Synthesis of Co3O4@SnO2@C core-shell nanorods with superior reversible lithium-ion storage†
Yue
Qi
,
Hui
Zhang
*,
Ning
Du
,
Chuanxin
Zhai
and
Deren
Yang
State Key Lab of Silicon Materials and Department of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027, People's Republic of China. E-mail: meszhanghui@zju.edu.cn; Fax: 86-571-87952322; Tel: 86-571-87953190
First published on 14th August 2012
Abstract
This paper describes a facile hydrothermal and subsequent carbonization approach for the synthesis of Co3O4@SnO2@C core-shell nanorods. The as-synthesized Co3O4@SnO2@C nanorods have been applied as anode materials for lithium-ion batteries, which exhibit improved cyclic performance and enhanced power capability. Both Co3O4 and SnO2 are electrochemically active materials, and the hybridization of Co3O4 and SnO2 into an integrated core-shell nanorod structure makes them an elegant synergistic effect when participating in the lithium-ion charge-discharge process. In addition, the carbon matrix has good volume buffering effect and high electronic conductivity, which may be responsible for the improved electrochemical performance.
1 Introduction
Rechargeable lithium-ion batteries are an excellent power source for energy storage devices and have conquered the portable electronic market.1 However, the limited gravimetric capacity (e.g., 372 mAhg−1) of the commercial carbon-based anode has prompted intensive research for alternative with high reversible capacity and enhanced cycle life.2 3d transition-metal oxides (MOs) have been considered as one of the most promising candidates to replace the commercial carbon-based materials since their discovery by Tarascon et al. in 2000.3 Among them, cobalt oxides (Co3O4, CoO) exhibited the best electrochemical properties as the anode in Li-ion batteries.4–6 However, cobalt oxides still suffer from poor capacity retention due to the low conductivity and large volume swings during charge/discharge cycling.7,8 Structural and morphological modifications provide a facile, versatile and powerful approach to further improve the electrochemical properties of cobalt oxide as the anode of Li-ion batteries such as anodic capacity, cycling performance, and rate capacity.Recently, much attention has been paid to hybridize nanostructured cobalt oxides with other active/inactive materials for enhancing lithium storage performance.9–11 SnO2 has been considered as an important anode material for lithium-ion batteries due to its high theoretical specific capacity. A large number of studies have focused on the improvement of the reversible capacity and cyclability of Co3O4 and SnO2 by combining them into delicate nanostructures.12–14 Moreover, the presence of Co nanoparticles at the interface between SnO2 and Co3O4 may improve the reversibility of the reduction reaction of Li2O and further enhance the reversible capacity.14 Hence, the hybridization of Co3O4 and SnO2 into an integrated nanostructure makes them an elegant synergistic effect when participating in the lithium-ion charge-discharge process. However, stability of these Co3O4 and SnO2 nanostructures still remains an existing issue. The breaking down of these nanostructures upon cycling often causes remarkable capacity fading. In recent years, extensive work has been focused on the synthesis of MOs–C (M = Sn, Ti, Co, Fe or Ni) core-shell hybrid nanostructures by introducing carbon layer as “buffering matrixes” owing to the excellent buffering effect and high electronic conductivity of carbon.15–18 As a result, Co3O4/SnO2–C core-shell hybrid nanostructures are expected to show enhanced electrochemical performance due to the synergistic effect between SnO2 and Co3O4 as well as the buffering matrix design of electrode materials. However, to the best of our knowledge, there is no report of the synthesis of Co3O4@SnO2@C core-shell nanorods as anodes for lithium-ion batteries with enhanced cyclic performance.
Herein, we report the synthesis of Co3O4@SnO2@C core-shell nanorods through a facile hydrothermal and subsequent carbonization approach. The as-synthesized Co3O4@SnO2@C nanorods have been applied as anode materials for lithium-ion batteries, which exhibit improved cyclic performance and enhanced power capability. Both Co3O4 and SnO2 are electrochemically active materials, and the hybridization of Co3O4 and SnO2 into an integrated core-shell nanorod structure makes them an elegant synergistic effect when participating in the lithium-ion charge-discharge process. Additionally, the carbon matrix has good volume buffering effect and high electronic conductivity, which may be responsible for the improved electrochemical performance, in particular cyclability.
