoctoral researchers. Each participant was conducting synthetic organic chemistry bench-top research.Skilled practitioner interviews
The eight skilled practitioners were asked to participate in two interviews. The first interview focused on collecting background information including former and current experiences as a chemist and general problem-solving experiences as a synthetic organic chemist. In the second interview, the skilled practitioners were asked to partially solve two to three of the developed problems and answer reflection questions related to their experience solving similar kinds of problems in their research practice. Problems were only partially solved because the purpose of the second interview was to stimulate discussion about similar problems the skilled practitioners had solved in their research experiences. Only six of the skilled practitioners completed the second interview; two participants opted out of the second interview due to scheduling conflicts. Data from these two participants' first interviews provided insight into the development of the seven ideas reported herein; therefore, data from these two skilled practitioners were included in data analyses.The choice, selection, and order of the problems partially solved by each skilled practitioner were made by the first author (JRR) in the context of the second interview. Decisions were made based on the skilled practitioner's background and reflections on problems partially solved. The author (JRR) therefore chose problems that would most likely lead to deep reflection and thus rich data. Four of the five problems were sampled three times; the total synthesis problem was sampled four times.
Data analysis
Data analysis originated with transcription of the audiotaped interviews and cataloging of problem-solving artifacts (i.e., participant written partial solutions). Notes were made during transcription on initial thoughts on the skilled practitioner problem-solving experiences. Interviews were first openly coded. Codes were condensed based on similarities and emerging ideas. Interviews and problem-solving artifacts were reviewed with the condensed coding scheme. Findings emerged for each problem on how to maintain authenticity to the practice of organic chemistry research and yet tailor the problems to the appropriate skill-level of undergraduate students, the ultimate goal of the adopted IDM.Revision of the skilled practitioner problems to problems for undergraduate students
The final stage of the instructional design methodology is to convert the skilled practitioner problems into novice learner problems. Defined by Jonassen and Hernandez-Serrano (2002) terminology, novice learners would be undergraduate organic chemistry students. Data from the skilled practitioners were used to transform the five skilled practitioner problems to five undergraduate-level problems. Two of these problems will be described in the Discussion section of this paper; details of all five problems can be found in Raker (2011). A synthetic chemist with experience teaching undergraduate students was consulted to ensure the undergraduate-level problems were appropriate and within the realm of authentic synthetic organic chemistry practice.The undergraduate problems were pilot tested with eight undergraduate students representing a broad array of majors and organic chemistry instructional experiences (i.e., courses at varying levels of difficulty and content). Undergraduate student participants were asked to provide information on their academic background and then attempt to solve three of the five problems in the same manner as the skilled practitioners. Data from these interviews provided an additional layer of analysis and understanding.
The seven ideas, presented in this paper, emerged from a meta-analysis of the skilled practitioner interviews, undergraduate interviews, and understanding from the science education and organic chemistry primary literature. Each idea will be described in the next section; appropriate data and literature references will be used to clarify each idea. To protect anonymity, skilled practitioner and undergraduate student participants will be referred to by pseudonyms and designation of their graduate student, postdoctoral researcher, or undergraduate student status.
Seven ideas for the development of undergraduate organic chemistry problems
Jonassen and Hernandez-Serrano (2002) have noted a lack of advice for instructional designers in developing practice-based problems. Rather than present the five developed undergraduate level organic chemistry problems from this study, we present seven ideas about how new problems can be developed or current problems can be refined to mirror the practice of synthetic organic chemistry. These ideas are meant to be curricular recommendations, not prescriptive in nature. In addition, these ideas need to be further explored as to how their implementation impacts student understanding of the practice of synthetic organic chemistry.(A). Project level problems include the identification and selection of large, complex, multiple functionality containing target molecules (a paraphrased statement of participant Alberto, a 4th year graduate student). Igantius, a 3rd year graduate student, stated that anything “that can be drawn [and] pretty much anything that has been isolated from nature can be made [synthesized] with enough effort.” Deslongchamps (1984) has noted a similar idea, that any natural product or research conceived molecule could be synthesized.
