A study of first-year chemistry students' understanding of solution concentration at the tertiary level
Kevin
de Berg
Avondale College of Higher Education, PO Box 19, Cooranbong NSW 2265, Australia. E-mail: kdeberg@avondale.edu.au
First published on 6th December 2011
Abstract
This paper reports on students' understanding of sugar concentration in aqueous solutions presented in two different modes: a visual submicroscopic mode for particles and a verbal mode referring to macroscopic amounts of sugar. One hundred and forty-five tertiary college students studying some form of first-year chemistry participated in the study. For problems of a similar nature, students were much more successful in solving solution concentration problems that were presented verbally than were presented using a submicroscopic representation of particles. The implications of this for chemistry education are outlined in the paper. One contributing factor to the poor success rate with submicroscopic representations (SMR) was possibly the fact that the SMR were presented in multiple-choice format whereas the verbal representations required a short-answer response. While the multiple-choice format may prove deceptive, on account of the way students interpret alternatives containing visual images, students agreed it also proved instructive in highlighting the importance of accounting for volume change in concentration calculations.
Introduction
We know generally that students have great difficulty understanding chemistry concepts at all levels of education. This is partly due to the ideas students bring to the study of chemistry which have been formed from their experience of the world and which differ from accepted scientific ideas (Mulford and Robinson, 2002). In addition to this, chemistry teachers and chemistry textbooks present chemistry knowledge over at least three levels of representation for which students are often not well prepared (Johnstone, 1982, 1991, 1993, 2000). These three levels of representation are most commonly referred to as macroscopic, submicroscopic and symbolic (Treagust, Chittleborough and Mamiala, 2003; Gilbert and Treagust, 2009). As far as a completely dissolved sugar solution is concerned, for example, the macroscopic representation refers to what can be seen or measured, that is, in this case, a glass containing a colourless liquid. The submicroscopic draws upon science's particle model and pictures the normally invisible particles of a solution as dispersed in all directions throughout the volume of the solution. The symbolic draws upon the chemist's use of chemical formulae and may label the solution as, C12H22O11(aq).The use of these three levels of representation, often called the chemist's triangle or triplet, has recently come under close scrutiny (Talanquer, 2011). Since chemical manipulations and measurements are not now confined to the macroscopic world, understood as the properties formed by a very large number of molecules, but include manipulations at the nanoworld level, Talanquer (2011, p. 187) prefers the following triplet classification.
Experiences: These refer to the actual empirical knowledge that we have or gather about chemical systems.
Models: These refer to the theoretical entities and underlying assumptions that we use to describe chemical systems.
Visualizations: These refer to the static and dynamic visual signs (from symbols to icons) developed to facilitate qualitative and quantitative thinking and communication about both experiences and models in chemistry.
Whichever triplet one chooses to adopt, the research evidence indicates that an instruction style that encourages students to form connections between all three levels of representation leads to enhanced achievement (Gabel, 1993; Sanger, 2000; Ardac and Akaygun, 2004; Davidowitz et al., 2010; Gilbert and Treagust, 2009).
Ebenezer (2001, p. 78) discusses how a hypermedia environment which integrates text, graphics, animation, video imaging and spatial modelling for solution chemistry enables “students to go forward and backward to see connections among different types of knowledge in chemistry…. In addition, a click on the button animated depiction of the solution process…and…the students were able to play the animation any number of times. Students could actually alternate between macroscopic and submicroscopic knowledge in this hypermedia environment”. Calik et al. (2005, p. 37) highlight the importance of such instructional strategies “to help students make connections between experience-based observations of chemical phenomena and chemists' abstract atomic and molecular models”. However, as a guide to teachers, Calik et al. (2010) suggest that one should not rely on only one instructional strategy such as hypermedia or worksheets if effective connections between the macroscopic, submicroscopic and symbolic are to be made. These authors outline a variety of presentation modes which might encourage teachers to expand and enrich the teaching and learning environment.
In addition to the description of chemical knowledge at three levels of representation, Paivio (1986) proposed for knowledge in general that the mind codes separately for knowledge expressed verbally and that expressed visually. This separate coding did not, however, mean that links could not exist between the two codes. Indeed, it was proposed that learning could be enhanced if connections were made. A verbal cue may automatically trigger a visual cue if the scenario has been embedded in experience. For example, if one says, “Six grams of sugar are dissolved in a litre of water”, a visual image of a spoon of white sugar being added to a tumbler of water may spontaneously be cued based on the event resting in common experience. However, for one not trained in the language and models of chemistry, the verbal cue above would most likely not trigger a visual molecular image based on the particle model or a visual image of a molecular model for sucrose or its formula. The role of chemistry education is thus to enhance the formation of triggers in verbal and visual cues which help to formulate links between the verbal and visual. In a study of dissolution with twenty-two Grade 9 students, She (2004) claimed that 76%–90% of the students underwent a conceptual shift towards the target curriculum knowledge when asked to verbally predict the outcome of an exercise such as adding sugar to water and then to draw a mental image of what they thought was happening during the exercise. Such multimodal instruction has been shown to enhance memory for simple tasks (Brunye et al., 2006) and the suggestion is made by Mayer (2001) that if students are presented even with complex topics in verbal and visual modes they will more likely be able to recall that information at a later time.
