Aminolysis-based surface modification of polyesters for biomedical applications
Yang
Zhu
,
Zhengwei
Mao
and
Changyou
Gao
*
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: cygao@mail.hz.zj.cn
First published on 8th November 2012
Abstract
The ester-containing polymers, in particular polyesters, are widely used as biomedical materials due to their industrial availability and, in many cases, degradability. However, the commercially available polyesters are usually absent of bioactive sites or motifs to meet the requirements of some specific biomedical uses, especially in the field of tissue engineering and regenerative medicine. Aminolysis is a convenient and versatile method to introduce –NH2 or other functional groups onto the polyester surface. Functional moieties can then be conjugated to or grafted from these active sites. Numerous studies have managed to fabricate a series of functional surfaces on various types and forms of polyesters. They are capable of improving cell adhesion, proliferation and cellular functions, domination of stem cell differentiation and isolation of certain subgroup of cells, demonstrating the versatility of the aminolysis-based polyester surface modification in biomedical applications. The mechanism and kinetics of aminolysis reaction, as well as its subsequent influence on materials properties are discussed in this review. The successive functionalization strategies and derivative applications of the aminolyzed surfaces are introduced. Finally, the review concludes with current challenges and future perspectives.
 Yang Zhu | Yang Zhu received his M.S. degree in polymer materials at Zhejiang University. He studied the fabrication of gradient biological cues based on aminolysis of polyester membranes and the gradient differentiation of bone marrow stem cells (BMSCs). He now works at McGowan Institute of Regenerative Medicine as a PhD student. |
 Changyou Gao | Changyou Gao is currently a professor of material science at Zhejiang University, a winner for the National Science Fund for Distinguished Young Scholars of China, and a Cheung Kong Scholar of Ministry of Education of China. He obtained his PhD in polymer chemistry and physics in 1996 under the supervision of Prof. Jiacong Shen at Jilin University, China. His research interests include self-assembled microcapsules, nano and colloid biomaterials and their interaction with cells, and biomaterials for tissue regeneration and cell migration. |
1. Introduction
A cascade of surface interactions, including water shell formation, protein adsorption and cell adhesion, occur when biomaterials contact with biological systems, e.g. blood flowing through a hemodialysis device, artificial teeth being implanted and damaged tissue being replaced with tissue engineering scaffolds.1 Since these interactions are closely related to biocompatibility and functions of biomaterials, the surface topography, chemical composition and biological cues are critical in determining the performances of biomaterials.2,3Polyesters, such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA) and poly(ε-caprolactone) (PCL), have been widely studied and adopted in tissue engineering, regenerative medicine and drug delivery, etc. for their excellent biocompatible, biodegradable and good processing properties.4,5 However, the utility of unmodified polyesters is often limited as a result of low hydrophilicity and absence of cell-recognition sites on the surface.6–8 A variety of methods, such as surface coating,9 layer-by-layer assembly,10 plasma treatment,11 laser treatment,12 UV irradiation,13 hydrolysis14 and aminolysis,15 have been developed to physically or chemically modify the polyester surfaces. Bioactive molecules, including peptides, proteins, and polysaccharides, can be further immobilized onto the polyester surfaces to create highly bioactive materials.4,16,17
Utilizing the classical principle of reaction between small primary diamines and esters, our group has developed an innovative aminolysis technique to modify the polyester surfaces with good effectiveness and versatility.15,18–21 Falling into the category of wet chemical reactions, the aminolysis is usually characterized by attacking on the backbone ester bonds by small diamine molecules at the interface between the diamine solution and bulk polyester material, endowing the polyester surface with amino (–NH2) and hydroxyl groups (–OH). The introduced –NH2 and –OH groups lay the foundation for subsequent conjugation of bioactive molecules. Compared to other surface modification methods, such as plasma or strong oxidation, the aminolysis has a clearer mechanism and predictable products. By adjusting the reaction parameters, the reaction rate and –NH2 density can be accurately tuned. In addition, as a method based on wet chemistry, the aminolysis is suitable for modifying the interior of complex structures like 3D scaffolds. Thanks to these advantages, the aminolysis has been extensively studied and used in surface modification of polyester biomaterials in various forms (Table 1).
| Polymers | Form of materials |
|---|---|
| PET | Fiber,27,35 membrane26 |
| PCL, PCL-PEG-PCL | Membrane,15,21,28,36,38,41,43,44,46 fiber,28 scaffold,37,45 particle42 |
| PLA, PLLA | Membrane,10,20,40 particle,19 scaffold39 |
| PLGA | Membrane,32 scaffold58 |
| Polyurethane (PU) | Scaffold18 |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) | Membrane33 |
This review summarizes the reaction mechanism and kinetics of aminolysis on the polyester surface. Its influence on the material properties shall be discussed and finally various applications in the biomedical field shall be introduced.
2. Mechanism and reaction kinetics
2.1 Mechanism of aminolysis
Aminolysis of small ester molecules was studied and reported over 50 years ago.22–24 Combining their own results and those from previous studies, Bunnett et al.24 proposed the reaction mechanism, as shown in Scheme 1. The whole aminolysis reaction is viewed as nucleophilic substitution at the carbonyl carbon, and corresponding evidence supports the assertion of tetrahedral intermediate formation.24 In this mechanism, a series of equilibrium steps are followed by a slow step featuring the lost of an alkoxy group. In addition, the overall reaction has been confirmed as base-catalyzed. Since the nature of aminolysis is the attack on the carbonyl carbon in the ester bond by amines, the roles of R′ and R′′ are less important.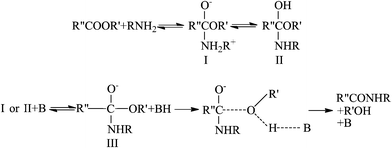 | ||
| Scheme 1 The mechanism of the aminolysis reaction of small molecules. R, R′ and R′′ represent alkyl groups and B represents a base molecule. Modified from the illustration of Bunnett et al.24 Reprinted with permission from ref. 24. Copyright 1960, American Chemical Society. | ||
In the aminolysis reaction with polyesters, the segments of polymer backbones can be viewed as R′ and R′′. The aminolysis reaction results in cleavage of ester bonds and simultaneous generation of amide bonds, exemplified here by the aminolysis of PCL (Scheme 2).25 Recently, we found that the rate of aminolysis reaction on the surface of PCL membrane is faster in basic solution than in acidic and neutral solutions (base catalyzation), indicating that the reaction mechanism of aminolysis with polyesters is essentially the same as the small esters.25
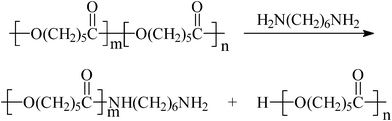 | ||
| Scheme 2 Aminolysis reaction of PCL with 1,6-hexanediamine.25 Reprinted with permission from ref. 25. Copyright 2012, Springer. | ||
Due to the intrinsic mechanism of aminolysis reaction, erosion of the polyester surface inevitably takes place, assisted by the invasion of reagents into a certain depth of the polyester surface. This phenomenon was confirmed by comparing the surface density of –NH2 groups determined by X-ray photoelectron spectroscopy (XPS) and a ninhydrin assay.25 The surface –NH2 group density given by the ninhydrin assay was far larger than that determined with XPS.25 Since XPS merely reaches ~10 nm depth of the surface, apparently –NH2 groups distribute deeper than 10 nm. Our group15 and Avadanei et al.26 independently reported that the –NH2 groups are able to reach deeper than 10 μm.
