Ensuring sustainability of tomorrow through green chemistry integrated with sustainable development concepts (SDCs)†
Mageswary
Karpudewan
*,
Zurida
Ismail
and
Wolff-Michael
Roth
Universiti Sains Malaysia and Griffith University, Queensland, Australia. E-mail: kmageswary@usm.my; mageswary_karpudewan@yahoo.com
First published on 28th February 2012
Abstract
The purpose of this article is to describe a best practice: an approach to teaching chemistry that our quantitative research has shown to produce large differences between experimental and control groups in terms of achievement, pro-environmental attitudes, values, and motivation. Our interest in teaching chemistry by focusing on sustainable development arises from the environmental concerns that as the country of this study, Malaysia is facing in many different areas—including rampant logging and pollution. As educators, we are interested in educating future generations so that they can cope with the environmental challenges that not only this nation but also the world as a whole is increasingly facing. The “green chemistry” approach we describe here may be just the answer that other developing nations and emergent economies in closing the gap with industrialized nations. We provide a detailed description of how green chemistry has been implemented in a curriculum for pre-service science teachers and in the curriculum of secondary school chemistry.
Introduction
The purpose of this paper is to describe a green chemistry approach—which we have used in the secondary school chemistry curriculum and in chemistry teaching methods courses—as an example of a “good/effective” practice, evidence for which our experimental work has provided (e.g., Karpudewan et al., 2009, 2011; Karpudewan et al., 2011, in press-a, in press-b). The unique feature of this paper is its concern for the nature of the practice rather than for the evaluation (which we provide elsewhere) in the hope that science educators generally but those working in countries with emergent economies especially are inspired to use the approach to prepare their nations for coping with pollution and environmental exploitation. We begin by outlining the societal and political background to our development of a green chemistry curriculum. We move to describing the fundamental ideas underlying the education for sustainable development and green chemistry. In the context of this paper, we provide and discuss some of the outcomes of the experimental work that we have obtained from studies on the integration of green chemistry as laboratory-based pedagogy in chemistry teaching methods course. We conclude this introduction with a sketch of the adaptations that we made to the regular chemistry curriculum to produce a green chemistry curriculum.Societal and political background
Malaysia is a developing nation with an emergent economy. The country is striving to achieve the status of a fully developed country by the year 2020, a goal explicitly stated in the Sixth Malaysian Plan (Economic Planning Unit, 1991). To achieve this status, the nation requires an annual growth of 7% (in real terms) over the 30-year period since the plan was articulated (1990–2020), leading to an eightfold increase of its 1990 GDP of RM115 billion. To achieve such an ambitious goal for economic growth, Malaysia draws on its natural resources, new constructions, the opening of new industries, and the development of power plants to support these industries. However, all of these activities potentially have tremendous impact on the environment. It is therefore of profound importance to manage resources and construction so that the development is sustainable over a time span that exceeds the 30-year plan. In addition to the environmental challenges from sanctioned developments, there are challenges that come from other activities, including the illegal logging of prime forests. To allow Malaysia—or all those developing nations and emergent economies in a similar situation—to set in place a sustainable economy also requires a population sufficiently informed about how to deal with the challenges to environment and environmental health.Educating citizens by introducing programs that target secondary school students and today's pre-service teachers who will teach future generations of students on the issue of sustainability appears to be a be viable approach to developing a pro-environmental orientation in a nation as a whole. We anticipate this based on observations in Switzerland, where the (pro-, anti-) environment-related discourses of 15–16-year old students reflect those of Swiss society as a whole, which means that there is a reproduction of adult discourses among the young who then have children themselves reflecting the discourses of their parents (Zeyer and Roth, 2009, 2011). Changing orientations toward the environment, therefore, requires targeting future generations (of parents) and their teachers, because these would then mediate the environmental and sustainability discourses of subsequent generations. With this goal in mind, we conducted empirical studies to test whether secondary school students and pre-service teachers would change toward more positive levels of their understanding, attitudes, values, and motivations toward environmental issues. Our effort of integrating green chemistry in teacher education curriculum is consistent with the calls for higher education to make sustainability education a requirement for all undergraduates; and adopting green chemistry into secondary school curriculum is consistent with the understanding that students have to have opportunities to be educated about sustainability from secondary school levels on (Rowe, 2007). In addition to the other effects we intend to bring about, the implementation of green chemistry also contributes to the improvement of science education (Alberts, 2005; Kennedy, 2007), to its goal of contributing to the building and sustaining of lively scientific communities that are able to address global problems, and to the maintenance of high levels of scientific literacy among the general public (van Eijck and Roth, 2007). Green chemistry also has the potential to contribute to the education of scientists who actively work in their community for bringing about sustainable practices related to the environment and environmental health (Roth, 2009). Green chemistry allows students to participate in decision making over real issues in their everyday worlds and, therefore, allows them to contribute to global environmental problems by acting appropriately on local matters.