Visualization (Gilbert, 2008) of the particle model of matter, which is so crucial for understanding the concept of solution concentration, is known to present difficulties for students. Students tend to assign to submicroscopic atoms and molecules the same properties observed for macroscopic entities (Calik et al., 2005; Ebenezer and Erickson, 1996; Ben-Zvi et al., 1986; Gabel et al., 1987; Harrison and Treagust, 1996; Bucat and Mocerino, 2009). Thus a copper atom is thought to be malleable, a water molecule thought to be wet, and a sugar molecule in solution thought to be white in colour. In addition to this, Bucat and Mocerino (2009) draw attention to the fact that macroscopic properties like solution concentration appear to be continuously variable and yet we explain this apparently continuously variable property using discrete, grain-size particles. These authors suggest that the only way a reconciliation of these anomalous ideas can be forthcoming is if students learn to recognize just how small the particles of the submicroscopic world are.
Students' understanding of solution chemistry has been studied from a number of different perspectives: the dissolution concept (Ebenezer and Erickson, 1996; Calik and Ayas, 2005b), the nature of solutions (Prieto et al., 1989), solubility (Ebenezer and Erickson, 1996), energy in solution processes (Liu et al., 2002), effects of temperature and stirring on solubility (Blanco and Prieto, 1997), conservation of mass during dissolution (Holding, 1987), structural characteristics (Liu and Ebenezer, 2002), saturated, unsaturated and supersaturated solutions and the effect of vapour pressure lowering on the boiling point (Pinarbasi and Canpolat, 2003). Previous studies have shown how difficult the topic of solutions proves to be across all levels of education. The process of a solid dissolving in water is sometimes regarded by students as ‘melting’ (Lee et al., 1993; Ebenezer and Gaskell, 1995; Valanides, 2000; Calik, 2005; Calik et al., 2010); as a chemical reaction with solvent (Valanides, 2000; Calik and Ayas, 2005a); and, in the case of sugar solutions, a process where sugar particles literally disappear (Butts and Smith, 1987). Smith and Metz (1996) noted that chemistry students at university level had great difficulty describing the concepts of strong and weak acids and bases at the particle submicroscopic level even though they could present a correct definition of a strong and weak acid and base. Pinarbasi and Canpolat (2003) found that university chemistry students had difficulty identifying saturated, unsaturated and supersaturated solutions in a set of particle drawings.
The concept of solution concentration is foundational to solution chemistry and is featured in the Chemical Concepts Inventory designed for General Chemistry students at College or University level (JCE Online, 1996). Calik (2005) administered a paper and pencil test on solution chemistry to 441 students from Trabzon in Turkey in years 7 to 10 and observed that for item 4 which was an open-ended question on solution concentration, only 10% of year 9 students and 5% of year 10 students demonstrated a sound understanding when asked to compare beaker A with one cube of sugar dissolved and beaker B with two cubes of sugar dissolved. Item 4 was one of four open-ended questions designed to determine students' conceptions of solutions. While these students were able to say that the solution in beaker A was dilute compared to the solution in beaker B, no students actually identified that the solution in beaker B was twice the concentration of the solution in beaker A. Devetak et al. (2009) undertook a study of aqueous solution chemistry with 408 sixteen year-olds from secondary schools in Slovenia by getting the students to draw a particle diagram for solutions of different concentrations. It was reported that the average achievement score on the four items dealing with solution chemistry was only 43% which was less than the average mark for the whole chemistry test. These studies highlight just how difficult the concept of concentration appears to be for students. Solution concentration is the focus of the study reported in this paper.
In view of the analysis given here in the introduction it was decided to undertake a study with first-year chemistry students at a tertiary college with the following two research questions in mind.
Research Question 1: To what extent is the ‘disappearing particles' conception, so prevalent in the early years of schooling, still prevalent at the beginning of tertiary study?
Research Question 2: In the case of solution concentration questions, how does the success rate of students compare when the question is presented verbally in a macroscopic context as opposed to when a similar question is presented visually in a submicroscopic context?
Methodology
Sample
One hundred and forty-five first-year college students from an Australian Higher Education Institution across a range of degree courses agreed to participate in the solution concentration study. All students were studying chemistry in some form as nursing students (N = 121) or science students (N = 24). A breakdown of the student population in terms of gender, age, and general academic background is given in Table 1.| Criteria | N (Nursing) | % of Nursing students (N = 121) | N (Science) | % of Science students (N = 24) | N (All students) | % of All students (N = 145) |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 23 | 19 | 15 | 63 | 38 | 26 |
| Female | 98 | 81 | 9 | 37 | 107 | 74 |
| Age | ||||||
| < 20 | 62 | 51 | 15 | 63 | 77 | 53 |
| 20 ≤ x ≤ 30 | 40 | 33 | 8 | 33 | 48 | 33 |
| ≥ 31 | 19 | 16 | 1 | 4 | 20 | 14 |
| Academic background | ||||||
| Sound | 12 | 10 | 5 | 21 | 17 | 12 |
| Average | 36 | 30 | 10 | 42 | 46 | 32 |
| Poor | 73 | 60 | 9 | 37 | 82 | 56 |
Academic background was assessed from the information students provided on entry to tertiary study. Students completing Year 12, the final year of secondary school in Australia, will have been given a tertiary admissions rank (ATAR) by the University Admissions Centre. A ranking of ≥75 means the student has been placed in the top 25% of the student population. Universities and Colleges will require a minimum ranking for direct admission. In the case of the institution represented in this study, students needed to have been given a ranking of at least 63 for direct admission. For the purpose of classifying academic background, students with a ranking ≥75 were classified as of sound academic background. Students with a rank ≥60 but less than 75 were classified as of average academic background. Students with a rank <60 or no year 12 grades reported were classified as of poor academic background. The significance of these rankings and classifications can be more clearly understood if one realises that a student presenting with a rank of 60 could be admitted to the institution but would be placed on academic probation until the student demonstrated a capacity to complete tertiary study.