(B). Synthetic planning problems include the retrosynthetic analysis of the target molecule (Corey, 1988, 1991; Corey and Cheng, 1995) and subsequent development of a synthetic pathway(s) while anticipating day-to-day problems. Synthetic planning problems are goal dependent; several were noted by the participants and confirmed in the organic chemistry literature: 100% selective reactions, inexpensive starting materials, efficient (Deslongchamps, 1984), environmentally friendly (Anastas and Warner, 1998), intricacy (Fuchs, 2001), and atom economy (Trost, 1991). Ignatius stated that these goals were not new, “the ideal, that's always been there. Still hasn't changed.”
One or more of these goals, as determined by the solver and research advisor, are utilized when solving synthetic planning problems. Alberto, a postdoc, couched his synthetic goals in terms of his ability to synthesize several compounds, potential drug leads, in a short period of time.
Synthesize as many compounds as you can in a very short time frame so that you can basically screen a whole panel of, of compounds to find the lead. So, that is specific to medicinal chemistry. It's a, a, an approach of chemistry that is specific to medicinal chemistry. Um… You have to find a simple but fast synthetic route from, you know, from… starting material to you[r] compound.
Alberto's goal of synthetizing compounds quickly were at the cost of achieving other potential synthetic goals such as using environmentally friendly reagents.
(C). Day-to-day problems arise while setting up physical apparatus, purifying products, characterizing products, or running property or biological activity testing. Aloysius, a 6th year graduate student, has defined these problems as a “challenge that keeps you from going to the next step.” Day-to-day problems are most commonly thought of in the situation of product characterization; these problems include byproducts, unexpected or no products; impure starting materials; insoluble products; instrumentation issues; reaction does not go to completion; and irreproducible reactions.
Another day-to-day problem is time management. Aloysius and Edmund, a postdoc, provided an insight into the demands of running reactions.
Some reactions are very easy; just mixing things together and everything will be fine. Some other reactions are much more demanding. And, very long in time and you have to, you know… So, if you take one hour off, and you should not at this time, then things go bad. (Aloysius)
We setup a reaction and if it's gonna go, if it takes 48 h for this reaction to go. Well if you stop it before that you gonna get a poor yield. There is nothing you can do sometimes. And sometimes if it, if you're supposed to stop a reaction at two in the morning. Because, if you leave it two hours longer it will decompose, well you're gonna have to do that. (Edmund)
For these practicing organic chemist participants, planning of reactions including anticipating when reactions will need to be worked up and the next reaction started was a very practical and ever-present problem.
Be able to make things from really simple materials. Like, what if you could [start with] CO2 and make sugar from it in a lab? That'd be really cool. I have no idea where to start. No. I don't think anybody knows where to start with that. As you can, you could take it to a carboxylic acid pretty easily. But, that's about all I would know how to do with that.
The problem, as Ignatius notes, is a synthetic planning problem: how to make the synthetic leap from carbon dioxide to a sugar.
Edelson (1998) stated that the learning context should reflect the use of the concepts and skills. Jonassen (2003) included context in defining his 11 problem types and advocated for instruction through problems; he believed that “when we learn something in the context of solving a problem, we understand and remember it better” (p. 18). Consider the common instructional problem of having students propose all the possible constitutional isomers for a given molecular formula. This type of problem is without any student-recognizable purpose. However, when coupled with spectroscopic identification of unexpected products, the task takes on the perspective of writing constitutional isomers for the purpose of identifying the obtained product.