Education for sustainable development
Various definitions were given for the term sustainable development. Basically, sustainable development is defined in a widely quoted statement: “the development that meets the needs of the present without comprising the abilities of future generations to meet their own needs” (World Commission on Environment and Development, 1987). Education is central for sustainability, because it effects the implementation of sustainable development plans, impacts the decision-making capacities of the society, and determines the quality of life. Education that is intended to support sustainable development is known as education for sustainable development. Education for sustainable development is a value-laden education, which centers on human beings and asks humankind to change its behaviors. The ultimate focus of education for sustainable development is to create a sense of responsibility that takes into account the social, economic, and environmental effects on human life forms (Burmeister et al., 2012). Education for sustainable development thereby becomes a guiding principle for classroom learning, life beyond the classroom, as well as for completing education. Therefore, education for sustainable development emphasizes active student participation as an important aspect that fosters a sense of responsibility to actively contribute to the development of a sustainable way of living.In an education for sustainable development, the content of the curriculum and pedagogy employed to deliver the subject matter are central to the particular values, worldviews, and attitudes that are fostered (Fien, 2000). The author recommends the curriculum to be holistic and pedagogy to be student centered. Both goals can be achieved when everyday life examples and knowledge are welcomed as the grounding for students' subsequent learning (Anderson, 2007). Integration of everyday life examples and knowledge leads to an interdisciplinary perspective on subject matter (Roth, 2003). This is so because sustainable knowledge systems bridge the gap between the knowledge and application (David et al., 2003). Similarly, sustainable development concepts also allow teaching and learning to integrate different curriculum subjects and, therefore, support students to participate in interdisciplinary problem statements and solution finding (Yencken et al., 2000). Suitable sustainable development concepts inclusive of concepts such as carrying capacity, steady-state economy, ecospace, ecological footprint, natural resource accounting, eco-efficiency, life-cycle analysis, sustainable consumption, local-global link, interdependence, intergenerational equity, intra-generational equity, interspecies equity, and basic human needs (Yencken et al., 2000).
Green chemistry
“Green chemistry” refers to the use of chemistry that prevents pollution. To prevent pollution, green chemistry employs materials, processes, or practices that reduce or eliminate the creation of pollutants or wastes. The term also refers to practices that reduce the use of hazardous and nonhazardous materials, energy, water, or other resources as well as protect natural resources through efficient use (EPA, 1996). The implementation of the green chemistry into practice is guided by 12 principles that underlie a green approach to chemistry (Anastas and Warner, 1998). Through application and extension of these 12 principles, green chemistry can contribute to sustainable development (Wardencki et al., 2005). The principles of green chemistry comprise the following: it is better to prevent waste than treating it; atom economy; using less hazardous material synthesis method; designing safer chemicals; design for energy efficiency; use of renewable feedstock; reduce derivatives; catalysis; design for degradation; real-time analysis for population prevention; and inherently safer chemistry for accident prevention (Anastas and Warner, 1998). In this, green chemistry is not a new branch of science. Rather, it is a new way of thinking about science in a responsible manner so that the lives of future generations are not compromised by today's actions. Implementing green chemistry into curriculum comes with the hope of building a foundation that leads to a sustainable chemical industry in support of a sustainable society (Braun et al., 2006).In schools, green chemistry allows students to make connections between the discipline of chemistry, other disciplinary subject matters, and aspects of their lives. For example, green chemistry has the potential to overcome a major barrier of current environmental education (Lianne, 2005), which separates pristine environments (nature) and the home, where the practices occur that impact the nature. Green chemistry provides students with the opportunity to act in an environmentally appropriate manner, because students understand how their chemistry directly affects the settings in which chemicals are used and where pollutants and wastes are dispersed. It has been shown that students who engage in environmental action tend to develop deep understandings of the sciences that they directly apply as part of their actions (Roth and Barton, 2004). Studies have suggested that implementation of green chemistry education can overcome the limitations of the current environmental education because it enhances critical thinking and problem solving skills as well as encourages students to look into sustainable development both locally and globally (Parrish, 2007). This study also lights that green chemistry enhances the communicative skills of the students. Students learn to address the environmental problems, as they felt empowered towards solving the problems (Haack et al., 2005).