Data collection
A diagnostic test instrument was constructed to provide a quantitative measure of understanding of the nature of solutions and proficiency in solution concentration calculations. Tasks involving visual submicroscopic representations (SMR) of the particle model for sugar solutions were tested using multiple-choice items. Multiple-choice items have served an important role alongside other research items such as open-ended questions for probing students' understanding of science concepts (Smith and Metz, 1996; Sanger, 2000 and 2005; Mulford and Robinson, 2002; Bunce and Gabel, 2002; Dori and Hameira, 2003; Pinarbasi and Canpolat, 2003; Ardac and Akaygun, 2004; Wood and Breyfogle, 2006). When Devetak et al. (2009) assessed students' understanding of solution concentration using a kind of open-ended question where students had to draw a submicroscopic picture of particles in solution, the students were also expected to show the particles dispersed in all directions throughout the volume of the solution. In the research reported here we wanted to test number and distribution of particles separately so as to focus on only one concept at a time. For this purpose the use of multiple-choice questions was deemed to be appropriate. In addition, multiple-choice items are commonly used in diagnostic tests and in General Chemistry examinations and we wanted to check on the effectiveness of such items for assessing an understanding of solution concentration.The SMR multiple-choice question for testing the student's understanding of the nature of a sugar solution, particularly the distribution of particles, is shown in Fig. 1. The point of this question was to include an option showing no particles to represent the ‘no particles’ misconception so prevalent in early school education. This option was added after the pilot testing of the instrument revealed little discrimination for this question. The SMR multiple-choice questions referring to solution concentration are given in Fig. 2. For these SMR multiple-choice questions, known misconceptions, such as that which ignores volume change in solution concentrations, were used in constructing some of the options. Andersson (1990) has drawn attention to the problematic visual SMR of a solution which shows only a relatively small number of molecules in a solution and the use of a horizontal line for the solution surface. However, the focus of the study is the concept of solution concentration and adding detail, even if the detail makes the concept more realistic, will add cognitive load and contribute to what Harp and Mayer (1997, 1998) called the ‘redundancy effect’. This effect draws the viewer's attention away from what is the central message. It was therefore decided to retain the horizontal line and deal only with a manageable number of particles. In the pilot testing of the instrument there was an attempt to show water molecules as well as sugar particles but students found this somewhat daunting and so it was decided to focus only on sugar particles in the final version. It is recognized that any representation of a solution which combines macroscopic phenomena like the outline of a container and a horizontal solution surface with a picture of submicroscopic particles lacks scale integrity. All chemical representations have this difficulty and one must always remind students of this problem.
 | ||
| Fig. 1 Multiple-choice question testing a student's understanding of the nature of a sugar solution at the submicroscopic level. | ||
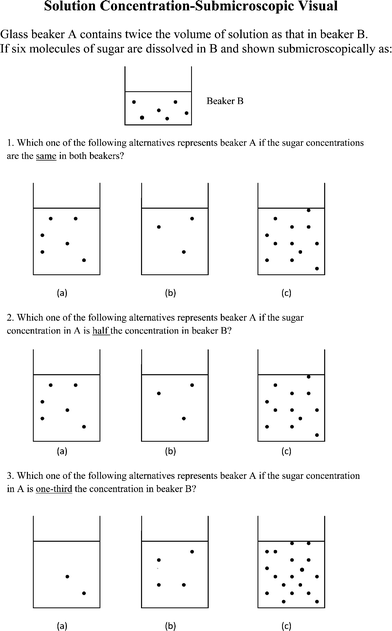 | ||
| Fig. 2 Three multiple-choice questions testing a student's understanding of solution concentration at the submicroscopic level. | ||
Solution concentration tasks involving verbal macroscopic representations of sugar solutions were tested using a short-written-answer format. These tasks are shown in Fig. 3. The test instrument was checked for content validity and presentation style by a PhD chemistry colleague of 45 years teaching and research experience and a science educator of 25 years high school science teaching experience and tertiary teaching experience in science curriculum studies. Responses to the three test items in Fig. 2 gave a Cronbach reliability coefficient of 0.833 and for the three test items in Fig. 3 a Cronbach reliability coefficient of 0.759. SPSS version 17 was used for the quantitative analysis. The Chi-Square Test for Goodness of Fit was applied to the data to determine any significant differences for the success rate on the items in Fig. 2 and 3. Students were given 15 min to complete the test instrument at the beginning of the semester during a lecture or tutorial time.
 | ||
| Fig. 3 Three short-answer questions on solution concentration of a more traditional type presented in verbal form rather than submicroscopic form. | ||
Qualitative insights into student responses to the test instrument were obtained by conducting individual interviews. Six nursing students agreed to a 20 min interview about two weeks after the test instrument had been administered. The students were chosen on the basis of their having demonstrated consistently a misconception across the multiple-choice questions. The idea here was to try to explore the student thinking behind the answers given to the test instrument and to gauge student reaction to the style of questions used in the test instrument. The only reason science students were not chosen for interview was that nursing students were more accessible for interview at the time of the research study. Summary notes were made of each interview and shown to the interviewee at the conclusion of the interview for validation. This helped ensure that the notes were a true reflection of comments made by the student. The interview notes were analysed with a view to determining common themes emerging from the study.
