Characterizing and representing student's conceptual knowledge of chemical bonding
Malka
Yayon
*,
Rachel
Mamlok-Naaman
and
David
Fortus
Weizmann Institute of Science, P.O. Box 26, Rehovot 76100, Israel. E-mail: malka.yayon@weizmann.ac.il
First published on 3rd April 2012
Abstract
Chemical bonding knowledge is fundamental and essential to the understanding of almost every topic in chemistry, but it is very difficult to learn. While many studies have characterized some of the central elements of knowledge of this topic, these elements of knowledge have not been systematically organized. We describe the development and testing of a matrix that represents: (A) a systematic organization of the conceptual knowledge on chemical bonding required at high school level and (B) a tool for representing students' conceptual knowledge of this topic. The matrix contains three strands: the structure of matter at the nanoscopic level, electrostatic interactions between charged entities, and energy aspects related to bonding. In each strand there are hierarchically ordered cells that contain fine grain elements of knowledge. Using various instruments, students’ conceptual knowledge of chemical bonding was assessed and mapped onto the matrix, generating graphical representations of their knowledge. New computational and online technologies enable automatic data collection and its analysis. Therefore, we believe that this organization and representation of small grain size elements of knowledge can be a useful for the development of a detailed diagnostic tool of knowledge of chemical bonding.
Introduction
Several researchers hypothesize that students' conceptual science understanding may be constructed through the acquisition of small-grain pieces of knowledge which are then ordered, organized, and linked to other already known concepts (Akçaoglu Küçüközer, 2005; diSessa, 1988; Galili and Hazan, 2000; Minstrell, 1992; Niedderer and Schecker, 1992; Reiner, 1992; Tiberghien, 2007). Accordingly, to characterize knowledge of a particular topic, one needs to specify the pieces of knowledge comprising this topic and the relations between them.diSessa viewed intuitive physics as consisting of a large number of fragments that he calls “p-prims” or phenomenological primitives, which are simple abstractions of common everyday experiences, for which one senses no need for explanation (diSessa, 1993). diSessa felt that p-prims are not selected by theory, but are basically short encoded scripts that have been reinforced over and over by experience; in other words, implicit and encapsulated knowledge elements based on prior experience that may be radically different from normative understanding.
Minstrell (1992, p. 112) described students' knowledge of physics in terms of facets of knowledge: “A facet is a convenient unit of thought, a piece of knowledge or strategy seemingly used by the student in addressing a particular situation”. Facets too are small-grain pieces of knowledge. However, unlike p-prims, they may be more than abstractions of everyday experience. They can be constructed as the result of instruction and may be considered an approximate understanding of some concept (Minstrell, 1992).
It may be possible that p-prims may feed into students' ideas about chemistry (Taber and García Franco, 2010), but it has also been shown, that the influence of student's naïve ideas on their understanding in chemistry is not as significant as in physics, since chemistry concepts are typically encountered for the first time in school (Taber, 2002a). However, the idea of knowledge being constructed in pieces seems very proper to explore and describe in detail the development of student's chemistry knowledge. This study takes the perspective that conceptual knowledge of chemical bonding can be decomposed into small pieces, which we define as elements of knowledge which are similar to Minstrell's facets in that they can be the result of instruction, but differ from them in that they all describe canonical ideas, while facets can describe also misconceptions.
We decided to chose the term “elements of knowledge”, neither “concepts” nor “facets” to describe basic, simple pieces of knowledge. Even though concepts can be also viewed as hierarchical subdivision of knowledge (Kelly, 1963), their description could also be wider (Gilbert and Watts, 1983) e.g. as active, constructive “actional concepts” or “ways of organizing our experiences” mentioned by Freyberg and Osborne (as cited in Gilbert and Watts, 1983). There is little agreement among experts about the definition of concept; concept is synonymous with idea however, ideas like love, chair or inch differ in several important aspects (Herron et al., 1977). The term “concept” is used in many ways, for example “electrons are negative” which we define as concept is elsewhere rather described as a proposition linking the two concepts ‘electron’ and ‘negative’ (Novak, 1990) or a “relational” view of a concept. In many studies “Chemical bonding” or “ionization energy” is referred as concepts. That is why instead of using such a broad term “concept”; we decided to use “elements of knowledge”.
The most basic elements of knowledge are descriptive (e.g. electrons are negative, or opposite charges attract each other). Other elements of knowledge typically build of a relationship between two or three more basic elements of knowledge (e.g., electrons are found around the nucleus at discrete distances). These can then combine with other additional elements of chemical bonding knowledge to lead to still larger elements of knowledge (e.g. for similar size bonded atoms, bond length increases as the difference in electronegativity decreases).
We propose a hierarchical organization of these elements of knowledge in which they are clustered and related to each other. Besides serving as a characterization of conceptual knowledge of chemical bonding, in this paper we use this organization of elements of knowledge to represent the development of two students' understanding of chemical bonding. In doing so, we demonstrate the usefulness of this organization as a tool to represent individuals' knowledge of the canon, its development over time, and it's potential as an aid in the development of a detailed automatic diagnostic tool for chemical bonding.
The rest of this article is divided into two sections. In the first section we describe the process and product of developing a small grain characterization of canonical chemical bonding knowledge. In the second section we describe how this characterization was used to create representations of students' knowledge of the canon and the development of this knowledge over time.
Constructing a small-grain characterization of conceptual chemical bonding knowledge
The process of constructing a fine grain characterization of conceptual chemical bonding knowledge involved the following steps:a. Identifying all the concepts deemed necessary to understand chemical bonding at the high school level.
b. Unpacking and elaborating the concepts into elements of knowledge.
c. Organizing the elements of knowledge into a matrix defined by categories and strands.
d. Organizing the elements of knowledge in each cell into clusters.
e. Expert validation and revision of the matrix.
We now elaborate each step.
Identifying the concepts needed to understand chemical bonding at the high school level
Much research has been done to determine and characterize the essential concepts that students should learn in chemical bonding (Coll and Taylor, 2002; Coll and Treagust, 2001; Gilbert, 2006; Harrison and Treagust, 2000; Herron, 1996; Kozma and Russell, 2005; Levy Nahum et al., 2007; Scalise et al., 2003; Taagepera et al., 2002; Taagepera and Noori, 2000; Taber, 2001; Taber and Coll, 2002; Teichert and Stacy, 2002). Since understanding of chemical bonding is fundamental and essential for the understanding of almost every topic in chemistry, but is very difficult to construct (Ritter, 2007), many researchers have studied it (Fensham, 1975; Gillespie, 1997; Hurst, 2002). In this section we mention only a few of the studies that helped us identify and organize the concepts that are relevant to an understanding of chemical bonding.Most researchers agree on the importance of a robust understanding of the particle nature of matter to understand more complex concepts of chemical bonding (Harrison and Treagust, 2000; Levy Nahum et al., 2007; Margel et al., (2007); Othman et al., 2008; Roseman et al., 2006; Taber, 2003a). Most chemistry teachers would argue that some basic elements of quantum mechanics are essential even at the high school level (Levy Nahum et al., 2010; Taber, 2002b; Tsaparlis, 2002), Shusterman and Shusterman (1997) claimed that very little knowledge of quantum mechanics is required; what matters is the appreciation of the quantum postulate that electron positions cannot be precisely defined as well as the consequential concept of partial charges. The first category of concepts we looked for dealt with structural aspects of materials at the nanoscopic level, including those dealing with the probabilistic nature of the electrons' locations, but not those dealing explicitly with quantum mechanics.
The importance of electrical interactions within and between molecular level systems in the understanding of bonding has been widely discussed (e.g., Othman et al., 2008; Shusterman and Shusterman, 1997; Taber, 2001; Talanquer, 2009; ten Hoor, 2004). We agree with both Taber and the Shustermans that students should develop a dynamic model of the atom in which the electron cloud is not static nor stiff, but flexible and interacting with other particles close to them and this model can be acquired without teaching quantum mechanics. As a result, the second category of concepts we looked for dealt with the coulomb interactions and partial charges.
The importance of the principle of minimum energy has also been discussed (Duit and Treagust, 2003; Levy Nahum et al., 2007; Roseman et al., 2006; Taber, 2001, 2003a; Teichert and Stacy, 2002). We accordingly included a third category of concepts dealing energy in our characterization of the chemical bonding knowledge.
Our decision to focus on nanostructure, electrostatic interactions, and energy aligns with Levy Nahum's work (Levy Nahum et al., 2007, 2008), which proposed a new “bottom-up” framework for teaching chemical bonding. This strategy relies on structure, coulomb forces, and energy at the atomic level to build a coherent and consistent perspective for dealing with all types of chemical bonds. As described by Levy Nahum et al. (2008, p. 1680): “It is possible to show how this diversity [of bond types] arises from a small number of fundamental principles instead of presenting it as a large number of disparate concepts.” Levy's proposed framework introduces the significant properties of isolated atoms, Coulomb's law, and the wave nature of electrons. It then follows by introducing chemical bonding between two atoms, presenting the different traditional categories of chemical bonds as extreme cases on a continuum of bonds. Equipped with this knowledge, students can then construct an integrated understanding of different molecular structures and properties.
We collected statements of concepts on chemical bonding from multiple sources. The first source we used was the Atlas of Science Literacy (AAAS, 2007). The Atlas does not include a specific map for chemical bonding, but the concepts related to the topic are shown in maps such as “Structure and Properties of Matter”, “Energy Transformations” and “Forces of Nature”.