Green chemistry laboratory manual
In the course of implementing green chemistry experiments into pre-service teachers' curriculum and secondary school chemistry curriculum, a laboratory manual was produced containing 27 activities (Karpudewan et al., 2011). These experiments/activities were obtained from various sources (e.g., Cann and Connelly, 2000; Warren, 2001; Kirchhoff and Ryan, 2002) and tailored to suit our Malaysian context. We also developed several new activities. Prior to the development of the manual, a series of workshops involving in-service teachers (Fig. 1), pre-service teachers and secondary school students (Fig. 2) were conducted to identify the feasibility of integrating these activities into the official curriculum. Through these workshops feedback was obtained from teachers and students, including their views in relation to the activities. The feedback was used to further improve the activities before the final version was completed. | ||
| Fig. 1 In service teachers working with the experiments. | ||
 | ||
| Fig. 2 Secondary school students involved in the experiment. | ||
The activities in the manual cover various topics that are an integral part of the secondary school chemistry curriculum. In the laboratory manual, the existing experiments in the chemistry curriculum were presented in such a way that environmental concerns were highlighted. To extend the learning beyond the walls of the classroom, and therefore to make the learning of chemistry relevant with the everyday life of the students, appropriate sustainable development concepts were also integrated into the curriculum. All the activities begin with pre-lab questions, which are followed by pre-lab discussions and a study of the procedures required for conducting the chemistry experiments at the heart of the activities. For answering the pre-lab questions, students have to review the relevant literature and discuss their answers within a peer group. The pre-lab questions thereby engage the student in reflection that set the stage for understanding the laboratory work in the applied context of a real-world problem. The pre-lab questions also introduce the materials and processes used in the experiment. The questions also require students to investigate the historical and current, real-world aspects of the experiment. The safety precautions and concerns as well hazardousness of the chemical and correct means of disposing the chemicals are discussed thoroughly in this section. The conversion of traditional experiments into green chemistry experiments also involved adding a new dimension to the students' tasks: they are asked to analyze and explain how their actions in relation to materials used in the experiment can and will contribute to sustainable development and the environmental health of the nation as experienced by future generations.
During the post-lab discussions students not only discuss the observation and findings of the experiments as they traditionally do but also in view of with the impacts of the pertinent chemistry on economy, environment, and society on Malaysia as well as on the world more generally. For this purpose the students have to draw on the sustainable development concepts, which therefore become the discursive resources they learn to deploy for the purpose of making arguments for a greener approach to the use of chemical processes. For example, the production of biodiesel from palm oil, an important resource produced in Malaysia, is one of the experiments included in the manual (see below). (It is evident that in other developing nations and emerging economies, curriculum developers will choose relevant local and national resources for contextualizing their green chemistry efforts.) Two sustainable development concepts are addressed: natural resource accounting and life cycle analysis (Yencken et al., 2000). In relating the experiment to life cycle analysis, students find all aspects included that are inherent in the process of producing biodiesel from the growing of palms to the use of biodiesel (including waste generation and management). This involves, for example, studying the starting materials (renewable or non-renewable sources used in the industrial process), energy and chemicals used to develop the biodiesel, or the wastes deriving from the production and consumption of biodiesel. Students evaluate the relevance of these factors to the local and national economy, environment, and the society as a whole.
In this way, green chemistry makes way for student-centered learning. In other words, studying the chemistry involved in the local context and with local relevance gives rise to deep learning, a process where learners do not rote memorize and regurgitate facts taught by teachers but engage with their hearts and minds (i.e., cognitive and affective domain) to enhance their understanding of issues that directly affect their everyday lives (Greeno, 1998). In the appendix, we exemplify green chemistry by means of a detailed description of one activity with local application: the production of biodiesel.