2.2 Influence of reagents
A polyester aminolysis reaction system typically includes a polyester bulk material and a diamine solution.15,19,27,28 For a certain kind of polyester material, the kinetics of aminolysis shall not be largely influenced by the forms of the material as long as the accessibility of small amine molecules is similar. Therefore, the membrane should be a good model for the kinetics analysis. In principle, it does not have to be a diamine, such as 1,6-hexanediamine. Those molecules bearing one or more than two nucleophilic –NH2 groups can be similarly used in the aminolysis as well. Secondary amines can also participate in the aminolysis, but their reactivity is usually negligible compared to the primary amines (–NH2). Diamine molecules are favorable due to the following reasons. 1) Two –NH2 groups offer the possibility that after one –NH2 group attacks one ester bond of the polyester, the other one can act as a potential anchoring site of bioactive molecules. Besides, the tethered –NH2 groups can be easily converted into other functional groups, such as –COOH, –SH, and –CHO.29 2) They are simpler in stoichiometry than polyamines in terms of both reactants and products.Since the aminolysis occurs on the interface between polyester and the amine solution, it is greatly influenced by the possibility of contact between diamine molecules and the carbonyl carbons in the ester bonds. Bech et al.27 compared the reactivity of four linear diamines possessing different molar mass with poly(ethylene terephthalate) (PET) fibers. The grafted amount of diamines decreased as their molar mass increased when the other parameters were kept constant.27 We found a similar influence pattern of molecular weight of diamines in the aminolysis reaction of PCL membranes.25 This is attributed to the less steric hindrance and better penetration ability of shorter diamines, favoring the reaction.
On the other hand, the solvent has a significant impact on the aminolysis reaction as well. Bech et al. found that aminolysis of PET fibers with 1,2-ethanediamine was faster in methanol than in water.27 Our results showed that the aminolysis rates of 1,6-hexanedimine with PCL membrane in different solvents are in the sequential order: methanol > ethanol > isopropanol > water.25 Basically, the better solvation of diamine molecules and wetting of the hydrophobic polyesters are beneficial to the contact of diamines and PCL molecules and, thereby, the aminolysis reaction.
Besides the factors mentioned above, there is also a difference in reactivity between different polyesters. For example, aminolysis of poly(L-lactic acid) (PLLA) membrane in 0.52 mol L−1 1,6-hexanedimine/n-propanol solution at 50 °C for 10 min resulted in 2.3 × 10−7 mol cm−2 surface –NH2 group density,30 while a similar density (2.0 × 10−7 mol cm−2) of –NH2 groups was obtained on PCL membrane surface after aminolysis in 0.43 mol L−1 1,6-hexanedimine/n-propanol solution at 30 °C for 10 min. Taking the effects of diamine concentration and reaction temperature (which will be discussed in the following paragraphs) into account, the aminolysis of PLLA membrane is slower than that of PCL membrane. Theoretically, the surface density of ester bonds is higher on the PLLA membrane surface due to the shorter repeat units, thus the aminolysis of PLLA should be faster. This might be attributed to the difference of the crystallinity of the polyester materials: PLLA is highly crystallized and its segments are more compactly packed, which more severely hinders the contact between diamine molecules and ester bonds than those of PCL. In another experiment we conducted, the aminolysis rate of sufficiently crystallized (annealed) PCL membranes was found slower than the randomly organized (quenched) counterparts. Besides the crystallinity, the morphology of the polyester substrate affects the aminolysis reaction rate too. Compared to the solvent-cast PCL membranes the nanofiber mats are aminolyzed at a much slower rate.25,28 It is probably due to the air trapped in the matrices, impeding the wetting and penetration of the diamine solution. Indeed, a smoother surface (the surface contact with the glass mold during casting) of the PCL membrane has a slightly higher (1.3 folds) –NH2 density than a relatively rough surface (the surface towards air during casting) after being aminolyzed in 1,6-hexanedimine/isopropanol solution.25
2.3 Reaction kinetics
Different from aminolysis of the small esters, aminolysis of polyesters is an interfacial reaction, resulting in surface erosion. In diamine aminolysis the surface –NH2 density increases with reaction time and reaches a plateau as the surface ester bonds are consumed and the balance between anchoring new diamine molecules and surface erosion is established.25 At the initial stage of aminolysis, the erosion is not significant and the reaction rate almost keeps constant, which allows convenient analysis of aminolysis kinetics. Bech et al. aminolyzed PET fibers with 1,2-ethanediamine at various temperature and showed that heating accelerates the aminolysis. The activation energy was determined to be 59 kJ mol−1.27 We also studied the influence of temperature on the aminolysis of PCL membrane by 1,6-hexanediamine in isopropanol and determined an activation energy of 54.5 kJ mol−1. Within a certain period of time, the reaction rate obeys a positively linear relationship with the diamine concentration (Fig. 1).25 Thus, the reaction rate of aminolysis can be easily tuned by temperature and diamine concentration, offering a fine control over the surface –NH2 density and subsequently the density of immobilized biomolecules.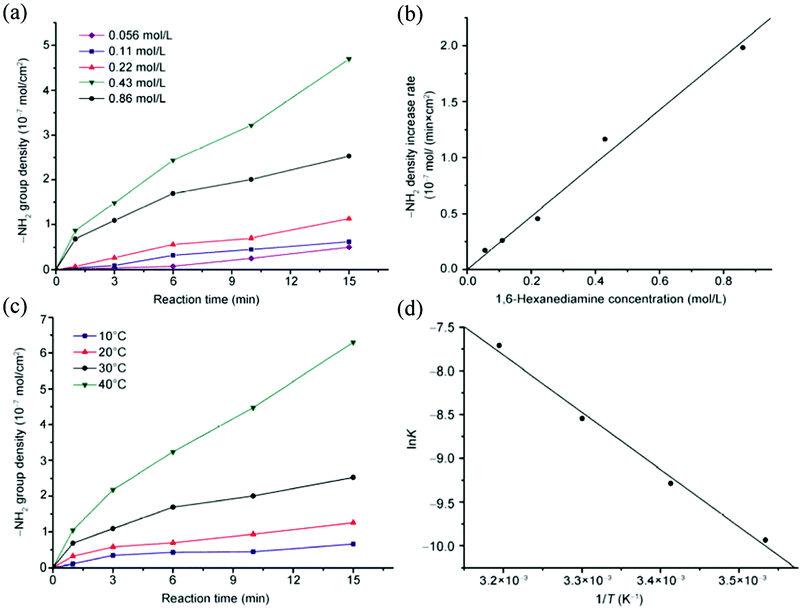 | ||
| Fig. 1 (a,c) –NH2 density as a function of reaction time with (a) different 1,6-hexanediamine concentration at 30 °C, and (c) different temperature at a 1,6-hexanediamine concentration of 0.43 mol L−1. (b) –NH2 density increase per unit time obtained from (a) as a function of 1,6-hexanediamine concentration, and (d) lnk vs. 1/T calculated from (c).25 Reprinted with permission from ref. 25. Copyright 2012, Springer. | ||