The second source we used for statements of concepts was the general literature on chemical bonding and textbooks (Coll and Taylor, 2002; Coll and Treagust, 2001; Ebbing and Gammon, 2002; Harrison and Treagust, 2000; Herron, 1996; Levy Nahum et al., 2006; Othman et al., 2008; Peterson et al., 1986; Scalise et al., 2006a; Taagepera and Noori, 2000; Taber, 2001; Taber and Coll, 2002; Teichert and Stacy, 2002; Zumdahl, 1989).
The last group of sources for statements of concepts was chemistry classes, students' interviews, exams, and chemistry teachers' workshops. Most of the concepts collected from this third category of source coincided with those collected from the first two sources, but not all. These were mostly rules of thumb that were useful in practical instruction related to the use of the periodic table of elements. Some examples are: non-metals form covalent bonds between themselves, metals form ionic bonds with non-metals, in general the size of an atom decreases along a row in the periodic table.
No misconceptions were included in the matrix; the matrix represents only canonical knowledge.
Unpacking and elaborating the concepts into elements of knowledge
After identifying the concepts, they were unpacked, when possible, into simpler and smaller elements of knowledge. Many of the statements collected were complex and dealt with multiple elements of knowledge, sometimes related to different topics. For example, benchmark 4D/H1 from the Atlas (AAAS, 2007):An atom's electron configuration, particularly the outermost electrons, determines how the atom can interact with other atoms.
This benchmark was unpacked and elaborated by decomposing it into phrases containing one or more simpler elements of knowledge:
• Atoms consist of nuclei surrounded by electrons.
• Electrons are found around the nucleus at discrete different distances (energy levels).
• The valence electrons are the electrons in the outer occupied energy level.
• The valence electrons in particular determine how an atom can interact with other atoms.
All the resulting fine-grain elements of knowledge obtained from different sources were compared to each other and reworded to use the same terms.
Organizing the elements of knowledge into a matrix defined by categories and strands
Following the identification of the particulate nature of matter, electrostatic interactions, and energy as the organizing categories for chemical bonding, we arranged the resulting elements of knowledge from the former section into these three categories. In addition to this organization into categories, the various elements of knowledge were ordered into seven different levels within each strand—the unbound atom, one bond between two atoms, one molecule, many bonds of the same type (lattice), many bonds of different types, and states of matter—so that elements of knowledge in higher levels were built from elements of knowledge situated in lower levels. The seventh level was not a higher level than the others; it includes elements of knowledge that provide an overview on bonding (e.g., there is a whole range of chemical bonds, or, in a diatomic system bond energy decreases from ionic > polar covalent > covalent > van der Waals for similar bond lengths), and other general elements of knowledge on the nature of chemical bonding (e.g., it is difficult to predict the relative strength of van der Waals and hydrogen bonds when the molecules involved are very different from each other).Table 1 shows one element of knowledge from each cell of the matrix, although in practice there are many in each cell, and in some cases the same element of knowledge is found in more than one cell.
| Nanoscopic structure | Interaction | Energy | |
|---|---|---|---|
| The unbound atom | Atoms consist of nuclei and electrons | There is an electrical repulsion between the electrons | The distance of an electron from the nucleus is determined by its energy |
| One bond between two atoms | The bond length is the distance between nuclei at which the atoms are in equilibrium | At bond length, attraction forces are equal to repulsion forces between the bound atoms | Energy is required to break a bond between atoms |
| A single molecule | Atoms bond into well defined shaped molecules | The shape of a molecule is defined by electric forces | The molecule's geometrical structure is such that it is at minimum energy |
| Many bonds of the same type (lattice) | In covalent, metallic, and ionic solids, molecular “building blocks” cannot be identified | In a covalent lattice, atoms are covalently bound | The structure of a lattice is such that it is at minimum energy |
| Many bonds of different types | Hydrogen bonds have a linear geometry (H:X–H) | The hydrogen atom participating in the hydrogen bond is covalently bound to a very electronegative atom, which attracts the hydrogen's only electron, resulting in big partial charges (Hδ+ δ−X–Hδ+) | The energy required to separate molecules increases with the number of permanent polar sites in the molecules |
| States of matter | In gases, molecules are separated on average from each other by distances that are large compared to the molecules' sizes | In gases average electrostatic interactions between molecules are negligible | In gases, particles move very fast and collide with each other |
| Overview | There is a whole range of chemical bonds which we can map on a continuous scale according to the strength of the interaction. | There is a whole range of chemical bonds, which we can map on a continuous scale according to the bond energy values. |
Organizing the elements of knowledge in each cell into clusters
We noticed that in some cases some of the elements of knowledge in a single cell were related to each other. In these cases, these elements of knowledge were grouped into clusters and given a heading that reflected the connection between them. See Table 2 for an example. These clusters became useful later when describing the characteristics of knowledge that a student has or doesn't have.| A single atom/nanostructure |
|---|
| Charged particles |
| Protons are positive |
| Electrons are negative |
| The charge of the nucleus equals the number of protons |
| In a neutral atom the number of electrons equals the number of protons |
| If the number of electrons differs from the number of protons, the atom is charged |
| If the number of electrons differs from the number of protons, the atom is called an ion |
| Positive ions have fewer electrons than protons; negative ions have more electrons than protons |
| Momentary partial charges (δ− and δ+) in the electron cloud occur because electrons do not have fixed positions |
| Structure |
| Nuclei consist of nucleons |
| Atoms consist of nuclei and electrons |
| Electrons are found around the nucleus at discrete distances in a simple model |
| The valence electrons are the electrons in the higher inhabited energy level |
| Electrons have no fixed position in the atom, but are everywhere in the electron cloud at once |
| An orbital is the space in which there is a high probability to find electrons |
| Electrons are distributed in the electron cloud in a probabilistic fashion |
| Periodic Table (PT) |
| The number of valence electrons equals the group number in the PT |
| The number of populated energy levels equals the row number in the PT |
| The atomic number equals the number of protons |
| In general the size of an atom decreases along the PT row |
Expert validation and revision of the matrix
After creating the matrix, all of its aspects were reviewed by a group of ten experts—chemists, chemistry and physics educators, and senior chemistry teachers. All were faculty, post-doctoral fellows, or graduate students at the institute where the authors work. Each expert commented on the choice and accuracy of the elements of knowledge, their breakdown into finer-grained elements of knowledge, their wording, and their location in the matrix. The matrix was revised according to their suggestions. Some examples of revisions made are:A) Original version: “When comparing a neutral atom with an ion of the same element, of which one has full energy levels that with the full energy level will be more stable.” The review of this statement led to a discussion regarding stability and reactivity, leading to a revised version: “Atom/ions with full orbitals are less reactive than those with partially full orbitals.”
B) Original version: “Atoms form a molecule if the energy of the bound atoms is lower than that of the separated atoms.” The review of this statement led to a discussion of the conditions that lead to the formation of molecules. Molecules do not always form when the energy of the bound atoms is lower than the sum of the separated atoms; there are additional conditions that influence whether a molecule will be formed. Also, atoms can bond into molecules which are instable. The statement was revised to include the conditional term “can”, which turned the statement into a less rigid one: “Atoms can form a molecule if the energy of the bound atoms (molecule) is lower than the sum of the energies of the separated atoms.”
C) The statement “Momentary partial charges (δ− and δ+) in the electron cloud occur because electrons do not have fixed positions” was originally located in the Structure strand of the map because it describes particle location, but was also located in the Interaction strand because it refers to partial charges and the reason for their existence.
An interesting finding from this stage was that there was broad agreement about the content and structure of the matrix. One size seemed to fit all. We don't claim that the matrix is complete in the sense that it represents a complete understanding of chemical bonding. No doubt there are some elements of knowledge that are missing. Also, the matrix treats chemical bonding knowledge as semantic and ignores its visual aspects, p-prims or misconceptions and its relation to a certain curricula. However, we believe that the matrix is a reasonable representation of many, if not most, of the main elements of knowledge comprising conceptual knowledge of chemical bonding at a high school level. Should researchers and practitioners find this representation useful, it will no doubt be enhanced and modified.
The matrix, as it appeared at present, is available at http://stwww.weizmann.ac.il/g-chem/the_matrix.doc, and in Appendix F.
Using the matrix to develop graphical representations of students' canonical knowledge
In this section we tested the matrix to see how it represented students' knowledge of chemical bonding as externalized in authentic classroom settings. Our goal here was not to uncover students' knowledge of chemical bonding but to see if the matrix serves as a beneficial method for researchers of representing their conceptual knowledge of the canon as revealed by using multiple conventional assessment methods.The study population
One tenth grade chemistry class of six students was observed while studying chemical bonding. The class is small compared to the other classes in Israel (15–35), but it was chosen because in such a small class students felt more comfortable to talk; improving the chance for discourse and argumentation. The teacher was a very experienced classroom practitioner who encouraged the students to ask questions, explain their ideas, and promoted conversation between the students. All the students had chosen to be chemistry majors, which mean that they would study Chemistry as a major subject till 12th grade and present the matriculation exam on Chemistry. (It does not mean they are applying to university to study chemistry when they complete school). They were defined by their teachers as having a range of abilities. All the results that will be presented are drawn from two students, Dan and Sandra, their interviews, written artifacts, and from the class discussions in which they participated. While the data obtained from the other students was different, the conclusions about the value of the matrix that could be reached from them were not.Instruments
Several studies have shown that it can be difficult to reveal what students understand about chemical bonding (Coll and Treagust, 2001; Taber, 2003b; Taber and Watts, 2000; Teichert and Stacy, 2002; Wu et al., 2001). Verbal and written explanations are often different: a written explanation allows students to edit and carefully choose their words (Kelly and Bazerman, 2003) while verbal explanations are more spontaneous. Some students express themselves better one way than the other. We used three data sources of students' knowledge of chemical bonding: exams, a quiz, written artifacts, verbal statements made by the students during class (all lessons were videotaped), and a semi-structured interview.Four exams were given by the teacher during the 9 months we followed the class. The topics assessed by the exams were atomic structure, a single bond between 2 atoms, a single molecule, lattices, substances and their properties, and phase change. These exams contained both open-ended and multiple-choice items, items that required the students to construct written explanations and items that required the students to make or interpret drawings. Examples of items from the tests appear in Appendix A; they represent standard questions given in high school chemistry exams. Each item was mapped onto the matrix to determine which elements of knowledge were assessed by the item, if an idea was not described in the matrix, and the idea seemed relevant, a new element of knowledge was added to the matrix. An example is given in Appendix B.