Toward a greener chemistry: the production of biodiesel
The production of biodiesel described in the appendix is one of the experiments that students conduct as part of the green chemistry curriculum. This description exemplifies our overall effort, which has led to substantial changes in students’ knowledge, pro-environmental attitudes, motivations to act pro-environmentally, and the values students adopt. That is, there is experimental evidence for the quality of this revised chemistry teaching practice. Thus, for example, after going through a course in green chemistry pre-service teachers' acquisition of environmental concepts (Traditional Environmental Concepts [TECs] and Sustainable Development Concepts [SDCs] improved and it appeared that the pre-service teachers developed deeper understandings of SDCs than TECs (Karpudewan et al., 2009). Understanding of SDCs is imperative, as it overcomes the limitation of TECs. For instance TECs basically imparts the knowledge about the environment and actions necessary to overcome pollution whereby in this scenario the environment is perceived differently than the human; however, SDCs describe the whole ecosystem as integral part of everyday living and the learners begin to relate the consequences of their actions on the whole ecosystem.In terms of environmental values, introduction of green chemistry experiments as a laboratory-based pedagogy changes pre-service teachers’ environmental value from initially being egocentric towards being more homocentric and ecocentric (Karpudewan et al., in press-a). The results of the study indicate that after going through series of green chemistry experiments pre-service teachers' egocentric value orientation decreased significantly, homocentric orientation increased, however, the increase is not significant. The ecocentric orientation value orientation of the pre-service teachers' improved significantly. Individual with egocentric value will engage in the activities that benefit themselves without considering whether or not the activities benefit the environment. Homocentric individual will justify their actions from the perspective of whether the actions benefit the humanity and ecocentric individual attempt to protect the environment for its intrinsic worth. Results of another study, involving different cohort of pre-service teachers indicates that pro-environmental attitude and self-reported behavior measured with New Ecological Paradigm scale and self-reported behavior survey changed substantially (Karpudewan et al., in press-b). An increase in the total pro-NEP stance measured in the percentage was obtained for the entire 15 items in the NEP scale among the pre-service teachers experienced green chemistry. Additionally, for the self-reported behavior statistically reliable differences were obtained between the pre-test and post-test means on every one of the eight pro-environmental items included in the survey.
A survey was conducted to identify the students' view/perceptions on the implementation of green chemistry as a laboratory-based pedagogy (Karpudewan et al., 2011). A majority of them agreed that the green chemistry experiments are accordance with syllabus requirement; it is easier to learn chemistry concepts with green chemistry experiments; the equipments and materials are readily available; sufficient time was allocated to conduct the experiments; and the experiments are safe to be conducted in schools. However, a majority also indicated that they are not sure whether they have better understanding of SDCs and how to apply in their everyday life. Additionally, these pre-service teachers are not sure whether the procedures to conduct the experiments are simple and easy to be followed.
The findings obtained from our effort in integrating green chemistry experiments into pre-service teachers' curriculum, as reported in various previously published work, explicitly indicates that there are substantial in evidence in green chemistry promoting pro-environmental attitudes, environmental values and knowledge (Karpudewan et al., in press-a, in press-b; Karpudewan et al., 2009).
Teaching methods that go with green chemistry
Green chemistry goes beyond simply doing the experiment and linking it to concepts. Throughout the unit, students are supported in making linkages to other aspects of their life. For example, to bring in the real-world situation, the experiment on biodiesel was further extended with activities comparing heat of combustion of petroleum diesel and biodiesel and comparison of energy required to travel for 100 km and the cost comparison between biodiesel and petroleum diesel. The integration of experiment on production of biodiesel as one of the green chemistry experiments is consistent with the claim that this will foster controversial discussions in the classroom and one possible way to improve students' motivation and attitude towards chemistry and its importance to society (Eilks, 2002). Fig. 3 shows another extension that allows students to understand that there is much more soot produced in the burning of petroleum diesel than in the burning of biodiesel. Through this hands-on activity students observe the differences in the burning of the two fuels.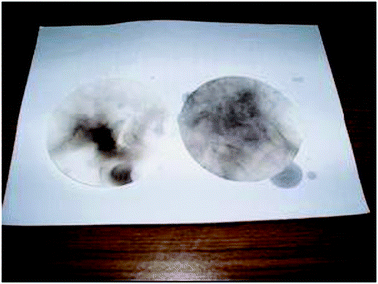 | ||
| Fig. 3 The soot developed from burning of biodiesel (left) and petroleum diesel (right). | ||
There are further extensions that involve students taking a particular side in a mock debate. The mock debate in our context is slightly different from previously reported studies on teaching of biodiesel (e.g.Eilks, 2002) and bioethanol usage (e.g.Feierabend and Eilks, 2011). The students were requested to provide opinion for the question of ecological evaluation based on a public debate concerning biodiesel that appeared in the newspaper (Eilks, 2002). For this debate, students review further relevant literature on the differences between these two fuels. They conduct a life-cycle analysis (similar to cradle-to-grave analysis used in Eilks, 2002) of both fuels, and based on that then they debate whether opting to use biodiesel is really green. For the purpose of debate, two groups are appointed. One group supports and argues that using biodiesel is green. The other group is tasked with arguing against the view. While debating, the students are intrinsically involved in understanding the arguments of the side they have to defend to convince the other side. In their argument the students draw on real-world examples, for example, on the fact that petroleum diesel adds to the air pollution due to forest burning in Sumatera and Indonesia, and which raises the air pollution index in Malaysia. The other group raises the question why the Indonesian (in Sumatera) burns the forest in the first place, which, as they find out, they do to plant palm trees for the purpose of biodiesel. That is, students learn that there are costs as well as benefits with the production of biodiesel from palm oil.