2 Experimental
All the chemicals were analytical grade without further purification. The fabrication process of Co3O4@SnO2@C core-shell nanorods is schematically shown in Fig. 1. It can be seen that the whole process involves in three steps: (1) synthesis of Co3O4 nanorods, (2) hydrothermal coating of SnO2 particles onto the Co3O4 nanorods, and (3) carbon coating on the Co3O4@SnO2 core-shell nanorods. The synthesis processes is detailed in the following sections. | ||
| Fig. 1 Schematic illustrating the fabrication of Co3O4@SnO2@C core-shell nanorods. | ||
2.1 Synthesis of Co3O4 nanorods
Co3O4 nanorods were prepared by a hydrothermal and subsequent annealing route. Briefly, 40 ml ethylene glycol was mixed with 25 ml concentrated ammonia hydroxide (28 wt %) and 2 ml 1 M Na2CO3 aqueous solution to form a transparent solution. After that, 10 ml 1 M Co(NO3)2 aqueous solution was dropped into the mixture solution under magnetic stirring. After vigorous stirring for 20 min, the final solution was transferred into a 100 ml Teflon-lined stainless steel autoclave, sealed, and maintained at 170 °C for 16 h in an electric oven. After the reaction was finished, the resulting solid products were centrifuged, washed with distilled water and ethanol to remove the ions possibly remaining in the final products, and then dried at 50 °C in air. Finally, the products were kept in a tube furnace at 230 °C for 3 h in air at a ramping rate of 5 °C min−1.2.2 Fabrication of Co3O4@SnO2@C core-shell nanorods
Co3O4@SnO2@C core-shell nanorods were prepared by a hydrothermal process and subsequent carbonization approach. Briefly, 0.3 g Co3O4 nanorods were dispersed in 62 ml ethanol/water (60 vol % ethanol) mixed solvents. Then 0.2 g Na2SnO3·4H2O and 1.8 g urea were added into the aforementioned suspension. After magnetic stirring for 10 min, the suspension was transferred to a 100 ml Teflon-lined stainless steel autoclave, sealed, and maintained at 170 °C for 1 h. After the reaction was finished, the resulting solid products were centrifuged, washed with distilled water and ethanol, and then redispersed in 40 ml 0.25 M aqueous glucose solution. After sonication for 20 min, the solution was transferred into a 50 ml Teflon-lined stainless steel autoclave, sealed, and maintained at 180 °C for 10 h in an electric oven. After the reaction was finished, the resulting products were centrifuged, washed with distilled water and ethanol to remove the ions possibly remaining in the final products, and then dried at 80 °C under vacuum. Finally, the products were kept in a tube furnace at 500 °C for 3 h under N2 at a ramping rate of 5 °C min−1.2.3 Characterization
The obtained samples were characterized by X-ray powder diffraction (XRD) using a Rigaku D/max-ga X-ray diffractometer with graphite monochromatized Cu-Kα radiation (λ = 1.54178 Å). The morphology and structure of the samples were examined by transmission electron microscopy (TEM, JEM-200 CX, 160 kV) and field emission scanning electron microscopy (FESEM, Hitachi S-4800) with energy-dispersive X-ray spectrometer (EDX). Thermogravimetric analysis (TGA) was tested on SDT Q600 V8.2 Build 100. The infrared (IR) spectra were measured with a Nicolet Nexus FTIR 670 spectrophotometer. The Raman spectroscopy measurement was carried out on a Labor Raman HR-800 Raman spectrometer system.2.4 Electrochemical measurements of Co3O4@SnO2@C core-shell nanorods
Electrochemical measurements were carried out using two-electrode cells with lithium metal as the counter and reference electrodes. The working electrode was composed of the active material (Co3O4@SnO2@C core-shell nanorods), conductive materials (acetylene black, AB), and binder (polyvinyldifluoride, PVDF) in a weight ratio of Co3O4@SnO2@C core-shell nanorods/AB/PVDF = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and pasted on Cu foil. The electrolyte solution was 1 M LiPF6 dissolved in a mixture of ethylene carbonate (EC), propylene carbonate (PC), and diethyl carbonate (DEC) with the volume ratio of EC/PC/DEC = 3