Ethics approval for the project was sought and granted by the institution's Research and Ethics Committee.
Results
Nature of solutions (Fig. 1)
While 63.4% of students chose the accepted dispersed particle model in (b), 27.6% of students chose (d), the choice representing the disappearance of particles. This illustrates how powerful is the influence of the macroscopic image of a sugar-water solution on the submicroscopic image. Alternatively, some students may have thought that even submicroscopic vision was not powerful enough to see very tiny sugar particles. To explain what appears to be a continuum of matter in terms of discrete particles still seems a little far-fetched for some students at the tertiary level (Bucat and Mocerino, 2009). The notion of discrete particles (atomism) in sugar-water solutions only increased from 0.0% in 4–5 year-olds to 12.9% in 12–13 year-olds according to Slone and Bokhurst (1992) in their study of 270 children. The suggestion was made that a concept like ‘atomism’ is therefore more than likely to be a product of schooling than a product of life experience. The fact that only a relatively small number of the cohort in the present study presented with a strong academic background may explain, therefore, why the option showing no discrete particles may have been attractive to some. However, this is an assumption that we were not able to verify in this study. It is so important to communicate how small the particles in an aqueous sugar-water solution are and it is this fact that makes the macroscopic solution seemingly grainless. It has been estimated, for example, that the number of water molecules in a teaspoon of water approximates the number of teaspoons of water required to empty the Atlantic Ocean. One must also consider the impact that multiple-choice test instruments have on some students. This became clear during the interviews.An analysis of the interview data led to the five categories with their frequency of occurrence shown in Table 2. Four out of the six students interviewed thought that option (d), showing no particles, best represented the sugar solution (Category 2). One of these students said that, “you cannot see molecules”; another said that, “when something dissolves you cannot see particles of what has dissolved even with a microscope”; and the other two students had seriously considered choosing option (b), the correct alternative, but thought that, given the discriminating nature of multiple-choice questions and their tendency to trick students, option (d) was probably more likely to be the answer. The two students who did choose option (b) stated that they also contemplated choosing option (d). Two students expressed an opinion about multiple-choice assessment. One student said that they, “preferred short-answer questions because you can usually get part marks, but in multiple-choice questions you are either right or wrong”. The other student said that they, “don't mind multiple-choice questions in words, but when pictures are presented they feel like they are taking pot-luck in choosing an alternative”. After having completed the solution concentration tasks outlined below, all students agreed with one student's comment that, “both multiple-choice and short-answer questions can help us understand a concept more fully even though multiple-choice assessment is sometimes tricky”. It was the multiple-choice items that highlighted the importance of volume in solution concentration determinations.
| Description Categories | Frequency of Occurrence (N = 6) |
|---|---|
| 1. In the multiple-choice responses there was a focus on number of particles rather than number and volume. | 6 |
| 2. Students thought that a picture showing no particles better represented the solution. | 4 |
| 3. Students found that the cognitive load for the SMR questions in Fig. 2 was much greater than for the questions in Fig. 3. | 4 |
| 4. The verbal questions in Fig. 3 were much easier to interpret than the SMR questions in Fig. 2. | 3 |
| 5. Students in interview were able to choose the correct SMR in Fig. 2 after scaffolding by the interviewer but they had difficulty giving a verbal explanation for their choice. | 4 |
Solution concentration (Fig. 2 and 3)
The percentages of students who scored correctly for the items in Fig. 2 and 3 are given in Table 3. The items in Fig. 2 are labelled F2 and the items in Fig. 3 are labelled F3. Devetak et al.'s (2009) results for SMR drawings are also included for comparison since their study also examined the same problem types as shown in Table 3 but at the high school level. Students were asked, however, to draw their own SMR particle diagrams in the Devetak et al. study.| Problem Type | SMR items (F2) | Verbal items (F3) | Devetak et al. (2009) SMR Draw items |
|---|---|---|---|
| Multiple-Choice | |||
| Same concentration | 31% | 76% | 71% |
| Half concentration | 24% | 75% | 66% |
| One-third concentration | 23% | 56% | 30% |
All three different modes (F2, F3, and Devetak et al.) show the same trend in scores for items with increasing difficulty from the same concentration problem to the one-third concentration problem reflecting the known difficulty students have with proportional reasoning (Mammino, 2008, p. 180). However, students performed much better on the traditional verbal mode (F3) than on the SMR mode (F2). This is consistent with the research findings of Nurrenbern and Pickering (1987) in a study of General Chemistry students' understanding of reaction stoichiometry. The study observed that students had far greater success solving traditional verbal questions than solving concept questions involving a pictorial representation of particles in a chemical reaction. The remarkable difference between student performance on SMR tasks and verbal tasks is also seen when one notes that only 16.6% of students (N = 145) gave correct responses to all three SMR tasks shown in Fig. 2 whereas 50.3% of students (N = 145) gave correct responses to all three verbal tasks shown in Fig. 3. A Chi-Square test showed that males significantly outperformed females on the SMR tasks in Fig. 2 [χ2 (1, N = 144) = 5.606, p < 0.05] but no significant dependence on gender was found for performance on the verbal tasks in Fig. 3. Gender dependence needs to be treated with some caution given the predominance of females in the sample (see Table 1), although Cohen's w of 0.467 still suggests that nearly 22% of the variance on SMR tasks can be accounted for by gender differences. It should also be noted that even though there was a gender imbalance in the sample, the Chi-Square test remains valid provided that no more than 20% of the expected frequencies are below 5 (Allen and Bennett, 2008, p. 224). This condition was met for the Chi-Square tests reported here. Bunce and Gabel (2002) also found that females scored significantly lower than males on chemistry questions which used visual models of particles. This gender difference disappeared, however, when females were specifically taught the particulate model. Male scores did not significantly improve during the teaching process.