The second context is the larger application of the problem. Especially when discussing project level problems, the practicing organic chemist participants were continuously referencing the application of their target molecules to medicinal treatments and other relevant applications. Robert, a 3rd year graduate student, limited the application of his natural product and human conceived target molecules to “just making pharmaceuticals really.” Aloysius, a 6th year graduate student, more broadly grouped current target molecules of synthetic interested as molecules with biological importance and bioactivity. The practicing synthetic organic chemist participants were able to state why their projects were important. For example, Edmund, a postdoc, works on the development of inhibitors of ‘big diseases,’ synthesizing libraries of biologically active compounds. (The actual diseases are not reported herein to protect the anonymity of the participant.) Most literature accounts of organic chemistry research include a section in the introduction for stating the application of the reported research. Bretz (2001), Edelson (1998), and Savery (2006) have spoken that knowledge learned should be meaningful; incorporation of solver relevant target molecules and applications is advantageous to peaking the meaningfulness of problems and learning for the solvers.
Several examples of this second idea exist in the chemistry education literature (e.g., Doxsee, 1990; Ferguson, 1980; Harrison, 1989; Kelley and Gaither, 2007). In each case, the focus was on providing an example of the application of the lecture material to a given field of study (e.g., medicine). However, these attempts of context incorporation remain within a topic-driven instructional model from the mid-twentieth century and not a practice of science driven instructional model. Therefore, these examples show the relationship between organic chemistry and a given field instead of the use of organic chemistry in the given field. Idea 2 advocates learning that emerges from trying to solve problems for fields such as medicine rather than give examples of how the theories and models from lecture apply to another context.
Literature references were made in the development of both the practicing organic chemist and undergraduate student problems. Ignatius, a 3rd year graduate student, noted in developing a revised synthetic pathway to solve one of the problems that obtaining the original paper would help him to generate new pathway ideas. In summarizing, he stated:
…a lot of times full papers will have failures in them also. Which is very, very useful. Because then you, you know well they exactly tried the exact things that you're thinking about that seems like a better idea. But it doesn't work for whatever reason. And sometimes you try it again anyway, because you think it didn't work because of their fault.
Inclusion of such references grounded the problem and applicable data explicitly in the record of organic chemistry research.
Overton and Potter (2008) would refer to this inclusion as a means to demonstrating the “authenticity” of the problems. Literature references can be a means for promoting lifelong learning; engaging the solver to explore the problem in more detail should the problem pique his or her interest. While the authors (JRR & MHT) acknowledge that an undergraduate student may be incapable of understanding every aspect of the literature references that could be made in problem prompts, the authors do feel strongly that literature references could generate further student interest in the field of synthetic organic chemistry. As undergraduate participant Julia noted in solving a methodology problem: “I think this is more of a real-world problem because, if I wanted to, I could look up this journal article.”
Searching for the literature from which sophomore-level organic chemistry instructional material is built can be difficult. Many of the models, such as substituent effects on electrophilic aromatic substitution, were reported in the early 1900's. This should not preclude practitioners for searching for the applicable literature and incorporating the references into their work.
Another implementation recommendation is for practitioners to build “adapted primary literature,” a process of pairing down and reframing primary literature into documents suitable for the knowledge and skill level of sophomore-level students (see Falk et al., 2008). This method has shown promise in exposing students to the literature down to even secondary students (Yarden et al., 2001).
The practicing synthetic organic chemist participants described their research as having open-ended character and confirmed that the problems provided during the problem-solving interview were open-ended. Xavier, a 4th year graduate student, noted that as the complexity of the target molecule increased that the open-endedness of the project-level problems increased. In comparing this to classroom problems that he had solved, Xavier reported that “you have something [a target molecule] that's much larger and… it's more open-ended. You don't have a starting material that you have to use.” Alberto, a postdoc, echoed the open-endedness of the targets by expressing that the number of possible synthetic pathways that could be posited for a given target molecule are at minimum in the “thousands.”
The open-ended problem characteristic allowed both the practicing organic chemist and undergraduate student participants to define the direction and answer format that they wished to take in solving the problems.
To increase the open-endedness of curricular problems, some dimension of the problem must be left to the discretion of the problem solver. One such dimension could be in the number of potential solutions. As the participants in the study discussed, having students develop synthetic pathways for given target molecules where more than one specific pathway is correct. Another suggestion would be to ask students to develop a research study to determine the stereochemical outcomes of substitution (SN1 and SN2) reactions; in this instance students have the ability to suggest what starting materials and reagents would provide the data to support a claim about the stereochemical outcomes of the chemistry.