10, and pasted on Cu foil. The electrolyte solution was 1 M LiPF6 dissolved in a mixture of ethylene carbonate (EC), propylene carbonate (PC), and diethyl carbonate (DEC) with the volume ratio of EC/PC/DEC = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The cell assembly was performed in a glovebox filled with pure argon (99.999%) in the presence of an oxygen scavenger and a sodium drying agent. The electrode capacity was measured by a galvanostatic discharge-charge method at a current density of 200 mAg−1 in the potential range of 0.01–2.5 V at 20 °C. Cyclic voltammetry (CV) were recorded on a MSTAT4 (Arbin Instruments) system in the potential range of 0.01–2.5 V at a scan rate of 0.1 mVs−1. The electrochemical impedance spectroscopy (EIS) of the electrodes was performed on a CHI660D electrochemical workstation with an ac signal of 5 mV in amplitude and the frequency ranged from 0.01 Hz to 100 kHz. Before the EIS measurement, the electrodes were cycled for five cycles, then discharged to 2.0 V and kept until the open-circuit voltage stabilized.
1. The cell assembly was performed in a glovebox filled with pure argon (99.999%) in the presence of an oxygen scavenger and a sodium drying agent. The electrode capacity was measured by a galvanostatic discharge-charge method at a current density of 200 mAg−1 in the potential range of 0.01–2.5 V at 20 °C. Cyclic voltammetry (CV) were recorded on a MSTAT4 (Arbin Instruments) system in the potential range of 0.01–2.5 V at a scan rate of 0.1 mVs−1. The electrochemical impedance spectroscopy (EIS) of the electrodes was performed on a CHI660D electrochemical workstation with an ac signal of 5 mV in amplitude and the frequency ranged from 0.01 Hz to 100 kHz. Before the EIS measurement, the electrodes were cycled for five cycles, then discharged to 2.0 V and kept until the open-circuit voltage stabilized.
3 Results and discussion
The Co3O4 nanorods were synthesized through a hydrothermal and subsequent annealing approach. Fig. 2 shows SEM and TEM images of the as-synthesized Co(CO3)0.5(OH)0.11H2O and Co3O4 nanorods. As can be seen from the Fig. 2 a and b, the rod-like products of Co(CO3)0.5(OH)0.11H2O nanorods were uniform with smooth surface and the diameter was measured to be about 30 nm. As previously reported,13 the nanorods were characteristic of a preferentially one-dimensional growth during hydrothermal process and the dominant growth orientation was fixed to be [100]. Compared with the Co(CO3)0.5(OH)0.11H2O nanorods, Co3O4 eventually remained the rod-like shape after annealing process in the air (Fig. 2c). Coating of SnO2 nanoparticles on the surface of Co3O4 nanorods was achieved by a hydrothermal process. Fig. 2d–f shows morphological and structural characterizations of the Co3O4@SnO2 core-shell nanorods after growth of SnO2 for 1 h. The SEM (Fig. 2d) and the TEM images (Fig. 2e) clearly demonstrate that most of the SnO2 nanoparticles were uniformly deposited on the surface of the Co3O4 nanorods and the structure of the nanorods was well maintained. Fig. 2f shows the magnified TEM image of an individual Co3O4@SnO2 core-shell nanorod. It is clear that the continuous SnO2 layers consisted of SnO2 nanoparticles with a size less than 5 nm and were uniformly coated on the surface of Co3O4, resulting in the formation of hybrid core-shell nanorods.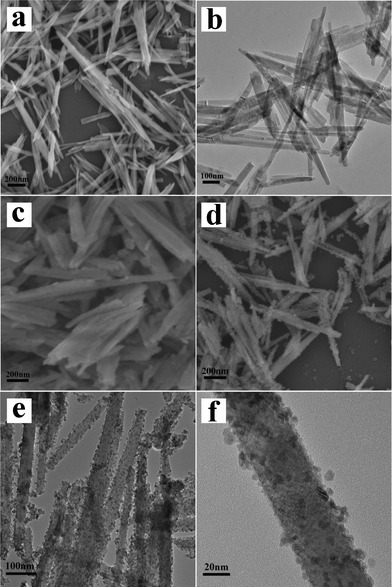 | ||
| Fig. 2 (a) SEM and (b) TEM images of Co(CO3)0.5(OH)0.11H2O nanorods. (c) SEM image of Co3O4 nanorods. (d) SEM, (e) TEM, and (f) magnified TEM images of Co3O4@SnO2 nanorods. | ||