2.4 Stability of the aminolyzed surface
Indicated by ninhydrin assay, the surface density of –NH2 groups reduced slowly when the aminolyzed PCL membranes were stored in air at 37 °C.25 By contrast, the density of –NH2 groups kept almost constant when the membranes were stored in air at −80 °C, which is below the Tg of PCL (−60 °C). On the other hand, the loss of surface –NH2 groups are even faster in phosphate buffered saline (PBS).25 This is attributed to the restructuring of the PCL membrane surface, which is similar to the phenomenon found on the surface of plasma-treated polytetrafluoroethylene (PTFE) and low-density polyethylene (LDPE).31 Basically, the PCL segments on the surface will undergo rearrangement to reduce the surface energy, and the –NH2 groups tend to move away from surface and get buried into the PCL matrix. This fact suggests that the aminolyzed polyester materials should be used immediately (for example, further modification) or stored at a very low temperature to freeze the segment mobility.3. Influence on material properties
3.1 Surface properties
The aminolysis cleaves hydrophobic ester bonds and replaces them with relative hydrophilic amide bonds and –OH groups. In the most commonly adopted aminolysis system consisting of diamines, one of the two –NH2 groups reacts with the carbonyl carbon and the other one is left free on the surface. All of these polar groups contribute to the increase of surface hydrophilicity of polyester materials.30,32,33 It is worth mentioning that pKa of –NH2 groups on PCL surface is smaller than 5, which is 4–5 units smaller than that in solution,25 probably due to the still low surface hydrophilicity.34Weight loss was recorded by a quartz crystal microbalance with dissipation (QCM-D) during PCL aminolysis and a decrease in fiber diameter was observed in PET aminolysis, which is solid evidence of the erosive effect of the aminolysis, especially on surface.25,27 As a result, the surface morphology may change. However, since the erosive effect is dependent on types of the polyesters and reaction conditions, the alternation of the surface morphology varies from system to system. For example, the surface morphology of PCL membranes does not significantly change at mild aminolysis conditions,25 while the surface roughness of PLLA membranes significantly increases after treatment.30 Morphology change featured with increased roughness was also observed on aminolyzed PET fibers with 1,2-ethanediamines.27 Avadanei et al. suggested that the aminolysis advances layer by layer on the PET membrane surface. As the amine molecules are accumulated deeper from the membrane surface, the delaminated layer becomes thicker and the uncovered surface becomes more uneven.26
Due to the erosive nature of the reaction, the aminolysis not only causes immediate surface degradation, but also compromises surface elastic modulus. Determined by nanoindentation, the surface modulus of the aminolyzed PCL membranes was significantly reduced.25
3.2 Bulk properties
Since the aminolysis has a great influence on the polyester surface properties, its influence on the bulk properties depends on the ratio between the “surface” (where diamines can reach and react) and bulk. For those extremely thin fibers and membranes, as well as scaffolds with high porosity and connectivity, the impact of aminolysis on the bulk properties is also significant.As the polyester molecules are cleaved by aminolysis, the average molecular weight of the polyesters reduces along with the prolonging of aminolysis time, accompanied by broadening of the distribution.25,35 Reduction of the molecular weight weakens the mechanical strength of the bulk materials, as listed in Table 2.35,36 However, the bulk mechanical properties do not decrease significantly in all cases. For example, aminolysis of the PCL membranes does not alter the Young's modulus and yield strength.25,37 This fact indicates that the influence of aminolysis on the bulk mechanical strength varies among different types of polyesters and different formulations. Therefore, it is still necessary to determine whether the mechanical strength is compromised in the circumstance where the mechanical properties are of great importance.
| Aminolysis time (h) | M v (g mol−1) | Relative breaking load (%) |
|---|---|---|
| 0 | 46894 | 100 |
| 4 | 41309 | 100 |
| 8 | 38485 | 91 |
| 16 | 34365 | 80 |
| 48 | 32045 | 79 |
| 96 | 26338 | 59 |
| 144 | 21082 | 43 |
| 168 | 19834 | 44 |
| 240 | 12315 | — |
| 336 | 6746 | — |
4. Applications
Thanks to its extraordinary controllability, versatility and convenience, aminolysis has been widely adopted in polyester surface modification for biomedical applications. Aminolysis introduces functional groups that function as reactive sites for the successive immobilization of various bioactive molecules. Extensive studies have demonstrated that these attached molecules maintain their functions and play important roles in modulating cellular behaviors, such as adhesion, proliferation and differentiation.4.1 Application to various polyesters
De Luca et al. found that the attachment and proliferation of Schwann cells were both improved on the aminolyzed PCL membranes compared to the pristine ones due to the increased hydrophilicity.38 Subsequent immobilization of bioactive molecules is very important to obtain functionalized polyester surfaces. Our group utilized the surface-introduced –NH2 groups to increase the hydrophilicity of PLLA, endowing the scaffold surface with appropriate properties for layer-by-layer assembly of polysaccharide and collagen.39 The multilayer-modified substrate is feasible for the attachment and proliferation of chondrocytes.Compared to the non-covalent modification of aminolyzed surfaces, the covalent immobilization of bioactive molecules is more often used and thus is focused on in the following sections.