Several lessons, while “bonding between two atoms” was being taught, were observed and audio-taped. Written and drawn artifacts made by the students during these lessons were collected. The recordings were transcribed and each student's statements were combined with the artifacts the made to create individual student records of these lessons.
Videotaped semi-structured individual interviews (Gilbert et al., 1985; White, 1985) were used to gather data on students' understanding of the structure of an atom and phase change. The outline of the interviews is given in Appendix C.
Data analysis
The data analysis was done in three stages. In the first stage the answers given by the students in the exams and the statements made by them in class (in writing or verbally) and during the interviews were mapped onto the matrix to show which canonical elements of knowledge were being used correctly, incorrectly, not used at all or to see if the matrix did not represent relevant elements of knowledge. Each exam, artifact, and interview was mapped separately for each student, thus creating a record of each student's conceptual knowledge as expressed through that instrument. For example, Table 3 shows the mapping of a segment from an interview. Each relevant utterance (in the left-hand column) is mapped onto elements of knowledge in the matrix, listed in the right-hand column. If an element of knowledge was used, but was not represented in the matrix, if it was considered important it was added to the matrix (Appendix B).
In the second stage all the answers from an exam or all the utterances in an interview, or all the statements made in class were summarized to determine which elements of knowledge were being used consistently in a correct manner by the student (marked black), sometimes correctly but not consistently so (marked grey), or incorrectly (marked with a diagonal line). An example of such a summary is presented in Table 4.

Table 4 is a representation of a student's knowledge of the canonical elements of knowledge as assessed through an interview. Similar representations were made for data collected with each instrument.
In the third stage, the answers given by the students in the exams and the statements made by them in class (in writing or verbally) and during the interviews were mapped onto the matrix to show which elements of knowledge were being used correctly, incorrectly, or not used at all. These representations of a student's conceptual knowledge were temporally ordered and placed side-by-side to create a representation of the development of the student's knowledge over time.
An example is given in Fig. 1 or enlarged in Appendix D (this figure is available online at http://stwww.weizmann.ac.il/g-chem/figure_1.pdf). Fig. 1 was not enlarged here because the purpose is to show the representation of a bird's eye view, enlargements of the specific elements of knowledge will be shown further. The elements of knowledge from the matrix that were assessed in at least one of the instruments are listed in the left-hand column. The next columns show the student's responses to various assessments, with the results of the earliest assessment on the left and progressively later ones to the right. There was an interval of about one month between each assessment. The heading of each column (shown enlarged in Table 5) indicates which instrument was used for that assessment. The numbers beneath these headings represent the dates on which the instruments were administered.
 | ||
| Fig. 1 Bird's eye view of the map that represents Sandra's conceptual knowledge over time. | ||
| Type of instrument used for the assessment | Interview | Test | Activity | Test | Test | Questionnaire |
| Date on which the instrument was administered | 11 2007 | 1 2008 | 2 2008 | 4 2008 | 5 2008 | 6 2008 |
Fig. 1 is a graphical representation of how one student (Sandra)'s conceptual knowledge of chemical bonding progressed over time. A close look can identify elements of knowledge with which, at a given time, the student is struggling or still not sure, and for which elements of knowledge there is no information.
Each table shows the elements of knowledge related to different strands; Electrostatic interactions (left), Nanostructure (middle) and Energy (right). The columns in each table show the student's responses to various assessments, the earliest on the left and progressively later ones to the right. An enlargement of the head columns that show the type of instruments is shown in Table 5. The elements of knowledge in the matrix, listed in the right-hand column are coded as following: correctly used elements of knowledge  , inconsistently used elements of knowledge
, inconsistently used elements of knowledge  and incorrectly used elements of knowledge
and incorrectly used elements of knowledge  . Some cells appear colored for further description in the text.
. Some cells appear colored for further description in the text.
The elements of knowledge from the matrix that were assessed in at least one of the instruments are listed in the left-hand column. The next columns show the student's responses to various assessments, with the results of the earliest assessment on the left and progressively later ones to the right. There was an interval of about one month between each assessment. The heading of each column indicates which instrument was used for that assessment. The numbers beneath these headings represent the dates on which the instruments were administered.
Fig. 1 is a graphical representation of how one student's conceptual knowledge of chemical bonding progressed over time. As one moves to the right, new cells become colored, grey and diagonal-lined cells become black, and in general the columns become darker, indicating that the student's knowledge of the canon is developing. A closer look can identify elements of knowledge with which, at a given time, the student is struggling or still not sure, and for which elements of knowledge there is no information.
Results
Fig. 1 shows a bird's eye view of the representation of a student's (Sandra) knowledge overtime, as a matrix. Sandra learns quickly and accurately. Most of the elements of knowledge that were assessed were consistently understood correctly (black cells). There are very few elements of knowledge that were used correctly, but not consistently so (grey cells), are also very few elements of knowledge that were used incorrectly (diagonal line), which indicates that there appears little confusion or contradictions in her thinking. Almost all of these elements of knowledge that were partially or incorrectly understood at one assessment point were understood consistently correctly at the next point that assessed the same elements of knowledge (they became black).Sandra constructed new knowledge steadily—over time new cells are colored, while crossed and graey cells become black. Her columns become progressively blacker as we move to the right.
Sandra correctly understood elements of knowledge related to nanostructure, electrostatic interactions, and was able to use all different kinds where appropriate. Some of the elements of knowledge that Sandra did not understand fully or at all at some assessment stage are shown enlarged in Table 6. It may surprise some that a capable student like Sandra had difficulty with a basic element of knowledge like the first one listed here, which is actually just a definition.

When we inspect the bird's eye view of the map of a different student (Dan) in Fig. 2 or the enlarged tables in Appendix E the differences between them quickly stand out (an online file can be enlarged at http://stwww.weizmann.ac.il/g-chem/malka/dan.pdf).
 | ||
| Fig. 2 Bird's eye view of the map that represents Dan's conceptual knowledge over time. | ||
Each table shows the elements of knowledge related to different strands: Electrostatic interactions (left), Nanostructure (middle) and Energy (right). The columns in each table show the student's responses to various assessments, the earliest on the left and progressively later ones to the right. The elements of knowledge in the matrix, listed in the right-hand column are coded as following: correctly used elements of knowledge  , inconsistently used elements of knowledge
, inconsistently used elements of knowledge  and incorrectly used elements of knowledge
and incorrectly used elements of knowledge  . Some cells appear colored for further description in the text.
. Some cells appear colored for further description in the text.
It is immediately apparent that Sandra (Fig. 1) has a better conceptual knowledge than Dan (Fig. 2): her map contains many more black cells (elements of knowledge that are consistently used correctly) and much fewer cells with diagonal lines (elements of knowledge that are used incorrectly) at any given assessment point. She also has fewer grey cells (elements of knowledge that are used correctly, but not consistently so). This is true from the very first assessment, and this difference between the two students only grew from one assessment to the next. While Dan's columns also become darker as we move to the right, since he too is learning, this happens much less consistently than in Sandra's map. Some of his black cells become grey or even crossed, meaning that while he exhibited knowledge of some elements of knowledge in an early assessment, this knowledge was not robust because in later assessment he didn't use this element of knowledge consistently correctly.
As mentioned earlier, Sandra demonstrated knowledge of structural, interactional, and energy-related elements of knowledge. Dan, on the other hand demonstrated consistent knowledge of many fewer interactional elements of knowledge. For example, as seen in Table 7, Sandra consistently used the following element of knowledge correctly: “The total attractive (and repulsive) forces increase as the distance between the bonding electrons and nuclei decreases.” Dan, on the other hand, used this element of knowledge as well, though not always correctly. Several other students' maps showed similar weaknesses regarding this element of knowledge. This can be an indication this element of knowledge requires remedial classroom treatment. Consider another element of knowledge “Positive ions have fewer electrons than protons, negative ions have more electrons than protons”. The incorrect understanding of these elements of knowledge, represented in Dan's map, was seen among many of the students in the class. There may be a common basis to these errors—e.g. perhaps not knowing the charges of the nucleus and the electrons. This is a basic element of knowledge and not knowing it can prevent a student from learning more advanced ideas such as partial charge and electron density distribution which have been described as central to bonding (Shusterman and Shusterman, 1997; Taagepera and Noori, 2000).
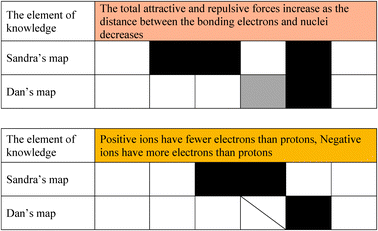
The difference in the number of occasions in which an element of knowledge was assessed (appeared black, grey or with the diagonal) differed in both cases because it depended on Sandra or Dan's explanations. Sandra, who is a better student, used more elements of knowledge in her arguments, while Dan used less elements of knowledge. A grade could be given only if the student used the element of knowledge in her/his explanations.