Yet another extension that allows students to understand chemistry concepts that have real-world pertinence: they determine, for example, the heat of combustion for biodiesel and petroleum diesel using the apparatus shown in Fig. 4. After they have calculated the combustion heat, energy level diagrams are drawn for both the fuels. They calculate bond energy: ΔH = bond broken − bond formed as well as the energy required to travel 100 km using either fuel. The differences in the energy are converted to number of moles and volume of biodiesel consumed.
 | ||
| Fig. 4 Apparatus used to determine heat of combustion. | ||
Finally, we end the discussion on biodiesel with a role-play. The implementation of role-play is consisted with notion that this form of expression raises learners’ awareness of the societal dimension of the issues being discussed; and it highlights the dialogues taking place and various options available for consideration and different special interest groups which take part in the process (Feierabend and Eilks, 2010). It is proposed that the government is planning to impose the use of biodiesel in the near future. Students are asked to respond to this based on the role they are assigned: a palm tree plantation worker, an active member of Greenpeace (a NGO of environmentalist), a CEO of a company that provides goods and transportation service in Malaysia, and as a member of the general public. As palm tree plantation worker, students provide their views to the governmental plan talking about, for example, why biodiesel is being produced commercially in Malaysia as well the future of biodiesel. As an active Greenpeace member, students imagine having just returned from the Bali Climate Talks and that they are getting worry about the condition of mother earth. As CEO of a transportation company, which consumes a large amount of diesel every year and expects that more fuel will be used due to the increase of customers in the coming year, they provide arguments about why or why not diesel-powered engine should be converted to biodiesel engines. Finally as a member of general public, the students comment on the government's plan and speculate about how the prices of other goods will be affected by the plan.
Implementation and evaluation of green chemistry
In this section, we describe where and how we implemented the green chemistry curriculum and evaluated its impact.Green chemistry in the teacher education curriculum
The chemistry teaching methods course is offered in the School of Educational Studies at Universiti Sains Malaysia. It is a compulsory course taken by the third-year students enrolled in the science education degree program. Upon completing this program, the pre-service teachers may be appointed as teachers, teaching chemistry to Form 4 and Form 5 (secondary school) students. Currently, the course content covers instructional theory, pedagogical content knowledge, and various instructional strategies used to teach chemistry. This includes constructivism, inquiry methods, project-based learning, active learning, and traditional laboratory-based pedagogy. The pre-service teachers are exposed to various instructional aids that may be used to teach chemistry effectively. Fundamentally, the course lays a foundation of pedagogical and pedagogical content knowledge as well as relevant educational theories.We began the implementation of green chemistry during the 2006/2007 and 2007/2008 semesters as a laboratory-based pedagogy. The first and second cohorts of the implementation consisted of 110 and 263 pre-service teachers, respectively. This effort constitutes a concrete implementation of the university's sustainability endeavors. During the semester, the pre-service teachers worked on ten experiments. They also simulated teaching a lesson in green chemistry, where students act in the role of the teacher. For this purpose they were required to prepare a lesson on the activity assigned to them. Upon completing the lesson, the students were required to submit a completed lab report. The students were continuously assessed on lesson plans, lab reports and simulated teaching. Two quizzes were administered, during the middle of semester (after completing the first five experiments) and towards the end of the semester (after completing all the 10 experiments). In their final written examinations, students were asked to justify the introduction of green chemistry in secondary school curriculum. Our empirical work shows large effects on the environmental values (Karpudewan et al., in press-a) and acquisition of sustainable development concepts (Karpudewan et al., 2009). Throughout the semester the students were exposed to the green chemistry experiments and effect of green chemistry experiments on environmental value change and acquisition of environmental concepts was measured for three times using repeated measure design. In all but one instance, statistically reliable effects were found (Table 1). Green chemistry also shows large effects on improving the environmental attitudes (Karpudewan et al., in press-b) when compared to students who did the traditional experiments on the same chemistry topic (Table 2).