| Crosslinking agent | Abbreviation |
|---|---|
| a A water-soluble, sulfo-NHS ester analog of this cross-linking agent is also available. b A long-chain sulfo-NHS ester analog is also available. c A long-chain analog is also available. | |
| (a) Reagents to crosslink –NH2 and –COOH | |
| 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide | EDC |
| 1-Cyclohexyl-3-(2-morpholinoethyl) carbodiimide | CMC |
| Dicyclohexyl carbodiimide | DCC |
| Diisopropyl carbodiimide | DIC |
| N-Ethyl-3-phenylisoxazolium-3′-sulfonate | Woodward's reagent K |
| N,N′-Carbonyldiimidazole | CDI |
| 4-(p-Azidosalicylamido)butylamine | ASBA |
| (b) Reagents to crosslink –NH2 and –NH2 | |
| Glutaraldehyde | GA |
| Formaldehyde | n/a |
| Dithio bis(succinimidylpropionate) | DSP |
| 3,3′-Dithio bis(sulfosuccinimidylpropionate) | DTSSP |
| Disuccinimidyl suberate | DSS |
| bis(Sulfosuccinimidyl)suberate | BS3 |
| Disuccinimidyl tartarate | DSTa |
| bis[2-(Succinimidyloxycarbonyloxy)ethyl] sulfone | BSOCOESa |
| Ethylene glycol bis(succinimidylsuccinate) | EGSa |
| Disuccinimidyl glutarate | DSG |
| N,N′-Disuccinimidyl carbonate | DSC |
| Dimethyl adipimidate | DMA |
| Dimethyl pimelimidate | DMP |
| Dimethyl suberimidate | DMS |
| Dimethyl 3,3′-dithiobispropionimidate | DTBP |
| N-Hydroxysuccinimidyl-4-azidosalicylic acid | NHS-ASAa,b |
| Sulfosuccinimidyl-2-(p-azido-salicylamido)ethyl-1,3′-dithiopropionate | SASD |
| N-Hydroxysuccinimidyl-4-azidobenzoate | HSABa |
| N-Succinimidyl-6-(4′-azido-2′-nitrophenylamino)hexanoate | SANPAHa |
| N-5-Azido-2-nitrobenzoyloxysuccinimide | ANB-NOS |
| Sulfosuccinimidyl-2-(m-azido-o-nitrobenzamido)-ethyl-1,3′-dithiopropionate | SAND |
| N-Succinimidyl(4-azidophenyl)-1,3′-dithiopropionate | SADPa |
| Sulfosuccinimidyl-4-(p-azidophenyl)butyrate | Sulfo-SAPB |
| Sulfosuccinimidyl-2-(7-azido-5-methyl coumarin-3-acetamide)ethyl-1,3′-dithiopropionate | SAED |
| Sulfosuccinimidyl-7-azido-4-methylcoumarin-3-acetate | Sulfo-SAMCA |
| p-Nitrophenyl diazopyruvate | pNPDP |
| (c) Reagents to crosslink –NH2 and –SH | |
| N-Succinimidyl 3-(2-pyridyldithio)propionate | SPDPb,c |
| 4-Succinimidyloxycarbonyl-α-methyl-α-(2-pyridyldithio)toluene | SMPTb |
| Succinimidyl 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate | SMCCa |
| m-Maleimidobenzoyl-N-hydroxysuccinimide ester | MBSa |
| N-Succinimidyl(4-iodoacetyl)-aminobenzoate | SIABa |
| Succinimidyl 4-(p-maleimidophenyl)butyrate | SMPBa |
| N-γ-Maleimidobutyryl-oxysuccinimide ester | GMBSa |
| Succinimidyl 6-[6-(((iodoacetyl)amino)-hexanoyl)amino]hexanoate | SIAXX |
| Succinimidyl 6-[(iodoacetyl)-amino]hexanoate | SIAX |
| Succinimidyl 6-((((4-(iodoacetyl)amino)methyl)-cyclohexane-1-carbonylamino)hexanoate | SIACX |
| Succinimidyl 4-((iodoacetyl)amino)methyl-cyclohexane-1-carboxylate | SIAC |
| p-Nitrophenyl iodoacetate | NPIA |
| 1-(p-Azidosalicylamido)-4-(iodoacetamido)butane | ASIB |
| N-[4-(p-azidosalicylamido)butyl]-3′-(2′-pyridyldithio)propionamide | APDP |
| (d) Reagents to crosslink –NH2 and –OH | |
| N,N′-Carbonyldiimidazole | CDI |
| N,N′-Disuccinimidyl carbonate | DSC |
| Bioactive molecule | Crosslinking agent |
|---|---|
| a Sulfo succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate. | |
| (a) Proteins | |
| Collagen | GA,15,18,19,30,36,40 DSC,28 EDC/NHS37 |
| Gelatin | GA15,18,30 |
| Fibronectin | DMP41 |
| Fibrinogen | DMP41 |
| BSA | EDC42 |
| CD105 antibody | EDC42 |
| (b) Polysaccharides | |
| Chitosan | GA,15,18,30 DSC28 |
| Chondroitin sulfate | EDC/NHS37 |
| Heparin | EDC43 |
| (c) Peptides | |
| RGD | EDC/NHS,44 DSC,28 sulfo-SMCCa45 |
| IKVAV | EDC/NHS44 |
| YIGSR | EDC/NHS44 |
On the other hand, initiators for surface-initiated radical polymerization can be anchored via the –NH2 groups and then polymers can be synthesized in situ on the surface, achieving various functions. This “grafting from” strategy has been adopted because it can also provide polymers with a much higher density of functional groups than that of the –NH2 groups introduced by aminolysis, enabling higher efficiency of attaching bioactive molecules. Xu et al. covalently immobilized atom transfer radical polymerization (ATRP) initiator 2-bromoisobutyryl bromide (BIBB) onto aminolyzed PCL membranes. A high density of –COOH groups was introduced subsequently by surface ATRP of sodium methacrylate, followed by gelatin coupling in order to improve cell adhesion (Scheme 3).20 In another study, zwitterionic poly(3-dimethyl(methacryloyloxyethyl) ammoniumpropane sulfonate) brushes were grafted via the surface-initiated ATRP on the PCL membranes for enhancing hemocompatibility.21
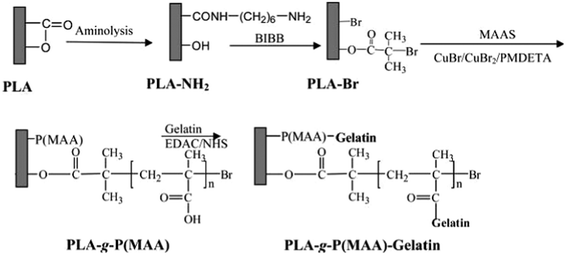 | ||
| Scheme 3 A schematic diagram illustrating the reaction of immobilizing 2-bromoisobutyrate bromide (BIBB) on the surface of aminolyzed PLA film, surface-initiated ATRP of methacrylic acid (MAA) to produce the PLA-g-P(MAA) surface, and gelatin immobilization on the PLA-g-P(MAA) surface to produce the PLA-g-P(MAA)-gelatin surface.20 Reprinted with permission from ref. 20. Copyright 2011, American Chemical Society. | ||