Co3O4@SnO2@C core-shell nanorods were synthesized by a simple hydrothermal and subsequent carbonization process with glucose as a reactant. Fig. 3 shows the morphological and structural characterizations of Co3O4@SnO2@C core-shell nanorods. As can be seen, the rod-like shape was retained after coating of a carbon-rich layer (Fig. 3a) on the surface of the Co3O4@SnO2 nanorods. The magnified TEM image (Fig. 3b) of an individual Co3O4@SnO2@C nanorod indicates that the uniform coating layer is smooth and continuous and the thickness of the layer is about 5–10 nm. The Co3O4@SnO2@C nanorods can be confirmed by the selected-area electron diffraction (SAED) pattern (Fig. 3b, inset). As observed, there are three diffraction rings corresponding to the (101), (110), and (211) planes of SnO2, respectively. HRTEM was employed to further characterize the Co3O4@SnO2@C core-shell nanorods. As can be seen from Fig. 3c, there are three kinds of lattice fringes with lattice spacings of about 0.335, 0.264 and 0.176 nm, corresponding to (110), (101) and (211) planes of SnO2 nanoparticles, respectively. In addition, the thickness of the carbon layer was measured to be about 6 nm. Fig. 3d shows the EDX spectrum taken from the Co3O4@SnO2@C core-shell nanorods. Obviously, the strong peaks for O, Co, Sn, and C elements are expected from the Co3O4 core, SnO2 nanoparticles, and carbon layer, respectively, while the Cu peaks come from the sample stage used in the FESEM measurements.
 | ||
| Fig. 3 Morphological and structural characterizations of Co3O4@SnO2@C core-shell nanorods: (a) TEM image, (b) magnified TEM image, (c) HRTEM image, (d) EDX spectrum of the Co3O4@SnO2@C core-shell nanorods. The inset in (b) corresponds to SAED pattern. | ||
The crystallographic structure of the products was characterized by XRD analysis (Fig. 4a). It can be seen from the diffraction pattern a and b, the crystalline phase can be assigned to orthorhombic Co(CO3)0.5(OH)0.11H2O (JCPDS: 48-0083) and cubic Co3O4 (JCPDS: 65-3103), respectively, indicating the phase transformation during the annealing process. After coating of SnO2 nanoparticles on the surface of Co3O4 nanorods, three diffraction peaks of tetragonal SnO2 (JCPDS: 41-1445) emerged from pattern c. Compared with Co3O4@SnO2, the diffraction peaks of Co3O4@SnO2@C nanorods remain unchanged due to the amorphous nature of the carbon layer (pattern d). TGA was performed to determine the carbon content in Co3O4@SnO2@C core-shell nanorods. As shown in Fig. 4b, the initial weight loss (up to 200 °C) is due to the evaporation of physically adsorbed water, while the weight loss between 200 and 600 °C could be attributed mainly to the removal of the carbon layer. As such, the carbon content of Co3O4@SnO2@C nanorods is determined to be about 19.0% by weight. FTIR spectroscopy was employed to further investigate the carbonization process. After glucose hydrothermal process, Co3O4@SnO2 nanorods were coated with glucose-derived carbon-rich polysaccharide (GCP) layer. Subsequently, the GCP layer was fully carbonized to a carbon layer under an inert atmosphere, resulting in the formation of the Co3O4@SnO2@C nanorods. It can be seen from Fig. 4c (curve a) that the stretching vibrations at 1700 and 1625 cm−1 can be attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations, respectively, indicating aromatization of glucose during the hydrothermal treatment.19,20 After thermal carbonization process, the GCP layer from Co3O4@SnO2@GCP has been fully carbonized to a carbon layer, leading to the disappearance of C