A re-reading of their statements showed that Dan's statements tended to be declarative while Sandra's were more explanatory. For example, in response to the question presented in Appendix B, Dan answered:
“From the periodic table the bond in Cl2 is stronger because Cl2's electron cloud is smaller than the electron cloud of Br2 and I2, according to Coulomb's law that if the distance is smaller the bond is stronger. The strength of the bond decreases as you go down the group because the electron cloud gets bigger.”
On the other hand, Sandra's answer to the same question was:
“From the periodic table the Cl2 bond is more energetic the Br2 or the I2 bond because the chlorine atom has the smallest number of electrons, the atoms can get closer to each other, and therefore the attraction forces between the bonding electrons and the nuclei are stronger and more energy is required to overcome the attraction forces between the bonding electrons and the nuclei. In Br2 and I2 there are more electrons, the repulsion forces between the bonding electrons are stronger than the attraction forces between the bonding electrons and the nuclei, and in order to break the bond less energy is required, therefore the bond energy is smaller.”
Previous papers characterized the Coulomb force principle as having greater explanatory power than the octet framework (Robinson, 1998; Taber, 1998, 2001, 2003a). Elements of knowledge related to Coulomb forces appear mainly in the electrostatic interactions strand in the matrix while elements of knowledge related to the octet framework are primarily in the nanostructure strand. Perhaps Dan's weaker knowledge of interactional elements of knowledge made it more difficult for him to construct explanatory statements?
The transition from a single molecule or atom to a lattice is perhaps difficult for students because it requires understanding of the interactions between many particles (Barke et al., 2009; Ben-Zvi et al., 1986; Coll and Taylor, 2002; Levy Nahum et al., 2007; Othman et al., 2008). Inter-molecular bonding is particularly difficult (De Posada, 1997; Peterson and Treagust, 1989). This can be seen in Dan's map in the exam that assessed knowledge related to lattices, which refers to a substance, not only a single molecule (4 2008) and was true for other students in the class as well; there appear many more declarations of incorrect knowledge than before. In the following exam, Dan demonstrated greater knowledge of the same elements of knowledge when they were retested.
Discussion
The matrix that represents our proposed characterization of conceptual knowledge of chemical bonding is the combined product of an analysis of the literature on chemical bonding and empirical research aimed at demonstrating the possibility of using the matrix to represent students' conceptual knowledge of chemical bonding. The matrix (Appendix F) is available on-line at http://stwww.weizmann.ac.il/g-chem/the_matrix.doc. and the different steps in its development are shown in Fig. 3.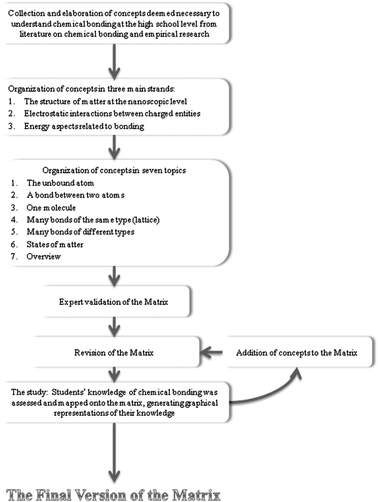 | ||
| Fig. 3 The steps in the characterization of students' conceptual knowledge of chemical bonding and in the development of the matrix. | ||
The idea that guided us when choosing the elements of knowledge to be represented in the matrix was that students who knew these elements of knowledge should have the basic knowledge expected from high school chemistry majors. The matrix is based upon elements of knowledge drawn from the ATLAS (AAAS, 2007), high school text books (e.g., Ebbing and Gammon, 2002; Zumdahl, 1989), research literature on high school chemistry (Ben-Zvi et al., 1986; Coll and Taylor, 2002; Coll and Treagust, 2001; Harrison and Treagust, 2000; Herron, 1996; Levy Nahum et al., 2006; Othman et al., 2008; Peterson et al., 1986; Scalise et al., 2006b; Taagepera and Noori, 2000; Taber, 2001; Taber and Coll, 2002; Teichert and Stacy, 2002), and verbal and written utterances made by students and teachers as they learned and taught about chemical bonding.
This study was undertaken in Israel, and since the matriculation exams in Israel do not assess all the elements of knowledge included in the matrix and many teachers typically teach only the “must know” elements of knowledge, the matrix represents a broader and deeper knowledge of chemical bonding than may be typically found in Israeli high school graduates. On the other hand, the matrix does not represent all the elements of knowledge required to understand chemical bonding at the undergraduate level. In other words, not all parts of the matrix would be considered canonical at all levels of chemistry (Sánchez Gómez and Martín, 2003). Since our research population consisted only of one class of high school students and their teacher, there are elements of knowledge represented in the matrix for which we could not obtain validating data.
The biggest conceptual differences between the matrix and other representations of knowledge such as concepts maps (Novak, 2010), V-diagrams (Mintzes and Novak, 1999) or construct maps (Wilson, 2005) are that the matrix represents knowledge of the cannon rather students' idiosyncratic ideas about bonding and does so in very fine detail; the other representations have, in most of the cases, a less fine grain-size when dealing with a broad topic and are concerned with students' knowledge of interrelationships between elements of knowledge, knowledge of the way conceptual knowledge is generated, or general qualitative aspects of students' knowledge, respectively. Each representation has it value and is useful for different purposes.
Mapping students' conceptual knowledge onto the matrix can provide a graphical representation of their knowledge and the connection of the knowledge of elements of knowledge according to the context being assessed which can be analyzed using proper items which should assess the relevant elements of knowledge.
The model of Sandra and Dan's knowledge (what elements of knowledge do they know or do not know) which was revealed from the matrix was similar to the model received from the protocols of the interviews, the exams and activities. Even if the model represents only conceptual canonical knowledge, the matrix may provide a good way to compare and analyze this knowledge which would be impossible to do by comparing the protocols.
As opposed to Sandra and Dan's representations, which were based on items that the teacher planned or on the discourse in class, the items in the diagnostic tool should cover enough relevant elements of knowledge in different contexts, in order to provide a valid and reliable graphical representation of the student's knowledge (as a matrix). This is necessary for these representations to be used for the diagnosis of areas of strength and weakness, to pinpoint difficult elements of knowledge, and to track knowledge development over time by comparing different student's matrices. This has been the basis in a further research (Yayon, 2011).
Promises
A. Cells that are diagonally crossed represent elements of knowledge that students incorrectly understood. An element of knowledge can be defined as difficult to learn if many students from different classes demonstrate incorrect knowledge of these elements of knowledge. By identifying elements of knowledge that are difficult, teachers and curriculum developers can decide which require special instructional attention. Teachers may gloss over some elements of knowledge, thinking them to be very simple to understand (Levy Nahum et al., 2007; Taber and Coll, 2002), forgetting that what they treat as simple may not be so for their students. Some elements of knowledge require more focused instructional attention than others, such as understanding energy levels and orbitals not only as “orbits” (Cervellati and Perugini, 1981; Stefani and Tsaparlis, 2009; Taber, 2005).B. It may be possible to identify essential elements of knowledge, such as that “electrons have no fixed position, but rather are distributed in a probabilistic fashion and that momentary partial charges (δ− and δ+) in the electron cloud are caused by momentary asymmetric changes in the distribution of the electrons” (Shusterman and Shusterman, 1997; Taagepera and Noori, 2000), without which the continued development of students' knowledge is obstructed (Herron et al., 1977). Without understanding the atom as a dynamic entity it is impossible to understand van der Waals (Coll and Taylor, 2002; Levy Nahum et al., 2007; Taber, 1998, 2001). These would be elements of knowledge represented by cells that mark the beginning of a “zone” of grey or crossed cells.
C. Since the elements of knowledge in the matrix are organized into topics and sub topics, the representation can show not only knowledge of independent elements of knowledge (a check list), but also the knowledge of sub topics and maybe even topics. If many elements of knowledge in a topic are used correctly we can assume that these are known, if on the other hand many elements of knowledge in a topic are used incorrectly we can assume that these are not known. Maybe common elements of knowledge in two topics could be known, and maybe we could see a pattern in which the knowledge of same elements of knowledge are used correctly in one topic and incorrectly in other among students from different classes.
D. Different teachers over or under-emphasize different elements of knowledge in their assessments. The mapping of exams onto the matrix can provide a way to identify elements of knowledge being over-assessed and others which are not being given enough attention.
E. The map shows the range of canonical elements of knowledge students know but not their ability to integrate different elements of knowledge together. We can conceive of an extra dimension to the matrix which would show, for each element of knowledge, to which other elements of knowledge it had been successfully connected by a student. Presumably as the number of connections that have been made to a element of knowledge increase, the more flexible and deeper the knowledge of that element of knowledge (Linn, 2006). From a similar point of view, since the items in the matrix deal with basic elements of knowledge at different stages, it means that elements of knowledge are examined in different contexts. This tool could be appropriate for investigating the influence of the context on the knowledge of a specific assessment item, or the knowledge of a specific assessment item in different contexts.
F. Learning progressions are descriptions of the ways knowledge of key disciplinary elements of knowledge develops over time (Duschl et al., 2007). The spread of dark cells in the matrix represents how a students' conceptual knowledge develops over time. By tracking many students learning from the same curriculum, one could identify typical paths in which knowledge develops, leading to an empirically-based description of a learning progression.