The undergraduate student participants were able to correctly identify the chemistry involved in their given problems. Champagne and Bunce (1991) have noted that a learner must be able to relate new information, ideas, and experiences to existing knowledge; the same could be said for relating new problems to existing knowledge of chemical problems. Familiarity is solver dependent, as was noted in the interviews with all the participants. A finding of Cartrette (2003) corroborates the solver dependence on familiarity: “problem solving is a personal endeavor” (p. 6). Bodner (2003) believed the difference between a problem and an exercise (or routine) to be couched in the element of familiarity; as will be mentioned in the next idea, a problem should not be entirely familiar to the solver and must contain some element of challenge.
 | ||
| Fig. 1 Example of a practicing organic chemist (left) and undergraduate student (right) appropriate total synthesis target molecule. | ||
Challenge was seen as a factor of the problem-solving experience and internal knowledge of the solver. Undergraduate student participants voiced their challenge with problems in the form of never having seen the problem format before, not knowing what method to use to solve the problem, inability to identify and apply known chemical concepts to new chemical systems, and utilizing multiple concepts in answering a single problem. A problem should not be entirely familiar; an element of “a gap” (Hayes, 1989) or “not knowing what to do” (Wheatley, 1984) must be present in order for the problem to not be considered a routine task.
Johnstone (1993) spoke of the importance of data being incomplete for higher order problems. However, the participants in this study treated incomplete data by identifying what data was necessary to solve the problem. In other words, if the data is not complete the solver will look up the data. Reference materials should include spectroscopic data tables, access to the literature, and textbooks of known reactions and mechanisms.
Discussion
In proposing these ideas, we recognize that the utility of actually achieving any goals of improving student understanding of the nature of science or persistence and interest in science have not been explored. However, we intend to make such a determination through subsequent research.In addition, we make no designation for whether all problems in a course should mirror the practice of science or whether problems that mirror the practice of science should be exclusively incorporated into graded or non-graded learning activities. In both these cases, it should be the learning goals of the specific instructor for a specific course that should ultimately dictate how practice-based problems should be incorporated into the curriculum.
To demonstrate the implementation of the seven ideas, we offer two examples of how the ideas could be used to revise or develop instructional problems. Both example problems were utilized for the undergraduate student interviews.
Example 1
First, synthetic planning problems are the most common of all three problem types found in synthetic organic chemistry research (Idea 1). In the instructional setting, these problems have the form of a provided starting material and final product. Students are tasked with finding a synthetic pathway between the starting material and final product within certain constraining parameters (e.g., all sources of carbon incorporated into the final product must originate in haloalkanes of six carbons or less). Synthetic planning problems for practicing chemists do not follow this pattern. Research synthetic planning problems are more open-ended (Idea 4): no defined starting material and constraints often center on starting material availability, cost, and degree of difficulty for completing the proposed reactions. Another form of research synthetic planning problems includes the revision of literature account pathways. Fig. 2 displays an example of how a synthetic planning problem is most frequently posed to a student. Fig. 3 then displays how that problem could be rephrased to most closely mirror the practice of synthetic organic chemistry. | ||
| Fig. 2 An example of a traditional synthetic pathway problem. | ||
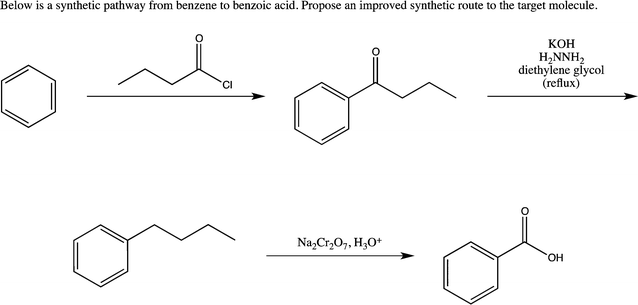 | ||
| Fig. 3 An example of a synthetic pathway problem that mirrors the practice of organic chemistry. | ||
In Fig. 2, the problem is straightforward, requiring a solver to develop a synthetic pathway based on their knowledge of potential reactions. In Fig. 3, the problem is phrased in the context of improving the pathway (Idea 2). A solver must then make a judgment about how the synthetic pathway could be improved before proposing their synthetic pathway.