4.2 Cell functions
Cell behaviors, such as adhesion, proliferation, migration and differentiation, can be effectively modulated by the surface properties of biomaterials especially the surface-tethered bioactive molecules.46–57 In order to impose desired influences on cells on the polyester substrates, corresponding bioactive molecules have been covalently attached to the aminolyzed surfaces and many positive results have been achieved.Improvement of the cytocompatibility of polyester materials is the most commonly seen application of the aminolysis-based surface modification approaches, which is usually reflected by the enhanced cell adhesion and proliferation. ECM proteins and their derivatives, such as peptides and antibodies, have been used for this purpose. Our group is among the earliest to work on this strategy. The 1,6-hexanediamine/isopropanol system has been used to introduce –NH2 groups on the solvent-cast PCL membranes, and gelatin, chitosan and collagen are subsequently anchored via glutaraldehyde (GA) coupling, respectively. Adhesion, proliferation and von Willebrand factor (vWF) secretion of human endothelial cells (human ECs) are all improved on the biomacromolecules-modified PCL membranes.10 Similar strategies have been adopted to improve the biocompatibility of PLLA membranes30 and polyurethane (PU) scaffolds.18 Hong et al. modified PLA microspheres with low and high surface density of –NH2 groups by controlling the aminolysis time in 1,6-hexanediamine/isopropanol solution.19 Collagen was subsequently coated via GA coupling and physical entanglement. Greater amount of collagen was immobilized on the microspheres with a higher density of –NH2. Chondrocytes could adhere and proliferate on the microspheres, especially on those with higher collagen density. Xu et al. grafted high density gelatin on a PLA surface by combining the aminolysis and ATRP techniques. Initiator BIBB was firstly attached to –NH2 groups on the aminolyzed PLA surface, followed by methacrylic acid (MAA) polymerization. Using the introduced –COOH groups in a large amount as the reactive sites, gelatin molecules were immobilized. In vitro culture of 3T3 fibroblasts revealed that the cytocompatibilty of the materials is significantly improved.20 The selective cell adhesion, i.e. cell sorting, is also achieved via special surface modification on the aminolyzed materials. Balmayor et al. fabricated magnetite-PCL (Fe3O4–PCL) microparticles and immobilized CD105 antibody on their surface through aminolysis and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)-mediated reaction. Taking advantage of the specific binding between CD105 antibody and its antigen expressed on the cell membrane of human adipose-derived stem cells (hASCs), the hASCs were isolated from the mixture of cells (Fig. 2).42
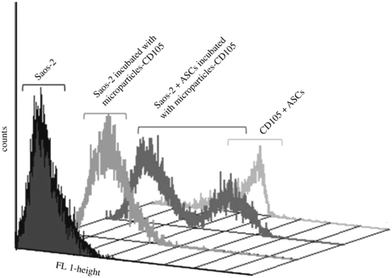 | ||
Fig. 2 Flow-cytometry analysis of CD105+ cells (ASCs) isolated from a heterogeneous cell mixture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Saos-2:ASCs) using amino-functionalized magnetic microparticles previously coupled with anti-CD105 antibody.42 Reprinted with permission from ref. 42. Copyright 2011, The Royal Society. 1, Saos-2:ASCs) using amino-functionalized magnetic microparticles previously coupled with anti-CD105 antibody.42 Reprinted with permission from ref. 42. Copyright 2011, The Royal Society. | ||
In addition to the large extracellular proteins and polysaccharides, short peptides are also employed to improve the cytocompatibility of polyester materials. Mattanavee et al. covalently immobilized RGD-containing peptides on the aminolyzed electrospun PCL matrixes, which promote cell adhesion and proliferation.28 Zhang et al. also conjugated the RGD-containing short peptides on the aminolyzed PCL scaffolds via sulfosuccinimidyl-trans-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (sulfo-SMCC). Rat bone mesenchymal stem cells (rBMSCs) displayed improved attachment and distribution on the RGD-modified PCL scaffolds. Integrin-mediated signal transduction FAK-PI3K-Akt pathway is significantly up-regulated by RGD and thereby the increase of cell survival and growth.45 Santiago et al. attached laminin-derived peptide sequences IKVAV, RGD, and YIGSR onto the aminolyzed PCL membranes, respectively, and found that IKVAV is more effective on improving adhesion of hASCs.44
In contrast to improving cell adhesion on the polyester surface, some surfaces are designed for reducing cell adhesion, which have specific applications. Jiang et al. grafted zwitterionic poly(3-dimethyl(methacryloyloxyethyl) ammoniumpropane sulfonate) (PDMAPS) onto the aminolyzed PCL membranes by ATRP. Significantly lower platelet and blood cell adhesion is achieved on the PDMAPS-modified surfaces, and this antifouling effect is dependent on the PDMAPS coverage (Fig. 3).21