G. Learning progressions are not developmentally inevitable; they are curriculum and instruction dependent (Golan Duncan and Hmelo-Silver, 2009). The matrix provides a simple tool for comparing between different instructional sequences. For example, consider two different approaches to teach bonding. The first is to present four different groups of substances—the ionic lattice, the molecular lattice, the covalent lattice, and the metallic lattice—and to elaborate on and discuss each of these structures, regarding the types of chemical bonds between the particles as described by Hurst (2002) and Sproul (2001). The second approach introduces the significant properties of isolated atoms, follows by introducing chemical bonds between two atoms, presenting the different traditional categories of chemical bonds as extreme cases on a continuum, and only after that discussing the characteristics of substances. The same elements of knowledge are taught but the curricular strategy and sequencing is very different (Levy Nahum et al., 2008).
H. It should be possible, in principle, to develop matrices representing conceptual knowledge for any topic, not just chemical bonding.
I. Maybe the matrix can be a base for a further tool which will show the ability to integrate different elements of knowledge as concept maps do.
Challenges
A. Knowledge of many of the relevant elements of knowledge that appear in the matrix was not assessed by the instruments used in this study. In addition, many elements of knowledge were assessed only once or twice, precluding the possibility of tracking the development of their knowledge. To reliably track the development of students' knowledge, it is important to use assessments that are designed to have an optimal level of spread and overlap, characteristics that were not considered when designing the instruments for this study.B. The process of mapping the items and statements onto the matrix is time-consuming. At present it could have value only to researchers. For it to become a useful tool for teachers the mapping process needs to be automated or a bank of pre-mapped items needs to be developed.
C. The normative approach followed gives only limited insight into student knowledge, as alternative conceptions and misconceptions are not represented. Analysis of conceptual structure as a means of informing teaching has been performed decades ago, but it is not common anymore, Gagne and White, (1978) described a set of six types of knowledge; one of them, propositions or informing teaching, which could be considered as elements of knowledge in our study. This approach became less common with the increasing influence of the constructivist perspective (Gilbert and Watts, 1983; Niedderer and Schecker, 1992). Learning is described as the change of elements or the change of cognitive processes using cognitive elements, which result from developmental processes of the cognitive system of the student interacting with external situations. These changes could include elements of knowledge that the student generated in an incorrect structure or schema. In other words, identifying the presence of what appears to be canonical knowledge does not imply the absence of contrary alternative conceptions.
D. We have tested in this study only the viability of the matrix as the basis of an analytical process and NOT the validity of its outcomes–this has been done in a further study (Yayon, 2011).
E. The matrix is a reasonable representation of many, if not most, of the main elements of knowledge comprising conceptual knowledge of chemical bonding at a high school level. It may be that some of them would not be considered canonical at all levels of chemistry (Sánchez Gómez and Martín, 2003).
F. As in chemistry, we know that the properties of a compound are different than the elements, or in other words, elements are not added to form a compound. Our assumption that we can add elements of knowledge and conclude about the knowledge of the concept is not necessarily correct, it can be “safer” to assume that if the elements of knowledge are not known, then the possibility of knowing the concept is lower.
To deal with both these challenges, we developed an on-line library of tests consisting of cloze items, each item assessing the knowledge of a predetermined set of elements of knowledge. Students take the tests on-line. The system automatically generates a map of a student or class's knowledge. This study is described in Yayon's PhD (Yayon, 2011).
Finally, as pointed out above, the matrix contains only canonical elements of knowledge and no alternative conceptions. This is a major limitation of the tool. But, as in every study, a researcher should have in mind which data can be collected by a certain instrument, so in this case, the matrix can diagnose only canonical conceptual knowledge and not alternative conceptions; it is important to know whether students know these elements of knowledge and topics (clustered elements of knowledge) and whether this knowledge is related to a certain context or not.
Appendix A—examples of items from tests
Question A
Describe a fluorine atom in detail—the particles from which it is composed, the forces acting between them, and their energy.Question B
Describe one molecule of ClF in detail—the particles from which it is composed, the forces acting between them, and their energy.Question C
Describe the substance ClF in a liquid state in detail—the particles from which it is composed, the forces acting between them, and their energy.Question D
When ClF turn from a liquid into gas, the molecules become farther away from each other but the covalent bond between the Cl and F does not break. Why?Question E
1. Draw the Lewis structure of Ethylene C2H4 and Ethane C2H6.2. The bond between the carbon atoms in C2H4 is shorter and stronger than the bond between the carbon atoms in C2H6. Explain by referring to the electrical forces between the particles.
Question G
The following questions deal with the elements of group 7 in the Periodic Table: fluorine, chlorine, bromine, & iodine.1. The element chlorine has two isotopes, one with mass number 35 the other 37.
i. How many protons, neutrons, & electron does each isotope have?
ii. Do these isotopes form identical chemical compounds or different ones? Explain.
2. How are the electrons populated in bromine?
3. Indicate the difference and the similarity in the way the electrons are populated in these four atoms.
Appendix B—the mapping of an item onto the matrix followed by a addition of elements of knowledge to the matrix
This appendix shows how an answer to a question of a test (given by the teacher) was mapped according to an expert and students' answers and how it succeeded a minor revision of the matrix. All the elements of knowledge in the answer were matched to those in the matrix (expert), but the “popular” answer among students, didn't have a “match'. Therefore the last element of knowledge shown in Table 9” In general, bond energy increases as the bond length decreases “was added. The addition of the element of knowledge followed the addition of similar elements of knowledge as well “In general, bond length decreases as the strength of a bond and bond energy increase” and “The strength of a bond increases as the bond energy increases”. Students seem to think that if they say a statement which has a relation between to factors, they are explaining the idea, instead of only claiming.Question
Table 8 shows the bond energy values of three elements of group 7.| The bond | Bond energy (kJ mol−1) |
|---|---|
| Cl–Cl | 243 |
| Br–Br | 193 |
| I–I | 151 |
| Element of knowledge in the matrix | Sub-item (expert) | Sub-item (students) |
|---|---|---|
| The number of populated energy levels equals the row number in the PT. | Iodine (I) is lower in the PT than Br which is lower than Cl. Therefore, I has more populated energy levels than Br which has more populated energy levels than Cl. | |
| The size of an atom in general increases as its number of energy levels increase. | The size of an atom is determined primarily by its number of populated energy levels, with size increasing with increasing number of energy levels, so I is larger than Br which is larger than Cl. | |
| The total attractive force (the bond strength) increases with decreasing radius of the bound atom. | The larger the atom, the weaker the attractive force it can apply to bonding electrons. Therefore, the bond force is strongest in Cl–Cl, then in Br–Br, and weakest in I–I. | |
| The energy required to break a chemical bond & separate the 2 atoms (bond energy) increases with increasing attractive forces. | As the bond strength increases, more energy is required to break the bond, that is, the bond energy increases. Therefore, the bond energy decreases from Cl–Cl to Br–Br to I–I. | |
| In general, bond energy increases as the bond length decreases. | Since the bond energy decreases from Cl–Cl to Br–Br to I–I, and in general the greater the bond energy the smaller the bond length, the bond length decreases from I–I to Br–Br to Cl–Cl. |
Answer the following questions by referring to the electrical forces between the particles.
1. Explain the trend in the bond energies.
Answer of an expert: The number of populated energy levels equals the row number in the PT. Iodine (I) is lower in the PT than Br which is lower than Cl. Therefore, I has more populated energy levels than Br which has more populated energy levels than Cl. The size of an atom is determined primarily by its number of populated energy levels, with size increasing with increasing number of energy levels, so I is larger than Br which is larger than Cl. The larger the atom, the weaker the attractive force it can apply to bonding electrons. Therefore, the bond force is strongest in Cl–Cl, then in Br–Br, and weakest in I–I. As the bond strength increases, more energy is required to break the bond, that is, the bond energy increases. Therefore, the bond energy decreases from Cl–Cl to Br–Br to I–I.
2. The bond lengths of these three molecules are 0.228 nm, 0.199 nm & 0.267 nm. Match each bond length to each molecule. Explain your reasoning.
Popular answer of students: Since the bond energy decreases from Cl–Cl to Br–Br to I–I, and in general the greater the bond energy the smaller the bond length, the bond length decreases from I–I to Br–Br to Cl–Cl.
Appendix C—outline of the interviews
• What does the symbol Br2(L) mean to you?• Can you make a drawing that represents liquid bromine at the molecular level?
• What is located at the spaces between the particles?
• Please explain what happens at the molecular level when liquid bromine is heated from room temperature until it boils.
• What do you think will happen if we continue heating the gaseous bromine to a very high temperature?
• Describe a single bromine atom to me.
• In what way do a bromine atom and a chlorine atom differ from one another?
• Chlorine boils at −35 °C while bromine boils at 58 °C. Explain what you think causes this difference in boiling points.