It is not surprising that students with a sound or average academic background scored significantly better on the SMR (F2) tasks [χ2 (2, N = 144) = 16.714, p < 0.001] and verbal (F3) tasks [χ2 (2, N = 144) = 18.046, p < 0.001] than those with a poor academic background. But why did students perform better on the verbal tasks (F3) compared to the SMR tasks (F2)? One of the possible reasons for the discrepancy was that 44% (N = 145) of the students chose items which focused on number only and did not account for volume change in the SMR questions (F2). That is, they chose the alternatives containing 6, 3, and 2 particles for the SMR questions in Fig. 2 instead of the alternatives showing 12, 6, and 4 particles. The selection of number only in the SMR questions (F2) showed no dependence on gender, age, or academic background. That is, students of sound academic background were just as likely to make this error as those of poor academic background, males were just as likely as females to focus on number only, and older students were just as likely as younger students to do the same. Devetak et al. (2009) estimated that on average 30% of students made the same mistake in their study when students were asked to draw their own particle diagrams. In a study where students were asked to identify the volume and the number and distribution of particles after two solutions were mixed together, Mammino (2008) found that students were generally correct in determining the final volume but invariably had difficulty in locating the number and distribution of solute particles. This again indicates the difficulty students seem to have in locating both number of solute particles and volume in concentration problems involving particulate representations in visual format.
Students also identified the ‘number to the exclusion of volume' problem when interviewed about their choices on the test instrument (Category 1 Table 2). Three students said that they, “focused on the pictures in order to reach a quick solution”, and felt that they, “didn't read the question carefully enough”. When students were questioned about their different success rate for SMR (F2) and verbal questions (F3), one student thought that “a calculation was not necessary for the SMR questions”. Their judgment was based simply on observing the pictures and choosing which one looked correct. Three students said that the “verbal questions (F3) were easier to interpret” (Category 4 Table 2) and it was obvious that a calculation was required for these questions. Such was not the case for the SMR questions (F2). One student “thought visual questions were easier than verbal questions but I guess I have been proved incorrect”. During interview four out of six students were able to choose the correct option in Fig. 2 after having worked through the importance of accounting for volume in these tasks with the interviewer. However, the students had difficulty giving a verbal explanation for their choice (Category 5 Table 2).
It appears that students often focused on which alternative looked correct visually for the SMR (F2) questions. In the verbal questions, however, they surmised that they were forced to do a calculation. Students who focused on particle number only, however, had to do a simple calculation of sorts even though they may not have realised it. The fact is they didn't carry the calculation through to include volume. In a sense they constructed a simple one-part calculation instead of a more complex two-part calculation. One student actually wrote on their test instrument: “Give me SMR questions rather than verbal questions any time”. This student proceeded to give a correct response for the verbal question and an incorrect response for the SMR question. It is interesting to note that, in comparison to the SMR questions (F2) where 44% of the students appeared to focus on number rather than number and volume, only 10% of students gave answers that reflected a focus on number only in the verbal questions (F3).
Different responses may also arise from the way a SMR of a solution might be presented. Compare, for example, the question in Fig. 4 taken from Mulford and Robinson (2002) and Question 2 of Fig. 2. Both questions refer to a two-fold dilution of a sample. The question in Fig. 4 focuses on a given volume subset of the sample in the diluted and undiluted case but Question 2 of Fig. 2 focuses on the number of particles in the total volume for the diluted and undiluted case. In the case of a two-fold dilution, then, one will halve the number of particles in the diluted case of Fig. 4 but retain the same number of particles in the diluted case of Fig. 2. This comparison did not feature in our study but is worthy of consideration for broadening the student's understanding of solution concentration.