| Measure | Categories | Statistic | Findings |
|---|---|---|---|
| Environmental Values | Overall values (combination of all the three categories of values) | Repeated measure one-way ANOVA | F(2,218) = 180.40, p < 0.0001 |
| Egocentric | F(2,218) = 12.53, p < 0.00333 | ||
| Homocentric | F(2,218) = 0.003, p > 0.0167 | ||
| Ecocentric | F(2,218) = 9.43, p < 0.00333 | ||
| Knowledge | Traditional Environmental Concepts | Repeated measure one-way ANOVA | F(2,208) = 3.784, p < 0.05 |
| Sustainable Development Concepts | F(2,208) = 59.56, p < 0.05. |
| Measure | Mean (M) and Standard Deviation (SD) | t-test results | |
|---|---|---|---|
| Control group | Experimental group | ||
| Environmental Attitudes | M = 2.68, SD = 0.42 | M = 3.13, SD = 0.29 | t(261) = 11.02, p = 0.004 |
Green chemistry in secondary school chemistry curriculum
The integration the sustainable development concepts into the green chemistry experiments was extended to the secondary school level, where they were introduced as part of the students' practical work. In Malaysia, practical work is compulsory and constitutes an essential component of teaching and learning chemistry. For the purpose of this research the experiments in relation to topic of rate of reaction and chemistry of carbon compound were introduced to the students. Table 3 indicates the list of experiments conducted by the high school students. The effect of the green chemistry on student motivation towards learning chemistry were measured using a qualitative approach and the effect on students understanding of chemistry concepts was measured using a previously validated chemistry achievement test (CAT). Again, there were large effect sizes (d = 3.27) when compared to the control group (t(65) = 14.89, p < 0.0001), where students did the equivalent chemistry experiments.| Week | Experiment |
|---|---|
| 1 | Effect of temperature on the rate of reaction |
| 2 | Effect of concentration on the rate of reaction |
| 3 | Effect of concentration on the rate of reaction |
| 4 | Biosynthesis of ethanol |
| 5 | Bromination of an alkene |
| 6 | Preparation and distillation of cyclohexene |
Conclusion
During the recent climate change meeting in Copenhagen (December 2009), Malaysia agreed to reduce carbon emissions from 187 million tones in 2005 to 74.8 million tones in 2020, a reduction to 40% of the original levels. To achieve such an ambitious target, the nation as a whole has to contribute both on personal and collective levels. The current secondary school students, who will be joining the workforce in the near future, require some fundamental understanding of sustainable development and what this implies for the individual and collective practices. Our work shows that green chemistry has a tremendous potential in changing relevant chemistry concepts, attitudes, motivations for acting pro-environmentally, and pro-environmental values. We fundamentally hope—though this remains to be studied—that our own endeavor of teaching green chemistry leads to sustainable change and development of the Malaysian people. We designed green chemistry in the hope that it will assist our people to actively contribute to sustainable growth and a planet that is made more livable because of how each of us behaves individually and contribute to collective decision-making.Appendix
Pre-lab questions
1. What is the characteristic of cooking oil that allows it to be used as starting material of biodiesel?2. Use structural features of cooking oil and methanol to describe how biodiesel is formed.
3. What are the differences between petroleum diesel and biodiesel in terms of their structures?
4. Describe what you know about the starting materials used for biodiesel production and to what extend it has been implemented in Malaysia.
5. What are the contributions of present generation for the future generations with the change from petroleum diesel to biodiesel?
Pre-lab discussion
In 1895 Rudolf Diesel designed an engine that run on vegetable oil. However, the high viscosity of vegetable oil and the lower production costs of petroleum diesel resulted in Diesel's modification of the engines to run on petroleum diesel. At the time, awareness about the pollution created by petroleum diesel did not exist. Today, however, it is well understood that the use of petroleum diesel contributes to air pollution; air pollution, in turn, impacts the climate both locally and globally as well as having immediate effects on human health (e.g., under smog conditions). Petroleum diesel forms particulates and carcinogenic compounds. It contributes to a net increase in greenhouse gases such as carbon dioxide, sulfur dioxide, and nitrous oxide. In addition, petroleum diesel is a product obtained by fractional distillation of petroleum, a non-renewable (fossil-fuel) resource. Furthermore, petroleum diesel is much more difficult to degrade than biodiesel is. A viable substitute for the demand for diesel fuel is biodiesel, a methyl ester of fatty acids found in vegetable oils. Biodiesel is produced through transesterification reaction (Fig. 5). During this reaction the bonding between glycerol and the fatty acids are cleaved. A methyl group is added to the end of the fatty acid, which is called biodiesel, and the other products are glycerol and the sodium hydroxide catalyst. Discarded cooking oil or palm oil is suitable starting materials for the production of biodiesel.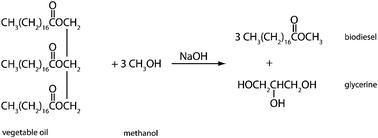 | ||
| Fig. 5 A transesterification reaction produces biodiesel (and glycerine) from the reagents vegetable oil and methanol in the presence of the sodium hydroxide catalyst. | ||
