Simple and green synthesis of monodisperse silver nanoparticles and surface-enhanced Raman scattering activity†
Zhengxia
Xu
and
Guoxin
Hu
*
School of Mechanical and Power Engineering, Shanghai Jiaotong University, Shanghai 200240, PR China. E-mail: hugx@sjtu.edu.cn; Fax: +86 21 34206569; Tel: +86 21 34206569
First published on 25th September 2012
Abstract
We describe a simple and green method for preparing silver nanoparticles with an average diameter in the range from 4.0 nm to 5.0 nm and with the standard deviation as low as 0.3 nm (polydispersity: 5.5%). The pre-synthesized silver oleate precursor in oleic acid was thermally reduced to obtain the monodisperse silver nanoparticles without a size selection procedure. This environmentally friendly chemistry approach requires only three reagents (silver nitrate, sodium oleate and oleic acid) which all come from renewable resources. The average diameter of these nanoparticles can be greatly adjusted by varying reaction time and finely regulated by varying reaction temperature at the same precursor concentration. The nucleation/diffusional growth model can be used to interpret the size and morphology difference of silver nanoparticles synthesized under different conditions. Raman spectra of graphene with and without an active layer of silver nanoparticles were measured to investigate the surface-enhanced Raman scattering properties of the silver nanoparticles. The Raman signal of graphene with an active layer increased by fifteen-fold in comparison to that without an active layer. The surface-enhanced Raman scattering activity can be explained using interparticle coupling induced Raman enhancement.
Introduction
Silver nanoparticles have been intensively investigated because of their catalytic, electronic, optical properties and their potential applications in catalysis (selective NOX reduction1 and ethylene oxidation2), materials (conductive films,3 dichroic coatings4) and analysis (biological sensors,5 surface-enhanced Raman scattering (SERS) substrate6). The synthesis of monodisperse silver nanoparticles is of key importance, because the properties of the silver nanoparticles depend strongly on their dimensions, which is broadly referred to as the size effect.7,8 In the past, colloidal silver nanoparticles were synthesized by either one of two methods. One is preparing hydrophilic surfactant-stabilized silver nanoparticles in aqueous solution through the chemical reduction of silver salt;9,10 the other is preparing hydrophobic surfactant-capped silver nanoparticles in organic solution through the thermal reduction of organosilver compounds.11,12 The aqueous phase approaches are undesirable due to some problems, such as broad size distribution,13,14 irreversible aggregation upon the addition of electrolytes15–17 and low silver nanoparticle content of the resulting solution.17,18 Through the thermal reduction of organosilver compounds, such as silver acetate,19 silver perfluorocarboxylates20 and silver oxalate,21 silver nanoparticles with a narrow size distribution can be obtained, but the organometallic compounds are extremely expensive and the solvent for thermal reduction is often toxic and expensive. So it is necessary to explore a novel method with low cost for preparing monodisperse silver nanoparticles.Among a number of methods for the synthesis of silver nanoparticles, purified natural materials are rarely used in their preparation. Some approaches do use purified natural materials (vitamin B2, vitamin E) as reduction agents for the synthesis of noble metal nanoparticles,22,23 but the size distributions of the nanoparticles synthesized are often broad. With the increasing emphasis on the topic of green chemistry and chemical processes over the past two decades, researchers have begun to develop more environmentally benign and sustainable methods for the synthesis of nanomaterials through the adoption of 12 principles in green chemistry.24 The main goals for the environmentally benign preparation of nanoparticles are  the choice of a nontoxic metal ion as the precursor,
the choice of a nontoxic metal ion as the precursor,  the choice of a purified natural solvent medium for synthesis,
the choice of a purified natural solvent medium for synthesis,  the choice of a environmentally benign reducing agent or no reducing agent,
the choice of a environmentally benign reducing agent or no reducing agent,  the choice of a nontoxic material to protect or passivate the nanoparticles surface. In this work, we aim at the development of an environmentally friendly process for synthesizing monodisperse silver nanoparticles.
the choice of a nontoxic material to protect or passivate the nanoparticles surface. In this work, we aim at the development of an environmentally friendly process for synthesizing monodisperse silver nanoparticles.