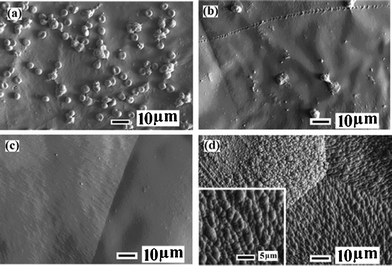 | ||
| Fig. 3 SEM images of (a) pristine PCL, (b) PCL-g-P(DMAPS)1, (c) PCL-g-P(DMAPS)2 and (d) PCL-g-P(DMAPS)3 (inset: enlarged view) surfaces after contact with fresh whole blood for 30 min. Reaction time for PCL-g-P(DMAPS)1, 2, 3 is 0.5 h, 1 h, 2 h, respectively.21 Reprinted with permission from ref. 21. Copyright 2011, American Chemical Society. | ||
Moreover, other functional molecules are used to modify the aminolyzed polyesters to adjust cell phenotypes and specific cell functions too. Gumusderelioglu et al. covalently grafted heparin onto aminolyzed PCL membranes. MC3T3-E1 preosteoblasts displayed stimulated alkaline phosphatase and osteocalcin secretion, as well as cell proliferation on the heparin-modified PCL.43 Chang et al. treated PCL scaffolds with 1,6-hexanediamine solution and subsequently grafted type II collagen and chondroitin sulfate. Enhanced expression of collagen and glycosaminoglycans (GAGs) was observed in the modified PCL matrices (Fig. 4).37
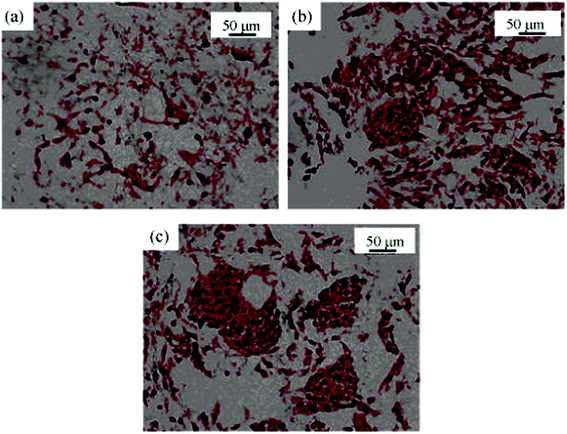 | ||
| Fig. 4 Histochemical images (Safranin-O staining for GAGs, ×200) of cartilage tissue in (a) the control PCL scaffold; (b) PCL-g-COL scaffold; (c) PCL-g-COL-g-CS scaffold. Cell seeding density is 2 × 106 cells per scaffold. Culture time is 2 weeks.37 Reprinted with permission from ref. 37. Copyright 2009, John Wiley and Sons. | ||
Rohman et al. fabricated growth factor-releasing surfaces. They first functionalized PLGA scaffolds with diamino-terminated poly(ethylene glycol) (diamino-PEG) and grafted heparin to the free –NH2 groups using EDC. The affinity between heparin and growth factors is utilized to immobilize and then perform sustained release of transforming growth factor beta (TGF-β).58 The aminolysis-based surface modification could also be used to increase gene transfection efficiency on a polyester surface. Subsequent to aminolysis-facilitated surface-initiated ATRP, Xu’s group adsorbed59 and covalently attached60 gelatin onto PCL membranes. The gelatin molecules not only promote cell adhesion, but also adsorb plasmid enhanced green fluorescent protein (pEGFP)/polyethyleneimine (PEI) complexes due to charge attraction, resulting in higher cell density and gene transfection efficiency.
Some studies listed above reported that the surface-tethered bioactive molecules are able to control cell behaviors in a density-dependent manner. Given that the density of the bioactive molecules can be modulated by the density of the underlying –NH2 groups, it is possible to fabricate gradient surfaces with a gradually changing density of functional molecules and to achieve spatial regulation over the cell behaviors. Employing an injection method, the –NH2 density gradients with diverse slopes are constructed and further transferred into collagen gradients.40 The attachment and spreading of chondrocytes are enhanced, along with the increase of collagen density (Fig. 5).40 The gradient surfaces are good models for studying the cells-materials interactions, which are critical in tissue regeneration and other physiological responses. Based on this paradigm, our group is now working on the directional migration and spatial differentiation of rBMSCs on the gradient materials.
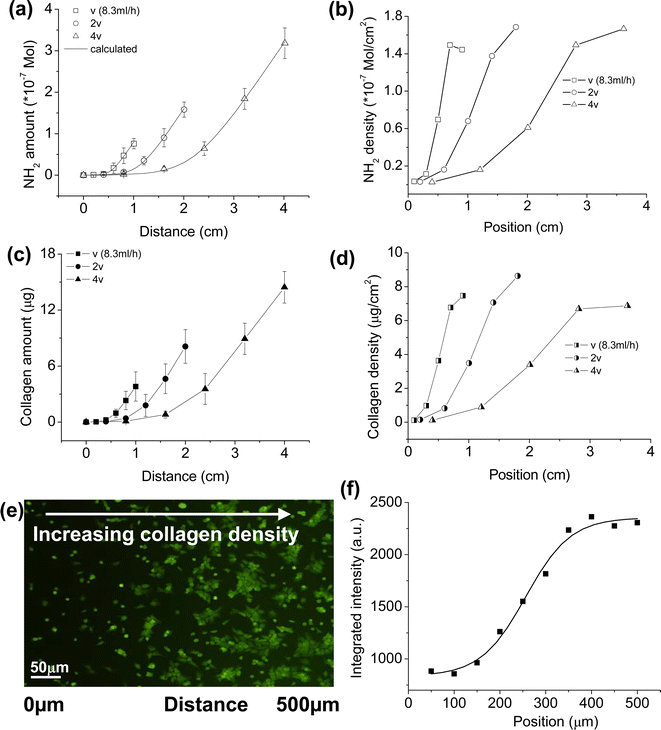 | ||
| Fig. 5 (a) Total –NH2 and (c) collagen amounts on a PLLA gradient surface as a function of distance, respectively. (b) –NH2 and (d) collagen densities calculated from (a) and (c), respectively, as a function of position. Here the whole gradient surfaces are regarded as five consecutive segments. The total –NH2 content of every segment could be obtained from experimental results (a) and then the average –NH2 density of every segment is calculated, as shown in (b). Position in (b) is the midpoint of every segment. (d) is calculated from (c) similarly. (e) A fluorescent image to show the chondrocyte distribution and morphology controlled by the collagen gradient. The image was taken after cell seeding for 24 h with a seeding density of 10 × 104 mL−1. (f) Integrated fluorescence intensity from (e) within a distance of 50 μm as a function of position.40 Reprinted with permission from ref. 40. Copyright 2008, Elsevier. | ||
5. Conclusions and perspectives