Purpose
1. To produce biodiesel from cooking oil.2. To identify the properties of biodiesel.
3. To compare and differentiate the properties of biodiesel and petroleum diesel.
Equipment and chemicals
100 mL Erlenmeyer flask, pestle and mortar, magnetic stir plate, hot plate, 20 mL graduated cylinder, 100 mL graduated cylinder, thermometer, stop watch, 125 mL separation funnel, retort stand, methanol, sodium hydroxides pellets, cooking oil.Procedure
1. Measure 14 mL of methanol into 100 mL Erlenmeyer flask.2. Weigh 0.5 g of sodium hydroxide. Crush the NaOH pellets into a powder using a mortar and pestle and transfer this powder into the Erlenmeyer flask containing methanol.
3. The NaOH can be dissolved with continuous stirring on magnetic stir plate for about 5 to 10 min.
4. Use a graduated cylinder to measure 60 mL of cooking oil and add this to the methanol solution in the Erlenmeyer flask.
5. Using a hot plate, gently heat the solution to a temperature between 35 °C to 50 °C for 20 min with continuous stirring so that the mixture does not separate into two layers.
6. Pour the warm reaction mixture into 125 mL separation funnel and allow the solution to cool and partition into two product layers.
7. Draw off the bottom layer, which contains glycerol, residual methanol; trace water and salts into a small weighed (tared) beaker.
8. The top layer in the separation funnel is the biodiesel. Gravity filtrations can be used to filter the biodiesel (Fig. 6).
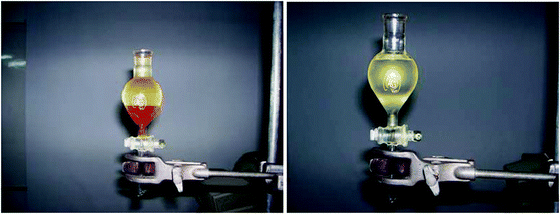 | ||
| Fig. 6 (left) Biodiesel being separated from the byproducts in a separation funnel. (right) Biodiesel developed after removal of glycerol and methanol (bottom layer). | ||
9. Measure the volume of biodiesel collected and calculate the percentage of biodiesel conversion based on the starting volume of oil and volume of biodiesel produced.
10. Compare the viscosity of vegetable oil and biodiesel.
Post-lab questions
1. Describe how the biodiesel production can be used to implement the concept of natural resource accounting: a strategy that helps household or government calculate its real wealth, i.e., the volume of total economic production minus the value of the natural and social capital consumed to achieve it.2. Life cycle analysis is a management tool for identifying the net flows of resource and energy used in the production, consumption and disposal of product or service in order to leverage eco-efficiency gains. Describe how this concept can be taught while teaching biodiesel production experiment.
3. What changes did you see between the characteristic of the starting materials and final oil?
4. Based on the answer for question three, explain why biodiesel is more easily degradable than petroleum diesel.
5. Is biodiesel really green? Explain at least one argument in support of the idea that biodiesel is a greener fuel. Also present one argument that biodiesel is not a greener fuel.
6. In the commercial production of biodiesel, 1200 kg of vegetable oil produces 1100 kg crude biodiesel. How does your yield compare to this?
7. Describe the green chemistry principle that could be incorporated into this experiment.
References
- Anastas P. T. and Warner J. C., (1998), Green chemistry: theory and practice, Oxford, UK: Oxford University Press.
- Anderson B., (2007), Curriculum content in the light of education for sustainable development, Retreived from www.unesco.org/education/desd on 21 May 2011.
- Alberts B., (2005), A wakeup call for science faculty, Cell, 123, 739–741.
- Burmeister M., Rauch F. and Eilks I., (2012), Education for sustainable development (ESD) and chemistry education, Chem. Educ. Res. Pract., 13(2) 10.1039/c1rp90060a.
- Braun B., Charney R., Clarens A., Farrugia J., Kitchens C., Lisowski C., Naistat D. and O'Neil, A., (2006), Completing our education, J. Chem. Educ., 83, 1126–1129.
- Cann M. C. and Connelly E. C., (2000), Real-world cases in green chemistry, Washington, DC: American Chemical Society.
- David C., William C., Frank A., Nancy D. and Noelle E., (2003), Science and Technology for Sustainable Development a special feature: A knowledge system for sustainable development, Retrieved from www.pnas.org/cgi/content/full on 21 May 2011.
- Eilks I., (2002), Teaching “Biodiesel”: A Sociocritical and problem-oriented approach to chemistry teaching and students' first views on it, Chem. Educ. Res. Pract., 3(1), 77–85.