 | ||
| Fig. 4 Represented here is a 1.0 L solution of sugar dissolved in water. The dots in the magnification circle represent the sugar molecules. In order to simplify the diagram, the water molecules have not been shown. Figure B represents the diluted sugar solution. | ||
In interview four students found the cognitive load of the SMR questions in Fig. 2 to be much greater than for the verbal questions in Fig. 3 (Category 3 Table 2). This was evident in such comments as: “I seemed to have to focus on too many things in the SMR questions. This was not the case for the verbal questions”. It is true that SMR tend to separate the fundamental concepts of volume and particle number in cognitive space whereas these tend to remain in the same cognitive space in the verbal questions. However, students agreed in interview that SMR nevertheless highlight the significance of the fundamental concepts as a result of their separation. There was universal agreement amongst the interviewees that both forms of assessments, SMR and verbals, provide valuable reinforcement of the concept of concentration. One student was prompted to ask the question, “What really is concentration?”, after having attempted the SMR. Such a question did not seem to arise from the attempts on the verbal questions. This may indicate that the SMR draw upon more conceptual thinking than do the verbal questions, although one should not discount the place of algorithmic learning at a certain level of chemistry (de Berg, 2008).
Discussion
In answer to Research Question 1, 27.6% of the student population chose a multiple-choice option corresponding to the ‘disappearing particles’ concept, or the ‘no particles’ concept, or the ‘molecules cannot be seen’ concept. Most of these students, however, did not come from a strong chemistry background suggesting that they may not have been schooled sufficiently in the particulate model of matter. In answer to Research Question 2, students were significantly more successful in answering solution concentration questions presented verbally than for questions presented visually in submicroscopic form. The students who were interviewed were most surprised by this result and concluded unanimously that presenting the concept of solution using both visual and verbal modes was superior to using only one mode, even though they were more successful with the verbal mode.It is interesting to note that visual representations have proved a success in physics problem solving, arguably more successful than in chemistry problem solving. Larkin and Simon (1987) demonstrated how diagrams in physics had the power to make explicit what was only implicit in a verbal representation and how this explicitness enhanced the efficiency of computation. The diagrams, however, had to be of a particular quality. This was borne out by Moreno et al. (2011) in a study of the problem solving capacity of 71 high school students during a unit on electricity. The use of concrete and abstract visual representations of electrical phenomena led to better problem solving performance than the use of only concrete or only abstract visual representations. The physics visualization represented was not the submicroscopic visualization of particles as was the case discussed in this paper on chemistry, but the visualization of macroscopic phenomena such as one finds in mechanics and electrical circuits. The submicroscopic representation of phenomena at the atomic and molecular level makes chemistry particularly difficult for students. As pointed out by Davidowitz et al. (2010) one can never accurately represent the atomic or molecular nature of chemical reactions or even physical processes like dissolution. Chemistry educators, however, see the need to persist with introducing students to SMR of matter as this enhances an understanding of the underlying chemistry and so proves more likely to enhance conceptual over and against algorithmic problem solving. Chemistry textbook authors like Silberberg (2009) now incorporate more SMR in the text and end-of-chapter exercises compared with previous editions.
Implications for teaching and learning
Solution concentration problems come in a variety of forms and it is suggested that problems of the type reported in this paper in Fig. 2 and 3 could form an initial introduction to the topic. The study reported here shows that these problems are simple enough but at the same time instructive enough to warrant spending some effort on them. Variations like that shown in Fig. 4 and an exercise similar to that shown in Fig. 2 except transitioning from a larger volume for Beaker B to a smaller volume sample for Beaker A would provide a variety of contexts that would enhance conceptual problem solving and help provide an answer to the student who enquired, “What really is concentration?”. An extension of the context to more realistic larger numbers of particles like 6 × 1020 particles would be important. Concentration unit conversions would ultimately need to be addressed with dimensional analysis offering one potential algorithmic support for an understandable conceptual framework. A possible presentation sequence for solution concentration is shown in Table 4 where the submicroscopic (SMR) and the macroscopic (MR) are presented in parallel. Difficulties with Stages 3, 4, or 5 may be addressed by revisiting the concepts in Stages 1 and 2.| Stage of Presentation | SMR | MR |
|---|---|---|
| Stage 1 | Small number of particles with simple volume and particle number changes. | Small whole numbers of mass with simple volume and mass changes. |
| Stage 2 | Large number of particles with simple volume and particle number changes. | Use of moles with simple volume and mole changes. |
| Stage 3 | Algorithm for concentration (c): (particle number(p)/volume(v)): with and without the calculator. Check for conceptual clarity. | Algorithm for concentration (c): (mass(m)/volume(v)) and (moles(n)/volume(v): with and without the calculator. Check for conceptual clarity. |
| Stage 4 | Change the unknown between c, p, and v. Dimensional analysis may help. | Change the unknown between m, n, c, and v. Dimensional analysis may help. |
| Stage 5 | Unit conversions: Dimensional analysis may help. | Unit conversions: Dimensional analysis may help. |
If it is true, as Paivio (1986) suggests, that questions should be attempted in both visual and verbal modes, our task as chemistry educators is to enhance the connections between these two modes by developing the skill of using multiple representations of chemical concepts (Gilbert and Treagust, 2009). This includes multiple representations within the verbal and within the visual as well as between the verbal and visual. The representations in Fig. 4 and in Fig. 2 are one example of two different ways of representing particles in dilution. We know the particle model and its representation proves difficult for students as borne out in this study and many others. There is certainly a case for exploring different ways of representing it in chemistry to provide the rich background necessary for an appreciation of the significance of chemistry. As far as assessment is concerned, using the particle model visually in multiple-choice questions can be discriminating but instructive and like all multiple-choice assessments care should be taken in their composition. The study reported here supports the efforts of chemistry educators to increase students' exposure to the submicroscopic representation of matter using the visual representation of particles even though students experience more difficulty with this mode compared to the traditional verbal mode.