In this paper, we present an inexpensive, environmentally friendly process for synthesizing oleic acid capped silver nanoparticles through the thermal reduction of a silver oleate complex in oleic acid. The silver oleate complex was synthesized by the reaction of silver nitrate solution (0.1 M) with sodium oleate solution (0.1 M). Only three reagents (silver nitrate, oleic acid, sodium oleate) are required in the synthesis process. Silver nitrate (the most common silver salt), sodium oleate (reaction product of sodium hydroxide and oleic acid) and oleic acid (component of vegetable oil) are all environmentally friendly, safe to use and come from renewable resources. The silver nanoparticles with an average diameter in the range from 4.0 nm to 5.0 nm and with the standard deviation as low as 0.3 nm (polydispersity: 5.5%) were synthesized in one pot without a size selection procedure. We have also investigated the effect of thermal reduction conditions (temperature, time and molar concentration of silver oleate in oleic acid) on the size and morphology of the silver nanoparticles. The silver nanoparticles show SERS properties with the enhancement factor of ∼15 by using graphene as an analyte. This inexpensive, environmentally friendly method for the synthesis of silver nanoparticles may be extended to prepare many other noble metallic nanostructures.
Experimental section
Materials
Silver nitrate and sodium oleate (chemically pure) were purchased from Sinopharm Chemical Reagent Co., Ltd. Oleic acid (analytical grade) was purchased from Aladdin reagent Co., Ltd (Shanghai, China). All other solvents and reagents were of analytical reagent grade or better and were used as received. All glass containers were rinsed with deionized water obtained from a Nanopure system (resistivity ≥18 MΩ cm) and dried in an oven before use.Preparation of oleic acid capped silver nanoparticles
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v) 30% H2O2/concentrated H2SO4 for 30 min, then rinsed thoroughly with deionized water and dried. The silver nanoparticles were dispersed in organic solvents, such as hexane and chloroform. A droplet of colloidal dispersion of silver nanoparticles was placed on a silicon wafer and dried. Then a droplet of graphene dispersion was drop-cast on a silicon wafer with an active layer of silver nanoparticles and dried. For comparison, a droplet of graphene dispersion was placed on a silicon wafer without an active layer of silver nanoparticles. Additionally, a droplet of colloidal dispersion of silver nanoparticles was directly placed on a paper-like graphene film, as a comparison with bare paper-like graphene film. The graphene dispersion and paper-like graphene film were prepared according to our previous study.6
3 (v/v) 30% H2O2/concentrated H2SO4 for 30 min, then rinsed thoroughly with deionized water and dried. The silver nanoparticles were dispersed in organic solvents, such as hexane and chloroform. A droplet of colloidal dispersion of silver nanoparticles was placed on a silicon wafer and dried. Then a droplet of graphene dispersion was drop-cast on a silicon wafer with an active layer of silver nanoparticles and dried. For comparison, a droplet of graphene dispersion was placed on a silicon wafer without an active layer of silver nanoparticles. Additionally, a droplet of colloidal dispersion of silver nanoparticles was directly placed on a paper-like graphene film, as a comparison with bare paper-like graphene film. The graphene dispersion and paper-like graphene film were prepared according to our previous study.6
Characterizations