C vibrations, respectively, indicating aromatization of glucose during the hydrothermal treatment.19,20 After thermal carbonization process, the GCP layer from Co3O4@SnO2@GCP has been fully carbonized to a carbon layer, leading to the disappearance of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations peaks, which was confirmed from the curve b. The phases of carbon and Co3O4 are further confirmed by Raman spectroscopy. In the Raman spectra of Co3O4@SnO2 and Co3O4@SnO2@C nanorods (Fig. 4d), the G band (∼1595 cm−1) corresponding to sp2-hybridized carbon, and the D band (∼1352 cm−1) originating from disordered carbon are both observed for Co3O4@SnO2@C (curve b).21 The peaks of Raman shift at 490 cm−1 and 669 cm−1 can be attributed to the F2g and A1g modes of Co3O4, respectively (curves a and b).22 These results clearly identify the existence of both carbon and Co3O4 in the as-prepared composites. All these aforementioned characterizations confirm the successful synthesis of Co3O4@SnO2@C core-shell nanorods.
O vibrations peaks, which was confirmed from the curve b. The phases of carbon and Co3O4 are further confirmed by Raman spectroscopy. In the Raman spectra of Co3O4@SnO2 and Co3O4@SnO2@C nanorods (Fig. 4d), the G band (∼1595 cm−1) corresponding to sp2-hybridized carbon, and the D band (∼1352 cm−1) originating from disordered carbon are both observed for Co3O4@SnO2@C (curve b).21 The peaks of Raman shift at 490 cm−1 and 669 cm−1 can be attributed to the F2g and A1g modes of Co3O4, respectively (curves a and b).22 These results clearly identify the existence of both carbon and Co3O4 in the as-prepared composites. All these aforementioned characterizations confirm the successful synthesis of Co3O4@SnO2@C core-shell nanorods.
 | ||
| Fig. 4 (a) XRD patterns of the Co(CO3)0.5(OH)0.11H2O, Co3O4, Co3O4@SnO2, and Co3O4@SnO2@C nanorods. (b) TGA analysis of the Co3O4@SnO2@C nanorods. (c) Infrared spectra of Co3O4@SnO2@C nanorods before and after carbonization. (d) Typical Raman spectrum of the Co3O4@SnO2 and Co3O4@SnO2@C nanorods. | ||
Electrochemical tests were conducted by using the as-synthesized core-shell nanorods as the anode and Li metal as the cathode. Fig. 5a shows the first three cyclic voltammogram (CV) curves of Co3O4@SnO2@C nanorods in the potential range of 0.0–2.5 V at a slow scan rate of 0.1 mVs−1. The CV curves are in good agreement with the previous reported SnO2 and Co3O4 anodes.10,16 It is generally accepted that the electrochemical process of SnO2 anodes can be described by the following principal reactions:
| SnO2 + 4Li → Sn +2Li2O | (1) |
| Sn + 4.4Li ↔ Li4.4Sn | (2) |
 | ||
| Fig. 5 (a) First three CV curves of the Co3O4@SnO2@C core-shell nanorods in the potential range of 0.0–2.5 V at a scan rate of 0.1 mVs−1. (b) Discharge capacity versus cycle number for the Co3O4, Co3O4@SnO2,and Co3O4@SnO2@C nanorods based anode materials at a current density of 200 mAg−1 at room temperature. (c) Nyquist plots of Co3O4@SnO2 and Co3O4@SnO2@C nanorods electrodes obtained by applying a sine wave with amplitude of 5 mV over the frequency range 100 kHz to 0.01 Hz. (d) Specific capacities of the Co3O4@SnO2@C core-shell nanorods for different discharge/charge cycles at various current densities. | ||
As for transition metal oxide Co3O4, the electrochemical reaction mechanism with Li can be described by: Co3O4 + 8Li ↔ 3Co + 4Li2O, which is fully reversible. It is noted that reversible decomposition and formation of the Li2O matrix could be electrochemically driven by the nano-sized transition metal particles formed during charged process.3,23 It is known that tin oxidation24 takes place at about 1.4 V (vs. Li+/Li) and cobalt oxidation25 commonly occurs at about 2.1 V (vs. Li+/Li). Hence, the presence of Co nanoparticles at the interface between SnO2 and Co3O4 may improve the reversibility of the reaction (eqn (1)) and further enhance the reversible capacity. In the first cycle, there is a clear reduction peak at the potential of 0.8 V, which can