Thanks to their good biocompatibility, biodegradability and processability, polyesters and their derivatives have been extensively adopted in various biomedical applications. Aminolysis has been demonstrated as a convenient, effective and versatile method for introducing –NH2 and other functional groups to the surface of bioinert polyester materials and further conjugating bioactive molecules. Based upon aminolysis, a series of surface modification techniques have been developed to improve the bioactivity of polyesters.Although the reaction mechanism and kinetics of aminolysis, influences of aminolysis on surface and bulk properties, methods of conjugating bioactive molecules and their utility in biomedical research and applications have been clarified so far, challenges still remain to thoroughly understand aminolysis and to enrich the subsequent surface modification methods, and finally expand their applications, some of which are summarized below. 1) Determination of the distribution of introduced functional groups (–NH2 groups in diamine aminolysis). Depth distribution of the aminolysis products, such as –NH2 groups, is characterized and even macroscopically shown. Yet under micrometer or even smaller scale the distribution pattern would be possibly different due to the heterogeneity of local material properties, such as crystallinity, leading to fluctuation of grafting amount and thereby cell behaviors. 2) Preservation of the activity of attached biomolecules. Smaller molecules, such as peptides, have a great potential to maintain their structure and particular functions, but proteins (especially growth factors) may undergo structural change during and after attachment, compromising their functionality. Introduction of a spacer, improvement of surface hydrophilicity of the polyesters and use of non-covalent immobilization methods might better retain the structure and activity of the immobilized biomacromolecules. 3) Application in surface modification of 3D scaffolds. Although aminolysis of 3D polyester scaffolds has been studied in some specific cases, there are still a lot of issues to be resolved. On one hand, infiltration of reaction solutions into the inner structure of the scaffolds through pores and channels is a complicated process, which would largely affect the distribution of attached molecules on the interior of the scaffolds. Accurate determination of the distribution of aminolysis products and careful design of scaffold structures are required. On the other hand, the much larger surface area of a 3D scaffold may result in a more significant impact on the properties of the bulk materials due to the faster and easier erosion.
Nevertheless, given the advantages of aminolysis and the series of surface modification methods developed upon it, deeper insights and more extensive biomedical applications of the aminolysis-based polyester surface modification are foreseeable.
Acknowledgements
This study is financially supported by the Natural Science Foundation of China (20934003) and the National Basic Research Program of China (2011CB606203).References
- B. Kasemo, Surf. Sci., 2002, 500, 656–677 CrossRef CAS
.
- Z. W. Ma, Z. W. Mao and C. Y. Gao, Colloids Surf., B, 2007, 60, 137–157 CrossRef CAS
.
- X. Liu, P. K. Chu and C. Ding, Mater. Sci. Eng., R, 2010, 70, 275–302 CrossRef
.
- Y. P. Jiao and F. Z. Cui, Biomed. Mater., 2007, 2, R24–R37 CrossRef CAS
.
- M. A. Woodruff and D. W. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217–1256 CrossRef CAS
.
- A. P. Zhu, M. Zhang, J. Wu and J. Shen, Biomaterials, 2002, 23, 4657–4665 CrossRef CAS
.
- K. B. Lee, K. R. Yoon, S. I. Woo and I. S. Choi, J. Pharm. Sci., 2003, 92, 933–937 CrossRef CAS
.
- T. W. Chung, M. G. Yang, D. Z. Liu, W. P. Chen, C. I. Pan and S. S. Wang, J. Biomed. Mater. Res., Part A, 2005, 72, 213–219 CrossRef
.
- R. A. Quirk, W. C. Chan, M. C. Davies, S. J. Tendler and K. M. Shakesheff, Biomaterials, 2001, 22, 865–872 CrossRef CAS
.
- Y. B. Zhu, C. Y. Gao, T. He, X. Y. Liu and J. C. Shen, Biomacromolecules, 2003, 4, 446–452 CrossRef CAS
.
- J. Yang, J. Z. Bei and S. G. Wang, Biomaterials, 2002, 23, 2607–2614 CrossRef CAS
.
- K. S. Tiaw, S. W. Goh, M. Hong, Z. Wang, B. Lan and S. H. Teoh, Biomaterials, 2005, 26, 763–769 CrossRef CAS
.
- F. Darain, W. Y. Chan and K. S. Chian, Soft Mater., 2011, 9, 64–78 CrossRef CAS
.
- J. S. Park, J. M. Kim, S. J. Lee, S. G. Lee, Y. K. Jeong, S. E. Kim and S. C. Lee, Macromol. Res., 2007, 15, 424–429 CrossRef CAS
.
- Y. B. Zhu, C. Y. Gao, X. Y. Liu and J. C. Shen, Biomacromolecules, 2002, 3, 1312–1319 CrossRef CAS
.
- U. Hersel, C. Dahmen and H. Kessler, Biomaterials, 2003, 24, 4385–4415 CrossRef CAS
.
- J. M. Goddard and J. H. Hotchkiss, Prog. Polym. Sci., 2007, 32, 698–725 CrossRef CAS
.
- Y. B. Zhu, C. Y. Gao, T. He and J. C. Shen, Biomaterials, 2004, 25, 423–430 CrossRef CAS
.
- Y. Hong, C. Y. Gao, Y. Xie, Y. H. Gong and J. C. Shen, Biomaterials, 2005, 26, 6305–6313 CrossRef CAS
.
- F. J. Xu, X. C. Yang, C. Y. Li and W. T. Yang, Macromolecules, 2011, 44, 2371–2377 CrossRef CAS
.
- H. Jiang, X. B. Wang, C. Y. Li, J. S. Li, F. J. Xu, C. Mao, W. T. Yang and J. Shen, Langmuir, 2011, 27, 11575–11581 CrossRef CAS
.
- R. L. Betts and L. P. Hammett, J. Am. Chem. Soc., 1937, 59, 1568–1572 CrossRef CAS
.