Five undergraduate participants (Isaac, John, Peter, Stan, and Stephanie) worked on developing solutions for this problem. Each made a statement about the goal of the problem. Peter called it developing a “better” synthetic pathway. Stan noted his limitation in knowledge about improving yield and selectivity; but, this did not completely deter him from posing ideas and solutions. Isaac, John, and Peter mentioned that the improved synthetic pathway would need to be less than three steps, the number of the steps in the pathway to be revised.
Each of the five participants took some time to understand the reaction scheme. For Isaac, Peter, and Stephanie, this time was spent talking through the pathway by naming reagents and reactions. John and Stan moved to critiquing the pathway. John noted that they pathway included installing functionality that was then removed: “they removed [a carbonyl group] and then added it again… It doesn't make sense to do that if you can avoid it, to be adding and removing things if you can skip it.”
Isaac was the only participant to generate a succinct solution in the time provided. He labeled his problem-solving process as just “playing around” and not putting “too much effort” into the process. Eventually he “stumbled across” the two synthetic pathways shown in Scheme 1.
 | ||
| Scheme 1 Isaac's synthetic pathways for problem 2. | ||
Isaac felt that he was unsure of what side reactions may occur in either of his solutions; therefore, he was unable to critique the improvement factor of this proposed synthetic pathways.
In reference to the problem solutions provided, Isaac and Stan commented on the use of literature-like resources for developing their pathways. Isaac stated that he would use his textbook to explore the requirements and limitations of reactions. Stan would consult his textbook for specific conditions for his reaction ideas.
Example 2
The focus of the next example will be on the incorporation of the ideas of providing contextual information (Idea 2), providing literature references (Idea 3), and including a familiar dimension to the problem (Idea 5). In this problem (see Fig. 4 for the problem prompt), a solver is directed to apply a provided reaction methodology to a series of target compounds. | ||
| Fig. 4 An example of reaction methodology problem. | ||
The prompt provides a reason for the desired syntheses, i.e., a physical organic chemistry's NMR study. Second, the prompt provides a literature reference for the reaction methodology, a reaction that should be familiar to the student even though not directly named (i.e., the Wittig reaction). In addition to the prompt, a solver is provided with experimental data for the reaction methodology (see Scheme 2 and Table 1). The prompt, reaction scheme, and reaction methodology results provide a complete data set for the solver to fulfill the desired task.
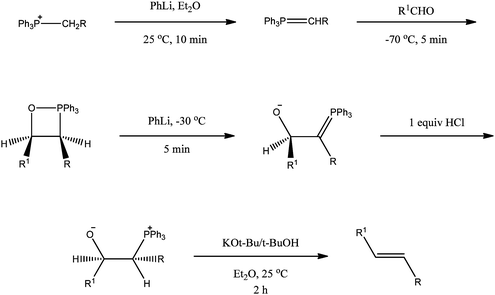 | ||
| Scheme 2 Olefin formation methodology (Schlosser and Christmann, 1966). | ||
| R | R1 | % yield |
trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis cis |
|---|---|---|---|
| CH3 | C5H11 | 70 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| C5H11 | CH3 | 60 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| C3H7 | C3H7 | 72 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| CH3 | Ph | 69 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| C2H5 | Ph | 72 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
While the nature of this problem borders on what an instructor might expect a student to prepare for a laboratory experiment, it is exactly the connection between lecture discussions (e.g., the Wittig reaction) and laboratory tasks (e.g., preparing experimental procedures) that demonstrate the linkage between theory and practice. It is in this linkage that the nature of science instruction emerges and Edelson's (1998) goal of making science learning mirror science practice is realized.