be attributed to the reduction of SnO2 and Co3O4, and the formation of Li2O and the SEI film.14 The peak in the cathodic process at 0.07 V is attributed to the formation of Li4.4Sn.16 In the first anodic process, three oxidation peaks at 0.57, 1.3, and 2.1 V are attributed to the de-alloying process (0.57 V), the re-oxidation of tin (1.3 V) and cobalt (2.1 V),5,18 respectively. In the second and third cycles, there are three characteristic pairs (cathodic, anodic) of current peaks at the potential of (0.07/0.57 V), (0.7/1.3 V) and (1.2/2.1 V), corresponding to the alloying/de-alloying process of Li4.4Sn, and the redox reactions of Sn/SnO2 and Co/Co3O4, respectively.
The discharge curve of the first cycle has an extended potential plateaux at about 1.0 V, followed by two sloping potentials at about 0.5 V and 0.2 V, as shown in Fig. S1†. The discharge capacities of the electrode in the 1st, 2nd, and 3rd cycles were 1697.5, 1165.9, and 1054.5 mAhg−1. Fig. 5b shows the discharge capacity versus cycle number for the Co3O4, Co3O4@SnO2,and Co3O4@SnO2@C nanorods based anode materials at a current density of 200 mAg−1 at room temperature. The Co3O4@SnO2, and Co3O4@SnO2@C nanorods show remarkably improved first reversible discharge capacities of 1292.6 and 1165.9 mAhg−1, much higher than that of Co3O4 nanorods (892.8 mAhg−1). The presence of nano-sized Co particles improved the reversibility of the reaction (eqn (1)), which may be responsible for the higher reversible capacity. The capacity of Co3O4@SnO2@C nanorods maintained at 864.6 mAhg−1 after 50 discharge/charge cycles with a capacity retention of 74.2% compared with the first reversible capacity, which was much higher than that of Co3O4@SnO2 (202.8 mAhg−1 with the capacity retention of 15.7%) and Co3O4 nanorods (182.2 mAhg−1 with the capacity retention of 20.4%). The uniform and continuous carbon buffering matrix, which can effectively alleviate volume expansion/contraction during cycling, may be responsible for the enhanced cycling performance of Co3O4@SnO2@C nanorods.26
EIS was used to understand the relevance of morphology and surface area of the Co3O4@SnO2 and Co3O4@SnO2@C electrodes with the electrochemical performance in terms of the total internal electrochemical impedances of a cell. The characteristic impedance curves (Nyquist plots) for the two electrodes are shown in Fig. 5c. In impedance spectroscopy, the high frequency semicircle is attributed to the SEI film and/or contact resistance, the semicircle in medium frequency region is assigned to the charge-transfer impedance on electrode/electrolyte interface, and the inclined line at an approximate 45° angle to the real axis corresponds to the lithium-diffusion process within electrodes.27,28 It is shown that the diameter of the semicircle in medium frequency region for the Co3O4@SnO2@C electrode is smaller than that of Co3O4@SnO2 electrode, revealing lower charge-transfer impedances. This result indicates that the charge-transfer process of the Co3O4@SnO2@C electrode was improved after the incorporation of carbon. The thickness of SEI film over active electrode affects the performance of a given electrode, since a very thick layer may prevent the effective charge transfer and diffusion process.29 The Co3O4@SnO2@C sample showed smaller high frequency semicircle, which indicated lower surface resistance and may be attributed to a thinner surface film. Due to the larger slope of inclined line in impedance spectroscopy, the lithium-diffusion process of the Co3O4@SnO2 electrode has also been improved after combination with the carbon layer electrode which leads to fast charge/discharge capability and enhanced electrochemical performance. The carbon can also keep the Co3O4@SnO2 active material stable during the charge-discharge cycling.