- Feierabend T. and Eilks I., (2010), Raising students' perception of the relevance of scientific teaching and promoting communication and evaluation capabilities using authentic and controversial socio-scientific issues in the framework of climate change, Sci. Educ. International, 21, 176–196.
- Feierabend T. and Eilks I., (2011), Teaching of the societal dimension of chemistry using a socio-critical and problem-oriented lesson plan based on bioethanol usage, J. Chem. Educ., 88, 1250–1256.
- Economic Planning Unit, (1991), Sixth Malaysian Plan 1991–1995, Kuala Lumpur: Percetakan Nasional Malaysia Berhad.
- Environmental Protection Agency (EPA), (1996), Green chemistry, Retrieved from www.epa.gov/greenchemistry on 12 May 2011.
- Fien J., (2000), Education for the environment: A critique–A Response, Env. Educ. Res., 6, 179–192.
- Greeno J. G., (1998), The situativity of knowing, learning, and research, Am. Psychol., 53(1): 5–26. DOI: 10.1037/0003-066X.53.1.
- Haack J., Hutchison J. E., Kirchhoff M. M. and Levy I. J., (2005), Going green: Lecture assignments and lab experiences for the college curriculum, J. Chem. Educ., 82, 974–978.
- Karpudewan M., Ismail Z. and Roth W.-M., (in press-a), The efficacy of a green chemistry laboratory-based pedagogy: changes in environmental values of Malaysia pre-service teachers, Int. J. Sci. Math. Educ. DOI:10.1007/s10763-011-9295-y.
- Karpudewan M., Ismail Z. and Roth W.-M., (in press-b), Promoting pro-environmental attitudes and reported behaviors of Malaysian pre-service teachers using green chemistry experiments, Env. Educ. Res. DOI:10.1080/13504622.2011.622841.
- Karpudewan M., Ismail Z. and Mohamed M., (2011), Greening a chemistry teaching methods course at the School of Educational Studies, Universiti Sains Malaysia, J. Educ. Sustain. Development, 5(2), 197–214.
- Karpudewan M., Ismail Z. and Mohamed M., (2011), Laboratory Manual. Green Chemistry: Approach addresses education for sustainable development, ISBN: 9789675467264.
- Karpudewan M., Ismail Z. and Mohamed M., (2009), The integration of green chemistry experiments with sustainable development concepts in pre-service teachers' curriculum: experiences from Malaysia, Int. J. Sustain. High Educ., 10, 118–35.
- Kennedy D., (2007), Science teaching roundup, Science, 317, 17.
- Kirchhoff M. and Ryan M., (2002), Greener approaches to undergraduate chemistry experiments, Washington, DC: American Chemical Society.
- Lianne F., (2005), Learning on environmental awareness in children: An empirical investigation, J. Env. Educ., 36, 39–50.
- Parrish A., (2007), Toward the greening of our mind: A new special topics course, J. Chem. Educ., 84, 245–247.
- Rowe D., (2007), Education for sustainable future, Science, 317, 323–324.
- Roth W.-M., (2003), Scientific literacy as an emergent feature of human practice, J. Curriculum Stud., 35, 9–24.
- Roth W.-M., (2009), Activism or science/technology education as byproduct of capacity building, J. Activist Sci. Tech. Educ., 1(1), 16–31.
- Roth W.-M. and Barton A. C., (2004), Rethinking scientific literacy, New York: Routledge.
- van Eijck M. and Roth W.-M., (2007), Improving science education for sustainable development, PLoS Biology, 5, 2763–2769.
- Warren D., (2001), Green chemistry, UK: Royal Society of Chemistry.
- Wardencki W., Curylo J. and Namiesnik J., (2005), Green Chemistry—current and future issues, Po. J. Environ. Stud., 14, 389–395.
- World Commission on Environment and Development, (1987), Our common future (The Brundtland report), Oxford: Oxford University Press.
- Yencken D., Fien J. and Sykes H., (2000), Environment, education and society in the Asia-Pacific: Local traditions and global discourses, London, England: Routledge.
- Zeyer A. and Roth W.-M., (2009), A mirror of society: a discourse analytic study of 14–15-year-old Swiss students' talk about environment and environmental protection, Cult. Stud. Sci. Educ., 4, 961–998.
- Zeyer A. and Roth W.-M., (2011), Post-ecological discourse in the making, Public. Underst. Sci. DOI:10.1177/0963662510394949.
Footnote |
| † This article is part of a themed issue on sustainable development and green chemistry in chemistry education. |
| This journal is © The Royal Society of Chemistry 2012 |