Fourier transform infrared spectra (FTIR) of silver oleate and oleic acid were recorded with a Nicolet 6700 (Thermo Fisher Scientific Inc., USA) in the wave number range from 400 cm−1 to 4000 cm−1. Due to the instability of the silver oleate sample, care should be taken to avoid excessive mercury lamp irradiation of the sample before the measurement of the FTIR spectrum. Ultraviolet-visible (UV-vis) absorption spectra were obtained using a EV300 UV-Visible Spectrophotometer (Thermal Electron Cor., USA). Transmission electron microscopy (TEM) images and selected area electron area diffraction (SAED) patterns were acquired using a transmission electron microscope (JEOL JEM 2000F) operated at 200 kV. TEM samples were prepared by placing a drop of the colloidal dispersion of silver nanoparticles onto an amorphous carbon-coated copper grid. The size distribution of the silver nanoparticles was analyzed by measuring nanoparticles based on TEM images using Image Software DigitalMicrograph (Gatan Inc., USA). Raman scattering spectra were recorded with a confocal Raman spectrometer (Senterra R200-L, Bruker Co., Germany), using the 532 nm line of a He–Ne laser as the excitation source.Results and discussion
We propose a simple, environmentally benign strategy to synthesize monodisperse silver nanoparticles without a size-selection procedure. The overall scheme for the synthesis of silver nanoparticles is depicted in Fig. 1 and the detailed procedures were described in the experimental section. Instead of using expensive organosilver compounds such as silver acetate11 and silver benzoate,25 we prepared the silver oleate complex by the reaction of silver nitrate with sodium oleate. The use of organic solvents as a reaction media for preparing metal oleate complexes26–28 is undesirable from the viewpoint of their environmental impact, while the use of water as a reaction media for preparing a silver oleate complex shows its advantages in environmental compatibility.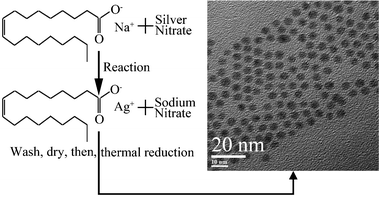 | ||
| Fig. 1 The overall schematic procedure for preparing silver nanoparticles. The silver oleate precursor was prepared by the reaction of silver nitrate with sodium oleate. The thermal reduction of the silver oleate precursor in the environmentally friendly solvent (oleic acid) produced monodisperse silver nanoparticles. | ||
The as-synthesized silver oleate complex in its white powder form is unstable at high temperatures (over 50 °C) and in light radiation (mercury lamp). Fig. 2 shows FTIR spectra of the silver oleate complex (prepared by reaction of sodium oleate with silver nitrate in aqueous medium) and oleic acid. The absorption bands in the γ(COO−) region for the FTIR spectrum of silver oleate are 1563, 1620, 1462, 1419 cm−1, while the absorption bands of the –COOH group for the FTIR spectrum of oleic acid are 1710, 1460, 1417, 1286 cm−1. The carboxylate coordination mode (ionic, unidentate, bidentate, and bridging) can be deduced from the position and separation (Δ) of γ(COO−) bands in the 1300–1700 cm−1 region.29–31 For Δ < 110 cm−1, a bidentate ligand is expected, whereas for Δ > 200 cm−1, it is a unidentate. For a bridging ligand, the range of Δ is from 140 to 200 cm−1. For silver oleate, the difference between the two bands at 1563 and 1419 cm−1 is 144 cm−1, indicating that bridging coordination is the major coordinate mode. The two shoulder peaks are at 1520 and 1462 cm−1. These shoulders demonstrate the presence of a minor coordinate mode, namely bidentate coordination (Δ is smaller than 101 cm−1).
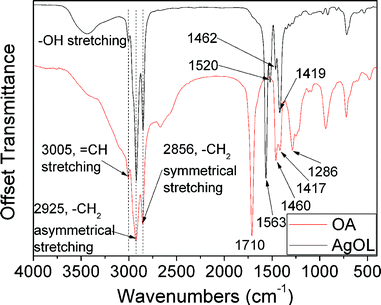 | ||
| Fig. 2 FTIR spectra of the silver oleate complex and oleic acid. | ||