The Co3O4@SnO2@C core-shell nanorods were also tested for their rate capability. Fig. 5d shows the discharge capacities of the two electrodes at current rates between 0.2 and 3.2 Ag−1. A good rate capacity is observed for the Co3O4@SnO2@C nanorods, giving a capacity retention of 48.8% between 0.2 and 3.2 Ag−1. Even at a current density as high as 3.2 Ag−1, the Co3O4@SnO2@C electrode is capable of delivering a stable capacity of about 400 mAhg−1. Upon decreasing the current rate from 3.2 Ag−1 to 0.2 Ag−1, nearly 90% of the initial capacity at 0.2 Ag−1 (about 900 mAhg−1) can be recovered. The structural stability and improved conductivity of the core-shell nano-electrode materials during cycling may be responsible for the enhanced power performance.15
Conclusions
In summary, we have successfully fabricated Co3O4@SnO2@C core-shell nanorods through a facile hydrothermal and subsequent carbonization approach. The Co3O4@SnO2@C core-shell nanorods exhibited good cycling performance (∼ 860 mAhg−1 after 50 cycles at 0.2 Ag−1) and improved power rate performance (48.8% of the capacity retained from 0.2 to 3.2 Ag−1), which can be ascribed to the synergetic effect between Co3O4 and SnO2, as well as the structural stability and improved electronic conductivity of the carbon matrix.Acknowledgements
The authors would like to acknowledge financial support from 863 Project (No. 2011AA050517), NSFC (No. 51002133) and Innovation Team Project of Zhejiang Province (2009R50005).References
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587 CrossRef CAS
.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS
.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496 CrossRef CAS
.
- K. T. Nam, D. W. Kim, P. J. Yoo, C. Y. Chiang, N. Meethong, P. T. Hammond, Y. M. Chiang and A. M. Belcher, Science, 2006, 312, 885 CrossRef CAS
.
- N. Du, H. Zhang, B. D. Chen, J. B. Wu, X. Y. Ma, Z. H. Liu, Y. Q. Zhang, D. R. Yang, X. H. Huang and J. P. Tu, Adv. Mater., 2007, 19, 4505 CrossRef CAS
.
- W. Mei, J. Huang, L. Zhu, Z. Ye, Y. Mai and J. Tu, J. Mater. Chem., 2012, 22, 9315 RSC
.
- X. W. Lou, D. Deng, J. Y. Lee, J. Feng and L. A. Archer, Adv. Mater., 2008, 20, 258 CrossRef CAS
.
- J. Zhu, T. Zhu, X. Zhou, Y. Zhang, X. W. Lou, X. Chen, H. Zhang, H. Hng and Q. Yan, Nanoscale, 2011, 3, 1084 RSC
.
- Y. Qi, N. Du, H. Zhang, X. Fan, Y. Yang and D. Yang, Nanoscale, 2012, 4, 991 RSC
.