Appendix D
Figs. 4–6 show enlargements of the electrostatic interactions, nanostructure and energy parts of Fig. 1 – the development of Sandra's conceptual knowledge over time. | ||
| Fig. 4 (Enlarged) the development of Sandra's conceptual knowledge over time – electrostatic interactions. | ||
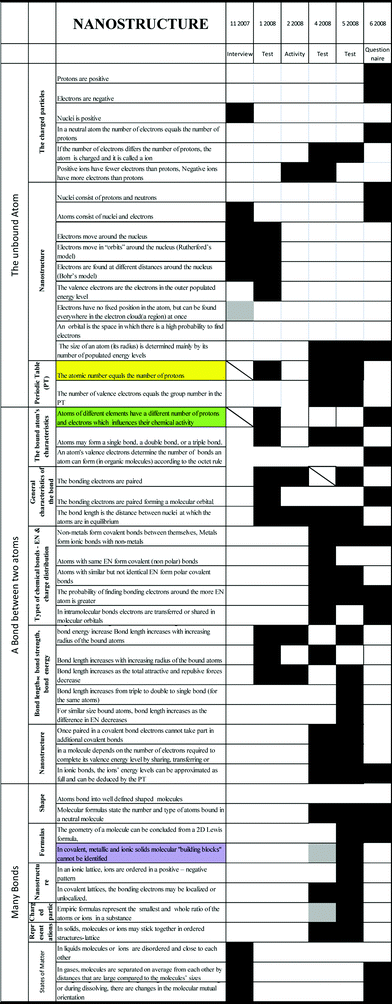 | ||
| Fig. 5 (Enlarged) the development of Sandra's conceptual knowledge over time – nanostructure. | ||
 | ||
| Fig. 6 (Enlarged) the development of Sandra's conceptual knowledge over time – energy. | ||
Appendix E
Figs. 7–9 show enlargements of the electrostatic interactions, nanostructure and energy parts of Fig. 2 – the development of Dan's conceptual knowledge over time.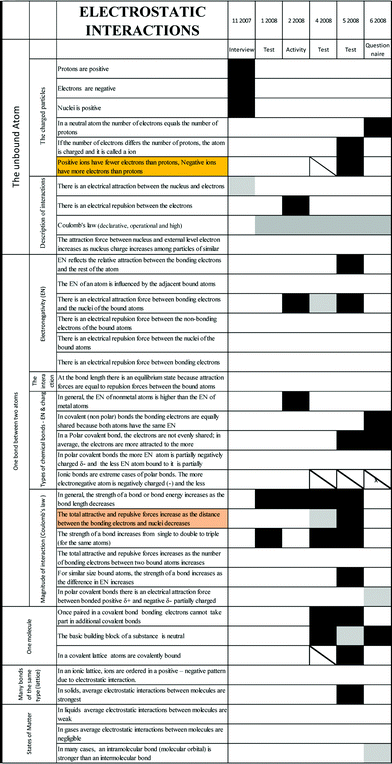 | ||
| Fig. 7 (Enlarged) the development of Dan's conceptual knowledge over time – electrostatic interactions. | ||
 | ||
| Fig. 8 (Enlarged) the development of Dan's conceptual knowledge over time – nanostructure. | ||
 | ||
| Fig. 9 (Enlarged) the development of Dan's conceptual knowledge over time – energy. | ||
Appendix F
Matrix: an unbound atom & a bond between two atoms (Table 10) and overview (Table 11).| Nanostructure | Electrostatic interactions | Energy |
|---|---|---|
| The unbound atom | ||
| The charged particles | Nanostructure–electron population technique | |
| Protons are positive | In each energy level there is a specific number of orbitals | |
| Electrons are negative | Each orbital is populated by 1 or 2 electrons at the most | |
| Nuclei are positive | Lower energy levels get filled before higher ones | |
| The charge of the nucleus equals the number of protons | Empty orbitals get filled before partially populated orbitals in the same energy level | |
| In a neutral atom the number of electrons equals the number of protons | The maximum number of electrons in the first energy level is 2, in the second energy level is 8, in the third energy level is 8 (till atomic number 18) | |
| If the number of electrons differs the number of protons, the atom is charged and it is called a ion | Energy levels in the atom | |
| Positive ions have fewer electrons than protons, Negative ions have more electrons than protons | Electrons are found around the nucleus in energy levels | |
| Momentary partial charges (δ− and δ+) in the electron cloud are caused because electrons have no fixed position | The distance of an electron from the nucleus is determined by its energy | |
| Momentary partial charges (δ− and δ+) in the electron cloud are caused due to momentary asymmetric changes in the distribution of the electrons | In average, electron populating lower energy levels are closer to the nucleus than those populating higher energy levels | |
| The valence electrons are the electrons populating the outer highest energy level | ||
| Particles with full energy levels are less reactive than those with partly full energy levels | ||
| Nanostructure | Description of interactions | Ionization energy |
|---|---|---|
| Nuclei consist of protons and neutrons | Energy is needed to separate an electron from its atom (ionization energy) | |
| Atoms consist of nuclei and electrons | There is an electrical attraction between the nucleus and electrons | The ionization energy increases as the attraction force between the nucleus and the electron increases |
| Electrons move around the nucleus | There is an electrical repulsion between the electrons | |
| Atoms are not small balls (they don't have a border) | ||
| Electrons do not move in “orbits” around the nucleus (Rutherford’s model) | ||
| Electrons are found at different distances around the nucleus (Bohr's model) | Bohr's model doesn't explain why electrons don't stick to the nuclei | |
| The valence electrons are the electrons in the outer populated energy level | ||
| Atoms are mostly vacuum | The attraction between the nucleus and electrons hold the atom together | |
| Electrons have no fixed position in the atom, but can be found everywhere in the electron cloud (a region) at once | Momentary partial charges (δ− and δ+) in the electron cloud are possible because electrons have no fixed position and _repel each other | |
| Electrons have no fixed position, but rather are distributed in a probabilistic fashion | ||
| An orbital is the space in which there is a high probability to find electrons | ||
| The shape of an atom is defined by the shape of its electron populated cloud | ||
| The size of an atom (its radius) is determined mainly by its number of populated energy levels | ||
| The size of an atom (its radius) decreases as the nucleus charge increases among atoms with same number of populated energy levels | ||
| The Lewis Formula represents the valence electrons |
| Periodic Table (PT) | Magnitude of interaction between nuclei and electrons (Coulomb's law) | The Periodic Table (PT) & ionization energy |
|---|---|---|
| Coulomb's law (declarative, operational and high) | ||
| The magnitude of the electrostatic force between two electric charges is inversely proportional to the square of the distance between the two charges. | ||
| The magnitude of the electrostatic force between two point electric charges is directly proportional to the product of the magnitudes of each of the charges | ||
| The number of valence electrons equals the group number in the PT | ||
| The number of populated energy levels equals the row number in the PT | The attraction force between a nucleus and an electron increases as the number of energy level in which the electron is decreases | Ionization energy of a valence electron decreases as the number of populated energy levels increases (along a column of the PT) |
| The attraction force between a nucleus and an electron increases as the distance between them decreases | Ionization energy decreases along a column in the PT as the distance between the nucleus and valence electrons increases | |
| The atomic number equals the number of protons | The attraction force between a nucleus and an external level electron increases as the atomic number increases among particles of similar size. | Along a row in the PT, the general trend is that ionization energy increases as the nucleus charge increases |
| The attraction force between nucleus and external level electron increases as nucleus charge increases among particles of similar size. | When comparing the ionization energy of similar size atoms (radius), the general trend is that the ionization energy of the valence electron occupying the same energy level increases as the nucleus charge increases | |
| The repulsion force between the electrons populating the same energy level increases as their number increases because the distance between them decreases | ||
| The general trend is that the size of an atom decreases along the PT row | The size of the atom (radius) decreases along the PT row due to the increase in attraction force between nucleus and electrons as nucleus' charge increases | |
| The number of valence electrons does not influence the attraction force between the nucleus and each valence electron |
| One bond between two atoms | ||
|---|---|---|
| The bound atom's characteristics | Electronegativity (EN) | |
| The number of protons doesn't change in chemical reactions. | ||
| Atoms of different elements have a different number of protons and electrons which influences their chemical activity | EN reflects the relative attraction between the bonding electrons and the rest of the atom | |
| Atoms may form a single bond, a double bond, or a triple bond. | EN is influenced among other, by the energy of ionization and the electron affinity | |
| An atom's valence electrons determine the number of bonds an atom can form (in organic molecules) according to the octet rule | The EN of an atom is influenced by the adjacent bound atoms | |
| General characteristics of the bond nanostructure | Description of interactions (general) | Description of the energy involved (general) |
|---|---|---|
| The bonding electrons are paired | There is an electrical attraction force between bonding electrons and the nuclei of the bound atoms | Energy is required to break a bond between atoms |
| The bonding electrons are shared forming a molecular orbital. | There is an electrical repulsion force between the non-bonding electrons of the bound atoms | |
| A molecular orbital represents the probability to find electrons in the space around the bound atoms | There is an electrical repulsion force between the nuclei of the bound atoms | |
| There is an electrical repulsion force between bonding electrons | The energy required to break a chemical bond, to separate the 2 atoms is the Bond energy | |
| There is mostly vacuum between nuclei in a bond | The energy required to break a bond into atoms is equal to the energy released when the bond is formed | |
| Empty molecular orbitals get filled before partially populated orbitals in the same energy level | ||
| The interaction between two atoms vs. distance between two nuclei (Coulomb's law) | ||
| The bond length is the distance between nuclei at which the atoms are in equilibrium | At the bond length there is an equilibrium state because attraction forces are equal to repulsion forces between the bound atoms | When 2 bound atoms are at bond length the system is at minimum energy |
| When two atoms move toward each other their orbitals overlap with each other forming a molecular orbital | Two atoms move toward each other because the attraction forces are stronger than the repulsion forces between them | Two atoms bond if the energy of the bound atoms is lower than that of the two separated atoms |
| At a closer distance than the bond length atoms move away from each other because, repulsion forces are stronger than attraction forces between them | When 2 bound atoms are at bond length, energy is required to separate or get the atoms closer to each other | |
| When the bond is broken, the atoms are separated and the molecule is broken | When the atoms are far from each other, any interaction–attraction or repulsion is negligible. | When the atoms are far from each other, the energy of the system is given by the sum of the energy of both atoms. |