Four participants (Alphonsus, Isaac, John, and Julia) worked on developing solutions for this problem. Each participant had varying levels of difficulty understanding the prompt. Alphonsus felt that the prompt was “vague.” Alphonsus and Julia asked about what were available starting materials. The four participants eventually settled on the goal to apply the given reaction scheme to synthesize the given targets.
The problem solution, as defined by the four participants, was mainly to define the R-groups of the necessary starting materials to synthesize the five target molecules. For Julia, this meant returning to the reaction scheme: “First, I'm gonna go back and identify what each of the two R [groups] are in diagram. And, then figure out where they came from in the mechanism”.
Conclusion
From a study on designing authentic problems based on the field of synthetic organic chemistry, seven ideas about how to design undergraduate-level organic chemistry problems emerged:1. Mirror the practice of organic chemistry.
2. Provide contextual information.
3. Provide literature references.
4. Make problems open-ended.
5. Include a familiar element in problems.
6. Make problems appropriately challenging.
7. Provide access to reference materials when students solve problems.
These seven ideas provide a framework for revising current and developing new curricular problems. Problems can be incorporated in classroom instruction, course assignments, formal assessments, and complementary laboratory exercises. While the ideas were developed from the perspective of synthetic organic chemistry, we envision that many of the ideas could be utilized in designing instructional materials for other chemistry and STEM disciplines. Further research needs to be conducted to demonstrate the utility of such curricular reformations on students' views of the nature of organic chemistry and further participation in science.
Acknowledgements
We would like to thank the 16 participants who gave of their time and energy for this study. And, we would like to thank the three professors who supplied the curricular problem sets that were used as data. We would also like to thank Ms. Nicole Becker and Mr. Daniel Nicponski for their thoughtful reviews and suggestions during the data analysis phase of this study.References
- Anastas P. T. and Warner J. C., (1998), Green Chemistry: Theory and Practice, New York: Oxford University Press.
- Bhattacharyya G., (2004), A recovering organic chemist's attempts at self-realization: How students learn to solve organic synthesis problems, PhD, Purdue University, West Lafayette, Indiana.
- Bodner G. M., (2003), Problem solving: The difference between what we do and what we tell students to do, University Chemistry Education, 7, 37–45.
- Bretz S. L., (2001), Novak's theory of education: Human constructivism and meaningful learning, Journal of Chemical Education, 78, 1107.
- Cartrette D. P., (2003), Using spectral analysis to probe the continuum of problem solving ability among practicing organic chemists, Masters of Science, Purdue University, West Lafayette.
- Champagne A. B. and Bunce D. M., (1991), Learning-theory-based science teaching. In S. M. Glynn, R. H. Yeany and B. K. Britton (ed.), The psychology of learning science (pp. 21–41), Hillsdale, New Jersey: Lawrence Erlbaum Associates.
- Corey E. J., (1988), Retrosynthetic thinking – Essentials and examples, Chemical Society Reviews, 17, 111–133.
- Corey E. J., (1991), The logic of chemical synthesis: Multistep synthesis of complex carbodenic molecules, Angewandte Chemie: International Edition in English, 30(5), 455–465.
- Corey E. J. and Cheng X., (1995), The logic of chemical synthesis, New York: Wiley.
- Deslongchamps P., (1984), The concept of strategy in organic synthesis, Aldrichimica Acta, 17(3), 59–71.
- Doxsee K. M., (1990), Development of an advanced synthesis laboratory course in Nobel prize winning chemistry, Journal of Chemical Education, 67(12), 1057–1060.
- Edelson D. C., (1998), Realising authentic science learning through the adaptation of scientific practice. In B. J. Fraser and K. G. Tobin (ed.), International Handbook of Science Education (pp. 317–331), Great Britain: Kluwar Academic Publishers.
- Education S. C. o., (2003), Exploring the molecular vision: American Chemical Society.