- Z.-S. Wu, W. Ren, L. Wen, L. Gao, J. Zhao, Z. Chen, G. Zhou, F. Li and H.-M. Cheng, ACS Nano, 2010, 4, 3187 CrossRef CAS
.
- Y. Qi, N. Du, H. Zhang, J. Wang, Y. Yang and D. Yang, J. Alloys Compd., 2012, 521, 83 CrossRef CAS
.
- Z. X. Yang, G. D. Du, Z. P. Guo, X. B. Yu, Z. X. Chen, T. L. Guo and R. Zeng, Nanoscale, 2011, 3, 4440 RSC
.
- Y. Wang, H. Xia, L. Lu and J. Y. Lin, Acs Nano, 2010, 4, 1425 CrossRef CAS
.
- G. Wang, X. P. Gao and P. W. Shen, J. Power Sources, 2009, 192, 719 CrossRef CAS
.
- Y. Wang, H. J. Zhang, L. Lu, L. P. Stubbs, C. C. Wong and J. Y. Lin, Acs Nano, 2010, 4, 4753 CrossRef CAS
.
- P. Wu, N. Du, H. Zhang, J. X. Yu, Y. Qi and D. R. Yang, Nanoscale, 2011, 3, 746 RSC
.
- C. Zhang, X. Peng, Z. Guo, C. Cai, Z. Chen, D. Wexler, S. Li and H. Liu, Carbon, 2012, 50, 1879 CrossRef
.
- Y. Qi, N. Du, H. Zhang, P. Wu and D. Yang, J. Power Sources, 2011, 196, 10234 CrossRef CAS
.
- X. Sun and Y. Li, Angew. Chem. Int. Ed., 2004, 43, 597 CrossRef
.
- S. Ikeda, K. Tachi, Y. H. Ng, Y. Ikoma, T. Sakata, H. Mori, T. Harada and M. Matsumura, Chem. Mater., 2007, 19, 4335 CrossRef CAS
.
- L. Xu, W. Zhang, Y. Ding, Y. Peng, S. Zhang, W. Yu and Y. Qian, J. Phys. Chem. B, 2004, 108, 10859 CrossRef CAS
.
- B. Li, H. Cao, J. Shao, G. Li, M. Qu and G. Yin, Inorg. Chem., 2011, 50, 1628 CrossRef CAS
.
- W. Zhou, C. Cheng, J. Liu, Y. Y. Tay, J. Jiang, X. Jia, J. Zhang, H. Gong, H. H. Hng, T. Yu and H. J. Fan, Adv. Funct. Mater., 2011, 21, 2439 CrossRef CAS
.
- I. A. Courtney and J. R. Dahn, J. Electrochem. Soc., 1997, 144, 2943 CrossRef CAS
.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, J. Power Sources, 2001, 97–98, 235 CrossRef CAS
.
- P. Wu, N. Du, H. Zhang, J. X. Yu and D. R. Yang, J. Phys. Chem. C, 2010, 114, 22535 CAS
.
- S. Yang, H. Song and X. Chen, Electrochem. Commun., 2006, 8, 137 CrossRef CAS
.
- X. H. Huang, J. P. Tu, C. Q. Zhang and J. Y. Xiang, Electrochem. Commun., 2007, 9, 1180 CrossRef CAS
.
- J. Zhu, Y. K. Sharma, Z. Zeng, X. Zhang, M. Srinivasan, S. Mhaisalkar, H. Zhang, H. H. Hng and Q. Yan, J. Phys. Chem. C, 2011, 115, 8400 CAS
.
Footnote |
| † Electronic Supplementary Information (ESI) available: The 1st, 2nd, and 3rd discharge curves of the Co3O4@SnO2@C nanorods based anode material at a current density of 200 mAg−1 at room temperature. See DOI: 10.1039/c2ra21765a |
| This journal is © The Royal Society of Chemistry 2012 |