References
- Allen P. and Bennett K., (2008), SPSS for the Health and Behavioural Sciences, Melbourne: Cengage Learning.
- Andersson B., (1990), Pupils' conceptions of matter and its transformations (age 12–16), Stud. Sci. Educ., 18, 53–85.
- Ardac D. and Akaygun S., (2004), Effectiveness of Multimedia-Based Instruction That Emphasizes Molecular Representations on Students' Understanding of Chemical Change, J. Res. Sci. Teach., 41(4), 317–337.
- Ben-Zvi R., Eylon B.-S. and Silberstein J., (1986), Is an atom of copper malleable? J. Chem. Educ., 63(1), 64–66.
- Blanco A. and Prieto T., (1997), Pupils' views on how stirring and temperature affect the dissolution of a solid in a liquid: A cross-age study (12 to 18), Int. J. Sci. Educ., 19(3), 303–315.
- Brunye T. T., Taylor H. A., Rapp D. N. and Spiro A. B., (2006), Learning Procedures: The role of working memory in multimedia learning experiences, Appl. Cognit. Psychol., 20, 917–940.
- Bucat R. and Mocerino M., (2009), Learning at the Sub-Micro Level: Structural Representations. In J. K. Gilbert and D. F. Treagust (ed.), Multiple Representations in Chemical Education, The Netherlands: Springer.
- Bunce D. M. and Gabel D., (2002), Differential Effects on the Achievement of Males and Females of Teaching the Particulate Nature of Chemistry, J. Res. Sci. Teach., 39, 911–927.
- Butts W. and Smith R., (1987), What do Students perceive as Difficult in HSC Chemistry?, J. Chem. Educ., 32, 45–51.
- Calik M., (2005), A Cross-Age Study of Different Perspectives in Solution Chemistry from Junior to Senior High School, Int. J. Sci. Math. Educ., 3, 671–696.
- Calik M. and Ayas A., (2005a), A Cross-Age study on the understanding of Chemical Solutions and their Components, Int. Educ. J., 6(1), 30–41.
- Calik M. and Ayas A. (2005b), A Comparison of Level of Understanding of Eighth-Grade Students and Science Student Teachers Related to Selected Chemistry Concepts, J. Res. Sci. Teach., 42(6), 638–667.
- Calik M., Ayas A. and Ebenezer J., (2005), A Review of Solution Chemistry Studies: Insights into Students' Conceptions, J. Sci. Educ. Technol., 14(1), 29–50.
- Calik M., Ayas A. and Coll R., (2010), Investigating the Effectiveness of Usage of Different Methods Embedded with a Four-Step Constructivist Teaching Strategy, J. Sci. Educ. Technol., 19(1), 32–48.
- Davidowitz B., Chittleborough G. and Murray E., (2010), Student-generated submicro diagrams: a useful tool for teaching and learning chemical equations and stoichiometry, Chem. Educ. Res. Pract., 11, 154–164.
- De Berg K. C., (2008), Conceptual Depth and Conceptual Usefulness in Chemistry: Issues and Challenges for Chemistry Educators. In I. V. Eriksson (ed.), Science Education in the 21st Century (pp. 165–182). New York: Nova Science Publishers.
- Devetak I., Vogrinc J. and Glazar S. A., (2009), Assessing 16-Year-Old Students' Understanding of Aqueous Solution at Submicroscopic Level, Res. Sci. Educ., 39, 157–179.
- Dori Y. and Hameira M., (2003), Multidimensional Analysis System for Quantitative Chemistry Problems: Symbol, Macro, Micro, and Process Aspects, J. Res. Sci. Teach., 40(3), 278–302.
- Ebenezer J. V., (2001), A Hypermedia Environment to Explore and Negotiate Students' Conceptions: Animation of the Solution Process of Table Salt, J. Sci. Educ. Technol., 10(1), 73–92.
- Ebenezer J. V. and Erickson G. L., (1996), Chemistry students' conceptions of solubility: A phenomenography, Sci. Educ., 80, 181–201.
- Ebenezer J. V. and Gaskell P. J., (1995), Relational conceptual change in solution chemistry, Sci. Educ., 79, 1–17.
- Gabel D. L., (1993), Use of the particle nature of matter in developing conceptual understanding, J. Chem. Educ., 70, 193–194.
- Gabel D. L., Samuel K. V. and Hunn D., (1987), Understanding the particulate nature of matter, J. Chem. Educ., 64(8), 695–697.
- Gilbert J. K., (2008), Visualization: An Emergent Field of Practice and Enquiry in Science Education. In J. K.Gilbert, M. Reiner and M. Nakhleh (ed.), Visualization: Theory and Practice in Science Education, The Netherlands: Springer.