- W. H. Watanabe and L. R. Defonso, J. Am. Chem. Soc., 1956, 78, 4542–4549 CrossRef CAS
.
- J. F. Bunnett and G. T. Davis, J. Am. Chem. Soc., 1960, 82, 665–674 CrossRef CAS
.
- Y. Zhu, Z. W. Mao, H. Y. Shi and C. Y. Gao, Sci. China: Chem., 2012, 55, 2419–2427 CrossRef CAS
.
- M. Avadanei, M. Drobota, I. Stoica, E. Rusu and V. Barboiu, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5456–5467 CrossRef CAS
.
- L. Bech, T. Meylheuc, B. Lepoittevin and P. Roger, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 2172–2183 CrossRef CAS
.
- W. Mattanavee, O. Suwantong, S. Puthong, T. Bunaprasert, V. P. Hoven and P. Supaphol, ACS Appl. Mater. Interfaces, 2009, 1, 1076–1085 CAS
.
-
G. T. Hermanson, Bioconjugate Techniques. Academic Press, New York, 1996 Search PubMed
.
- Y. B. Zhu, C. Y. Gao, X. Y. Liu and J. C. Shen, Tissue Eng., 2004, 10, 53–61 CrossRef CAS
.
- R. C. Chatelier, X. M. Xie, T. R. Gengenbachand and H. J. Griesser, Langmuir, 1995, 11, 2576–2584 CrossRef CAS
.
- T. I. Croll, A. J. O'Connor, G. W. Stevens and J. J. Cooper-White, Biomacromolecules, 2004, 5, 463–473 CrossRef CAS
.
- I. Keen, P. Broota, L. Rintoul, P. Fredericks, M. Trau and L. Grondahl, Biomacromolecules, 2006, 7, 427–434 CrossRef CAS
.
- D. V. Vezenov, A. Noy, L. F. Rozsnyai and C. M. Lieber, J. Am. Chem. Soc., 1997, 119, 2006–2015 CrossRef
.
- S. A. Holmes, J. Appl. Polym. Sci., 1996, 61, 255–260 CrossRef CAS
.
- F. Causa, E. Battista, R. Della Moglie, D. Guarnieri, M. Iannone and P. A. Netti, Langmuir, 2010, 26, 9875–9884 CrossRef CAS
.
- K. Y. Chang, L. H. Hung, I. M. Chu, C. S. Ko and Y. D. Lee, J. Biomed. Mater. Res., Part A, 2010, 92, 712–723 CrossRef
.
- A. C. de Luca, G. Terenghi and S. Downes, J. Tissue Eng. Regener. Med., 2012 DOI:10.1002/term.1509
.
- Y. H. Gong, Y. B. Zhu, Y. X. Liu, Z. W. Mao, C. Y. Gao and J. C. Shen, Acta Biomater., 2007, 3, 677–685 CrossRef CAS
.
- H. P. Tan, L. Wan, J. D. Wu and C. Y. Gao, Colloids Surf., B, 2008, 67, 210–215 CrossRef CAS
.
- H. Bramfeldt and P. Vermette, J. Biomed. Mater. Res., Part A, 2009, 88, 520–530 CrossRef
.
- E. R. Balmayor, I. Pashkuleva, A. M. Frias, H. S. Azevedo and R. L. Reis, J. R. Soc. Interface, 2011, 8, 896–908 CrossRef CAS
.
- M. Gumusderelioglu, A. Karakecili and T. T. Demirtas, J. Bioact. Compat. Polym., 2011, 26, 257–269 CrossRef CAS
.
- L. Y. Santiago, R. W. Nowak, R. J. Peter and K. G. Marra, Biomaterials, 2006, 27, 2962–2969 CrossRef CAS
.
- H. Zhang, C. Y. Lin and S. J. Hollister, Biomaterials, 2009, 30, 4063–4069 CrossRef CAS
.
- V. Gribova, R. Auzely-Velty and C. Picart, Chem. Mater., 2012, 24, 854–869 CrossRef CAS
.
- S. Khan and G. Newaz, J. Biomed. Mater. Res., Part A, 2010, 93A, 1209–1224 CrossRef CAS
.
- M. Bachle and R. J. Kohal, Clin. Oral Implants Res., 2004, 15, 683–692 CrossRef
.
- J. D. Wu, Z. W. Mao and C. Y. Gao, Biomaterials, 2012, 33, 810–820 CAS
.
- A. Pulsipher and M. N. Yousaf, ChemBioChem, 2010, 11(745–753), 730 CrossRef
.
- R. H. Fu, Y. C. Wang, S. P. Liu, C. M. Huang, Y. H. Kang, C. H. Tsai, W. C. Shyu and S. Z. Lin, Cell Transplant., 2011, 20, 37–47 CrossRef
.
- E. Martinez, A. Lagunas, C. A. Mills, S. Rodriguez-Segui, M. Estevez, S. Oberhansl, J. Comelles and J. Samitier, Nanomedicine, 2009, 4, 65–82 CrossRef CAS
.
- R. Ayala, C. Zhang, D. Yang, Y. Hwang, A. Aung, S. S. Shroff, F. T. Arce, R. Lal, G. Arya and S. Varghese, Biomaterials, 2011, 32, 3700–3711 CrossRef CAS
.
- J. M. Curran, F. Pu, R. Chen and J. A. Hunt, Biomaterials, 2011, 32, 4753–4760 CrossRef CAS
.
- R. Peng, X. Yao and J. D. Ding, Biomaterials, 2011, 32, 8048–8057 CrossRef CAS
.
- E. D. Miller, K. Li, T. Kanade, L. E. Weiss, L. M. Walker and P. G. Campbell, Biomaterials, 2011, 32, 2775–2785 CrossRef CAS
.
- K. A. Kilian, B. Bugarija, B. T. Lahn and M. Mrksich, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4872–4877 CrossRef CAS
.
- G. Rohman, S. C. Baker, J. Southgate and N. R. Cameron, J. Mater. Chem., 2009, 19, 9265–9273 RSC
.
- C. Y. Li, W. Yuan, H. Jiang, J. S. Li, F. J. Xu, W. T. Yang and J. Ma, Bioconjugate Chem., 2011, 22, 1842–1851 CrossRef CAS
.
- W. Yuan, C. Y. Li, C. Zhao, C. G. Sui, W. T. Yang, F. J. Xu and J. Ma, Adv. Funct. Mater., 2012, 22, 1835–1842 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2013 |