| Electric forces between electrons and nucleus hold bound atoms together at the bond length. | ||
| Types of chemical bonds–EN & electron distribution | Types of chemical bonds–EN & charge distribution | |
| Thumb rule: Non-metals form covalent bonds between themselves, Metals form ionic bonds with non-metals | In general, the EN of nonmetal atoms is higher than the EN of metal atoms | |
| Atoms with same EN form covalent (non polar) bonds | There are permanent partial charges (δ− and δ+) in the electron cloud due to the asymmetric distribution of the electrons. | |
| In covalent (non polar) bonds the probability of finding bonding electrons around both atoms is equal | In covalent (non polar) bonds the bonding electrons are equally shared because both atoms have the same EN | |
| Atoms with similar but not identical EN form polar covalent bonds | In a polar covalent bond, the electrons are not evenly shared; in average, the electrons are more attracted to the more electronegative atom | |
| In polar covalent bonds the more EN atom is partially negatively charged δ− and the less EN atom bound to it is partially positively charged δ+ | In polar covalent bonds the more EN atom is partially negatively charged δ− and the less EN atom bound to it is partially positively charged δ+ | |
| The probability of finding bonding electrons around the more EN atom is greater | The polarity of the bond increases as the difference in the EN of the bound atoms increases | |
| In intramolecular bonds electrons are transferred or shared in molecular orbitals | Permanent partial charges are created due to the asymmetric distribution of electrons in chemical bonds. | |
| Atoms with very different EN form ions, a negative nonmetal ion, a positive metal ion | In ionic bonds negative nonmetal ions and positive metal ions can be assumed to exist since in bonds between atoms with very different EN bonding electrons are not shared, but are mostly by the electronegative atom | |
| In ionic bonds the more EN atom is a negative ion, the less EN atom is a positive ion. | Ionic bonds are extreme cases of polar bonds. The more electronegative atom is negatively charged (−) and the less electronegative atom bound to it is positively charged (+) |
| Bond length ∝ bond strength, bond energy | Magnitude of interaction (Coulomb's law) | Bond energy ∝ bond strength, bond length |
|---|---|---|
| In general, bond length decreases as the strength of a bond and bond energy increase | The strength of a bond increases as the bond energy increases | In general, bond energy increases as the bond length decreases |
| In general, the strength of a bond or bond energy increases as the bond length decreases | ||
| The strength of a bond is evaluated by its bond energy | The bond energy represents the bond strength | |
| The bond strength indicates the total attractive (and repulsive) forces between the particles in the bound atoms | ||
| Bond length increases with increasing radius of the bound atoms | The total attractive (and repulsive) forces (the bond strength) increase with decreasing radius of the bound atoms | Bond energy increases with decreasing radius of the bound atoms |
| Bond length increases as the total attractive and repulsive forces decrease | The total attractive (and repulsive) forces (the bond strength) increase as the distance between the bonding electrons and nuclei decreases | Bond energy increases with increasing attractive and repulsive forces |
| Bond length increases from triple to double to single bond (for the same atoms) | The strength of a bond increases from single to double to triple (for the same atoms) | The bond energy increases from single to double to triple |
| For similar size bound atoms, bond length increases as the number of lone pairs decreases | The total attractive (and repulsive) forces increases as the number of bonding electrons between two bound atoms increases | |
| For similar size bound atoms, bond length increases as the difference in EN decreases | For similar size bound atoms, the strength of a bond increases as the difference in EN increases | For similar size atoms, the bond energy increases as the difference in EN increases |
| In polar covalent bonds there is an electrical attraction force between bonded positive δ+ and negative δ− partially charged atoms | ||
| In an ionic bond, there is an electrical attraction force between positive and negative ions |
| Overview | ||
|---|---|---|
| Generally speaking, a diatomic system can be mapped in a continuous scale from Van der Wals to non-polar covalent to polar covalent to ionic for similar atomic radii bound atoms | ||
| according to the bond length | according to the bond energy values that reflect the strength of the electrostatic forces | according to the bond energy values |
| There is a whole range of chemical bonds which we can map on a continuous scale according to the | ||
| strength of the interaction | bond energy values | |
| Chemical bonds can be understood, among others, by: attractive and repulsive forces, the equilibrium point, the difference in EN values, the atomic EN values, bond length and energy | ||
| Chemical bonds can be explained by quantum theory, but in practice, much can be qualitatively explained without explicitly using this theory | ||
References
- AAAS, (2007), ATLAS of Science Literacy (Vol. 1 and 2). Washington, DC: National science teachers association.
- Akçaoglu Küçüközer, H. A., (2005), L'étude de l'évolution de la compréhension conceptuelle des élèves avec un enseignement: Cas de la mécanique en 1ère S. Unpublished Doctoral dissertation, Université Lumière Lyon 2 Lyon.
- Barke, H. D., Hazari, A. and Yitbarek, S., (2009), Structure-property relationships. In H. D. Barke, A. Hazari and S. Yitbarek (Ed.), Misconceptions in chemistry (pp. 103–144). Berlin: Springer.
- Ben-Zvi, R., Eylon, B.-S. and Silberstein, J., (1986), Is an atom of copper malleable? J. Chem. Educ., 63(1), 64–66.
- Cervellati, R. and Perugini, D., (1981), The understanding of the atomic orbital concept by Italian high school students, J. Chem. Educ., 58(7), 568–569.
- Coll, R. K. and Taylor, N., (2002), Mental models in chemistry: Senior chemistry students' mental models of chemical bonding, Chem. Educ. Res. Pract. Eur., 3(2), 175–184. Retrieved from http://www.uoi.gr/cerp/2002_May/pdf/08Coll.pdf.
- Coll, R. K. and Treagust, D. F., (2001), Learners' mental models of chemical bonding, Res. Sci. Educ., 31(3), 357–382.
- De Posada, J. M., (1997), Conceptions of high school students concerning the internal structure of metals and their electric conduction: Structure and evolution, Sci. Educ., 81, 445–467.
- diSessa, A. A., (1988), Knowledge in pieces. In G. Forman and P. Pufall (Ed.), Constructivism in the computer age (pp. 49–70). Hillsdale, NJ: Erlbaum.
- diSessa, A. A., (1993), Toward an epistemology of physics, Cognit. Instruct., 10, 105–225.
- Duit, R. and Treagust, D. F., (2003), Conceptual change: A powerful framework for improving science teaching and learning, Int. J. Sci. Educ., 25(6), 671–688.
- Duschl, R. A., Schweingruber, H. A. and Shouse, A. W., (2007), Learning progressions. In R. A. Duschl, H. A. Schweingruber and A. W. Shouse (Ed.), Taking science to school: Learning and teaching science in grades K-8 (pp. 213–250). Washington, DC: National Academy Press.
- Ebbing, D. D. and Gammon, S. D., (2002), Atomic and molecular structure. In K. Prancan (Ed.), General chemistry (pp. 278–439). Boston: Houghton Mifflin.
- Fensham, P., (1975), Concept formation. In D. J. Daniels (Ed.), New movements in the study and teaching of chemistry (pp. 199–217). London: Temple Smith.
- Gagne, R. M. and White, R. T., (1978), Memory Structures and Learning Outcomes, Rev. Educ. Res., 48(2), 187–222.
- Galili, I. and Hazan, A., (2000), The influence of an historically oriented course on students' content knowledge in optics evaluated by means of facets-schemes analysis, Am. J. Phys., 68, S3–S15.
- Gilbert, J. K., (2006), On the nature of “Context” in chemical education, Int. J. Sci. Educ., 28(9), 957–976.
- Gilbert, J. K., Watts, D. M. and Osborne, R. J., (1985), Eliciting student views using an interview-about-instances technique. In L. H. T. West and A. L. Pines (Ed.), Cognitive structure and conceptual change (pp. 11–27). London: Academic Press.
- Gilbert, J. K. and Watts, D. M., (1983), Concepts, misconceptions and alternative conceptions: changing perspectives in science education, Stud. Sci. Educ., 10, 61–98.
- Gillespie, R. J., (1997), The great ideas of chemistry, J. Chem. Educ., 74(7), 862–864.
- Golan Duncan, R. and Hmelo-Silver, C. E., (2009), Learning progressions: Aligning curriculum, instruction, and assessment, J. Res. Sci. Teach., 40(6), 606–609.
- Harrison, A. G. and Treagust, D. F., (2000), Learning about atoms, molecules, and chemical bonds: A case study of multiple-model use in grade 11 chemistry, Sci. Educ., 84(3), 352–381.
- Herron, J. D., (1996), The chemistry classroom: Formulas for successful teaching. Washington, DC: American Chemical Society.
- Herron, J. D., Cantu, L., Ward, R. and Srinivasan, V., (1977), Problems associated with concept analysis, Sci. Educ., 61(2), 185–199.
- Hurst, O., (2002), How we teach molecular structure to freshmen, J. Chem. Educ., 79(6), 763–764.
- Kelly, G., (1963), A theory of personality: The psycology of personal construct. New York: W. W. Norton.
- Kelly, G. and Bazerman, C., (2003), How students argue scientific claims: A rhetorical-semantic analysis, Appl. Linguist., 24(1), 28–55.
- Kozma, R. B. and Russell, J. W., (2005), Students becoming chemists: Developing representational competence. In J. K. Gilbert (Ed.), Visualization in Science Education (pp. 121–146). Dordrecht, The Netherlands: Springer.
- Levy Nahum, T., Mamlok-Naaman, R., Hofstein, A. and Krajcik, J. S., (2007), Developing a new teaching approach for the chemical bonding concept aligned with current scientific and pedagogical knowledge, Sci. Educ., 91(4), 579–603.