- Gilbert J. K. and Treagust D. F., (2009), Macro, Submicro, and Symbolic Representations and the Relationship between them. In J.K. Gilbert and D.F. Treagust (ed.), Multiple Representations in Chemical Education, The Netherlands: Springer.
- Harp S. F. and Mayer R. E., (1997), The role of interest in learning from scientific text and illustrations: on the distinction between emotional interest and cognitive interest, J. Educ. Psychol., 89, 92–102.
- Harp S. F. and Mayer R. E., (1998), How seductive details do their damage: a theory of cognitive interest in science learning, J. Educ. Psychol., 90, 414–434.
- Harrison A. and Treagust D. F., (1996), Secondary Students' mental models of atoms and molecules: Implications for teaching chemistry, Sci. Educ., 80(5), 509–534.
- Holding B., (1987), Investigayion of School Children's Understanding of the Process of Dissolving with Special Reference to the Conservation of Matter and the Development of Atomistic Ideas, Unpublished PhD thesis, University of Leeds.
- JCE Online, (1996), Conceptual Questions and Challenge Problems: Chemical Concepts Inventory, http//jchemed.chem.wisc.edu/JCEDLib/QBank/collection/CQandChP/CQs/ConceptsInventory.
- Johnstone A. H., (1982), Macro- and micro-chemistry, Sch. Sci. Rev., 64, 377-379.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7, 701–703.
- Johnstone A. H., (1993), The development of chemistry teaching, J. Chem. Educ., 70(9), 701–705.
- Johnstone A. H., (2000), Teaching Chemistry: Logical or Psychological? Chem. Educ. Res. Pract. Eur., 1(1), 9–15.
- Larkin J. H. and Simon H. A., (1987), Why a diagram is (sometimes) worth ten thousand words, Cognit. Sci., 11, 65–100.
- Lee O., Eichunger D. C., Anderson C. W., Berkheimer G. D. and Blakeslee T. D., (1993), Changing middle school students' conceptions of matter and molecules, J. Res. Sci. Teach., 30, 249–270.
- Liu X. and Ebenezer J., (2002), Descriptive categories and structural characteristics of students' conceptions: An exploration of the relationship, Res. Sci. Technol. Educ., 20, 111–132.
- Liu X., Ebenezer J. and Fraser D., (2002), Structural characteristics of university engineering students' conceptions of energy, J. Res. Sci. Teach., 39, 423–441.
- Mammino L., (2008), Teaching Chemistry With and Without External Representations in Professional Environments with Limited Resources. In J.K. Gilbert, M. Reiner and M. Nakhleh (ed.), Visualization: Theory and Practice in Science Education, The Netherlands: Springer.
- Mayer R. E., (2001), Multimedia learning, New York: Cambridge University Press.
- Moreno R., Ozogul G. and Reisslein M., (2011), Teaching with Concrete and Abstract Visual representations: Effects on Students' Problem Solving, Problem Representations, and Learning Perceptions, J. Educ. Psychol., 103(1), 32–47.
- Mulford D. R. and Robinson W. R., (2002), An Inventory for Alternate Conceptions among First-Semester General Chemistry Students, J. Chem. Educ., 79, 739–744.
- Nurrenbern S. C. and Pickering M., (1987), Concept learning versus problem solving: is there a difference? J. Chem. Educ., 64, 508–510.
- Paivio A., (1986), Mental representations: A dual-coding approach, New York: Oxford University Press.
- Pinarbasi T. and Canpolat N., (2003), Students' Understanding of Solution Chemistry Concepts, J. Chem. Educ., 80(11), 1328–1332.
- Prieto T., Blanco A. and Rodriguez A., (1989), The ideas of 11 to 14-year old students about the nature of solutions, Int. J. Sci. Educ., 11, 451–463.
- Sanger M. J., (2000), Using particulate drawings to determine and improve students' conceptions of pure substances and mixtures, J. Chem. Educ., 77, 762–766.
- Sanger M. J., (2005), Evaluating Students' Conceptual Understanding of Balanced Equations and Stoichiometric Ratios Using a Particulate Drawing, J. Chem. Educ., 82(1), 131–134.
- She H., (2004), Facilitating Changes in Ninth Grade Students' Understanding of Dissolution and Diffusion through DSLM Instruction, Res. Sci. Educ., 34, 503–525.
- Silberberg M. S., (2009), Chemistry: the molecular nature of matter and change (5th ed.), Boston: McGraw Hill.
- Slone M. and Bokhurst F. D., (1992), Children’s understanding of sugar water solutions, Int. J. Sci. Educ., 14(2), 221–235.
- Smith K. J. and Metz P. A., (1996), Evaluating student understanding of solution chemistry through microscopic representations, J. Chem. Educ., 73, 233–235.
- Talanquer V., (2011), Macro, Submicro, and Symbolic: The many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33(2), 179–195.
- Treagust D. F., Chittleborough G. and Mamiala T., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25(11), 1353–1368.
- Valanides N., (2000), Primary student teachers' understanding of the particulate nature of matter and its transformation during dissolving, Chem. Educ. Res. Pract. Eur., 1, 249–262.
- Wood C. and Breyfogle B., (2006), Interactive Demonstrations for Mole Ratios and Limiting Reagents, J. Chem. Educ., 83(5), 741–748.
| This journal is © The Royal Society of Chemistry 2012 |