- Falk H., Brill G., Yarden A., (2008), Teaching a biotechnology curriculum based on adapted primary literature, International Journal of Science Education, 14(17), 1841–1866.
- Ferguson L. N., (1980), Content and structure of the chemistry course: New approaches, Journal of Chemical Education, 57(1), 46–48.
- Fuchs P. L., (2001), Increase in intricacy - A tool for evaluating organic syntheses, Tetrahedron, 57, 6855–6875.
- Garratt J., (1997), Virtual investigations: Ways to accelerate experience, University Chemistry Education, 1, 19–27.
- Harrison A. M., (1989), Medicinal chemistry/pharmacology in sophomore organic chemistry, Journal of Chemical Education, 66(10), 825–826.
- Hayes J. R., (1989), The complete problem solver (2nd edn), Hillsdale, NJ: Lawrence Erlbaum Associates.
- Hendrickson J. B., (1978), A logical characterization of organic chemistry, Journal of Chemical Education, 55(4), 216–220.
- Johnstone A. H., (1993), In C. Wood and R. Sleet (ed.), Creative problem solving in chemistry, London: The Royal Society of Chemistry.
- Jonassen D. H., (2003), Learning to solve problems: An instructional design guide, San Francisco: Pfeiffer.
- Jonassen D. H. and Hernandez-Serrano J., (2002), Case-based reasoning and instructional design: Using stories to support problem solving, Educational Technology Research & Development, 50(2), 65–77.
- Kelley C. and Gaither K. K., (2007), Integrating pharmacology into the organic chemistry course: Understanding the synergy of biology and chemistry, Journal of College Science Teaching, 30(7), 450–453.
- King P. M. and Kitchener K. S., (1994), Developing reflective judgments: Understanding and promoting intellectual growth and critical thinking in adolescents and adults, San Francisco: Jossey-Bass.
- Lindsay H. A. and McIntosh M. C., (2000), Early exposure of undergraduates to the chemistry research environment: A new model for research universities, Journal of Chemical Education, 77(9), 1174–1175.
- Nersessian N. J., (1992), How do scientists think? Capturing the dynamics of conceptual change in science. In R. Giere (Ed.), Minnesota Studies in the Philosophy of Science, Minneapolis, MN: University of Minnesota Press.
- Nersessian N. J., (1995), Should physicists preach what they practice? Science & Education, 4, 203–226.
- Overton T. and Potter N., (2008), Solving open-ended problems, and the influence of cognitive factors on students success, Chemistry Education Research and Practice, 9, 65–69.
- Raker J. R., (2011), Connecting scientific research and classroom instruction: Developing authentic problem sets for the undergraduate organic chemistry curriculum, Doctor of Philosophy, Purdue University, West Lafayette, Indiana.
- Raker J. R. and Towns M. H., (2010), Benchmarking problems used in second year level organic chemistry instruction, Chemistry Education Research and Practice, 11(1), 25–32.
- Raker J. R. and Towns M. H., (2012), Problem types in synthetic organic chemistry research: Implications for the development of curricular problems for second-year level organic chemistry instruction, Chemistry Education Research and Practice 10.1039/c2rp90001g.
- Savery J. R., (2006), Overview of problem-based learning: Definitions and distinctions, The Interdisciplinary Journal of Problem-based Learning, 1(1), 9–20.
- Schlosser M. and Christmann K. F., (1966), Trans-selective olefin syntheses, Angewandte Chemie: International Edition in English, 5(1), 126.
- Shepard L. A., (2000), The role of assessment in a learning culture, Educational Reseacher, 4–14.
- Trost B. M., (1991), The atom economy-A search for synthetic efficiency, Science, 254(5037), 1471–1477.
- Wheatley G. H., (1984), Problem solving in school mathematics, West Lafayette, IN: Purdue University, School Mathematics and Science Center.
- Yarden A., Brill G. and Falk H., (2001), Primary literature as a basis for a high-school biology curriculum, Journal of Biological Education, 35(4).
| This journal is © The Royal Society of Chemistry 2012 |