- Levy Nahum, T., Mamlok-Naaman, R., Hofstein, A. and Kronik, L., (2008), A new “bottom-up” framework for teaching chemical bonding, J. Chem. Educ., 85, 1680–1685.
- Levy Nahum, T., Mamlok-Naaman, R., Hofstein, A. and Taber, K. S., (2010), Teaching and learning the concept of chemical bonding, Stud. Sci. Educ., 46(2), 179–207.
- Levy Nahum, T., Shwartz, Y. and Bar-Dov, Z., (2006), Chemical bonding and quantitative relations in the materials world. Rehovot, Israel: Weizmann Institute of Science.
- Linn, M. C., (2006), The knowledge integration perspective on learning and instruction. In R. K. Sawyer (Ed.), The Cambridge handbook of the learning sciences (pp. 243–264). New York: Cambridge University Press.
- Margel, H., Eylon, B. and Scherz, Z., (2007), A Longitudinal Study of Junior High School Students' Conceptions of the Structure of Materials, J. Rs. Sci. Teach., 45(1), 132–152.
- Minstrell, J., (1992), Facets of student's knowledge and relevant instruction. In R. Duit, F. Goldberg and H. Niedderer (Ed.), Research in physics learning: Theoretical issues and empirical studies (pp. 110–128). Kiel, Germany: Institut für die Pädagogik der Naturwissenschaften.
- Mintzes, J. J. and Novak, J. D., (1999), Assessing science understanding: The epistemological Vee diagram. In J. J. Mintzes, J. H. Wandersee and J. D. Novak (Ed.), Assessing science understanding: A human constructivist view (pp. 42–71). San Diego, CA: Academic Press.
- Niedderer, H. and Schecker, H., (1992), Towards an explicit description of cognitive systems for research in physics learning. Paper presented at the Research in physics learning: Theoretical issues and empirical studies, Kiel, Germany.
- Novak, J. D., (1990), Concept maps and Vee diagrams: two metacognitive tools to facilitate meaningful learning, Instruct. Sci., 19(1), 29–52.
- Novak, J. D., (2010), Learning, creating, and using knowledge: Concept maps as facilitative tools in schools and corporations (2nd edn). New York: Routledge.
- Othman, J., Treagust, D. F. and Chandrasegaran, A. L., (2008), An investigation into the relationship between students' conceptions of the particulate nature of matter and their understanding of chemical bonding, Int. J. Sci. Educ., 30(11), 1531–1550.
- Peterson, R. and Treagust, D. F., (1989), Grade-12 students' misconceptions of covalent bonding and structure, J. Chem. Educ., 66, 459–460.
- Peterson, R. F., Treagust, D. F. and Garnett, P., (1986), Identification of secondary students' misconceptions of covalent bonding and structure concepts using a diagnostic instrument, J. Res. Sci. Educ., 16(1), 40–48.
- Reiner, M., (1992), Patterns of thought on light and underlying commitments. Paper presented at the Research in physics learning: Theoretical issues and empirical studies, Kiel, Germany.
- Ritter, S. K., (2007), Chemical & engineering news: The chemical bond-whether it's sextuple bonds or bonds involving no shared electrons, chemists chase down new modes of bonding, Sci. Technol., 85(5), 37–40.
- Robinson, W. R., (1998), An alternative framework for chemical bonding, J. Chem. Educ., 75(10), 1074–1075.
- Roseman, J. E., Caldwell, A., Gogos, A. and Kurth, L., (2006), Mapping a coherent learning progression for the molecular basis of heredity. Paper presented at the National Association for Research in Science Teaching meeting. from http://www.project2061.org/publications/articles/papers/narst2006.pdf.
- Sánchez Gómez, P. J. and Martín, F., (2003), Quantum versus ‘classical’ chemistry in university chemistry education: A case study of the role of history in thinking the curriculum, Chem. Educ. Res. Pract., 4(2), 131–148.
- Scalise, K., Claesgens, J., Krystyniak, R., Mebane, S., Wilson, M. and Stacy, A. M., (2003), Perspectives of chemists: Tracking conceptual understanding of student learning in chemistry at the secondary and university levels. From http://www.cchem.berkeley.edu/amsgrp/ed_pages/AERA2004Paper_FINAL.pdf.
- Scalise, K., Claesgens, J., Wilson, M. and Stacy, A. M., (2006a, October). ChemQuery: An assessment system for mapping student progress in learning chemistry. Paper presented at the National STEM Assessment conference.
- Scalise, K., Claesgens, J., Wilson, M. and Stacy, A. M., (2006b), Contrasting the expectations for student understanding of chemistry with levels achieved: A brief case-study of student nurses, Chem. Educ. Res. Pract., 7(3), 170–184.
- Shusterman, G. P. and Shusterman, A. J., (1997), Teaching chemistry with electron density models, J. Chem. Educ., 74(7), 771–776.
- Sproul, G., (2001), Electronegativity and bond type: Predicting bond type, J. Chem. Educ., 78(3), 387–390.
- Stefani, C. and Tsaparlis, G., (2009), Students' levels of explanations, models, and misconceptions in basic quantum chemistry: A phenomenographic study, J. Res. Sci. Teach., 46(5), 520–536.
- Taagepera, M., Arasasingham, R., Potter, F., Soroudi, A. and Lam, G., (2002), Following the development of the bonding concept using knowledge space theory, J. Chem. Educ., 79(6), 756–762.
- Taagepera, M. and Noori, S., (2000), Mapping students' thinking patterns in learning organic chemistry by the use of knowledge space theory, J. Chem. Educ., 77(9), 1224–1229.
- Taber, K. S., (1998), An alternative conceptual framework from chemistry education Int. J. Sci. Educ., 20(5), 597–608.
- Taber, K. S., (2001), Building the structural concepts of chemistry: Some considerations from educational research, Chem. Educ. Res. Pract. Eur., 2(2), 123–158. Retrieved from http://www.uoi.gr/cerp/2001_May/pdf/09Taber.pdf.
- Taber, K. S., (2002a), Chemical misconceptions-Prevention, diagnosis and cure: Volume II Classroom resources (Vol. 2). London: Royal Society of Chemistry.
- Taber, K. S., (2002b), Conceptualizing quanta: Illuminating the ground state of student understanding of atomic orbitals. Chem. Educ. Res. Pract. Eur., 3(2), 145–158. Retrieved from http://www.uoi.gr/cerp/2002_May/pdf/06Taber1.pdf.
- Taber, K. S., (2003a), Lost without trace or not brought to mind? A case study of remembering and forgetting of college science, Chem. Educ. Res. Pract. Eur., 4(3), 249–277. Retrieved from http://www.uoi.gr/cerp/2003_October/pdf/03Taber.pdf.
- Taber, K. S., (2003b), Mediating mental models of metals: Acknowledging the priority of the learner's prior learning, Sci. Educ., 87(5), 732–758.
- Taber, K. S., (2005), Learning quanta: Barriers to stimulating transitions in student understanding of orbital ideas, Sci. Educ., 89, 94–116.
- Taber, K. S. and Coll, R. K., (2002), Bonding. In J. K. Gilbert, O. D. Jong, R. Justi, D. F. Treagust and J. H. Van Driel (Ed.), Chemical education: Towards research-based practice (pp. 213–234). Dordrecht, The Netherlands: Kluwer.
- Taber, K. S. and García Franco, A., (2010), Learning processes in chemistry: Drawing upon cognitive resources to learn about the particulate structure of matter, J. Learn. Sci., 19(1), 99–142.
- Taber, K. S. and Watts, M., (2000), Learners' explanations for chemical phenomena, Chem. Educ. Res. Pract. Eur., 1(3), 329–353. Retrieved from http://www.uoi.gr/cerp/2000_October/pdf/05Taber.pdf.
- Talanquer, V., (2009), On cognitive constraints and learning progressions: The case of “structure of matter”, Int. J. Sci. Educ., 31(15), 2123–2136. Retrieved from http://www.informaworld.com/10.1080/09500690802578025.
- Teichert, M. A. and Stacy, A. M., (2002), Promoting understanding of chemical bonding and spontaneity through student explanation and integration of ideas, J. Res. Sci. Teach., 39(6), 464–496.
- ten Hoor, M. J., (2004), The polar bond, School Sci. Rev., 86(315), 123–126.
- Tiberghien, A., (2007), Construction of students' knowledge in relation to a teaching sequence: Hypothesis on learning and knowledge, Paper presented at the Guided Construction of Knowledge in Classrooms from http://escalate.org.il/construction_knowledge/papers/Tiberghien.pdf.
- Tsaparlis, G., (2002), Quantum-chemical concepts: Are they suitable for secondary students? Chem. Educ. Res. Pract. Eur., 3(2), 129–144.
- White, R. T., (1985), Interview protocols and dimensions of cognitive structure. In L. H. T. West and A. L. Pines (Ed.), Cognitive structure and conceptual change (pp. 51–59). London: Academic Press.
- Wilson, M., (2005), Constructing measures: An item response modeling approach, Mahwah, NJ: Lawrence Erlbaum Associates.
- Wu, H.-K., Krajcik, J. S. and Soloway, E., (2001), Promoting understanding of chemical representations: Students' use of a visualization tool in the classroom, J. Res. Sci. Teach., 38(7), 821–842.
- Yayon, M., (2011), The characterizing and diagnosis of chemical bonding knowledge. Unpublished Doctoral dissertation. Rehovot, Israel: Science teaching department. Weizmann Institute of Science.
- Zumdahl, S. S., (1989), Chemistry (2nd edn). Lexington, DC: Heath.
| This journal is © The Royal Society of Chemistry 2012 |