In a typical synthesis of silver nanoparticles, the pre-synthesized silver oleate was added to oleic acid and dissolved at 90–100 °C. The oleic acid acts as both the solvent to provide the thermal condition for the thermal reduction of the silver oleate precursor and the surfactant to cap on silver nanoparticles. When the solution was heated to 150 °C, the color of the solution changed from light yellow to dark yellow and to dark brown in less than 10 min, indicating the formation and the concentration change of silver nanoparticles. Although the results of the tests (SAED pattern and UV-vis spectra in Fig. 3) indicate that the silver nanoparticles can be synthesized from the silver oleate precursor, the key questions now are how this formation occurs and when the reaction ends. To tackle these questions, we performed experiments aiming to track the timing course of the formation process. Fig. 4 shows TEM images of silver nanoparticles obtained by the thermal reduction of silver oleate at 150 °C for a designated time with 10 mM of silver oleate in oleic acid. As shown in Fig. 4D, the appearance of fringes with spacings of 0.23 ± 0.01 nm, a value that corresponds to the [1 1 1] interplanar distance for face centered cubic silver, indicates that the nanoparticles are crystalline. The average diameter of the silver nanoparticles increased from 4.0 ± 0.3 to 5.0 ± 0.3 nm with reaction time from 15 to 60 min (Fig. S1 and Fig. S3 in ESI†, Table 1). The average diameter of silver nanoparticles obviously increased from 4.0 ± 0.3 to 5.0 ± 0.6 nm with time from 15 to 30 min (Fig. S1 and Fig. S2 in ESI†, Table 1), while there was no obvious size change with time from 30 to 60 min (Fig. S2 and Fig. S3 in ESI†, Table 1). The evolution of the silver nanoparticles can be explained by the classical nucleation/diffusional growth model.12,32,33 The fixed number of seeds are initially formed by the burst nucleation of silver atoms, and then seed particles continue to grow by the diffusion-driven deposition of silver atoms, as shown by the previous model and experiments.33–35 The parameters in the diffusional growth stage significantly affect the resulting particle size and size distribution. If the deposition rate of silver atoms is linearly dependent on the concentration of silver oleate precursor, an exponential growth curve will be obtained.
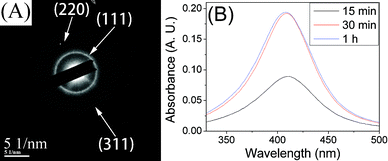 | ||
| Fig. 3 (A) SAED pattern of silver nanoparticles synthesized at 170 °C, 10 mM in oleic acid, 30 min. (B) The surface-plasmon absorbance spectra of silver nanoparticles synthesized at 150 °C, 10 mM in oleic acid. | ||
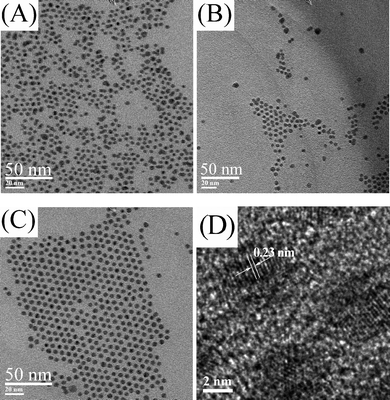 | ||
| Fig. 4 TEM images of silver nanoparticles synthesized at 150 °C, 10 mM in oleic acid for reaction times of (A) 15 min, (B) 30 min, (C, D) 1 h. | ||
| Conditions | Average diameter | Standard deviation | Correlation coefficient | Peak position |
|---|---|---|---|---|
| nm (particle number) | nm | nm | ||
| 10 mM, 150 °C, 15 min | 4.0 (1885) | 0.3 | 0.99 | 412 |
| 10 mM, 150 °C, 30 min | 5.0 (396) | 0.6 | 0.77 | 409 |
| 10 mM, 150 °C, 1 h | 5.0 (1217) | 0.3 | 0.99 | 408 |
| 10 mM, 160 °C, 30 min | 4.8 (1459) | 0.3 | 0.99 | 407 |
| 10 mM, 170 °C, 30 min | 4.8 (2251) | 0.3 | 0.97 | 411 |
| 10 mM, 160 °C, 1 h | 4.9 (1554) | 0.3 | 0.98 | 412 |
| 10 mM, 170 °C, 2 h | 4.7 (1889) | 0.3 | 0.96 | 410 |
| 100 mM, 150 °C, 30 min | polydisperse | — | — | 426 |
| 100 mM, 160 °C, 1 h | polydisperse | — | — | 418 |
The nucleation/diffusional growth process has also been tested at other reaction temperatures and reaction times. Fig. 5 shows TEM images of silver nanoparticles at 160 and 170 °C for a reaction time of 30 min with 10 mM of silver oleate in oleic acid. The average diameter and the standard deviation of these nanoparticles based on the analyses of TEM images are displayed in Fig. S4 and Fig. S5 in the ESI†, and are summarized in Table 1. As the reaction temperature increased from 150 to 170 °C in the reaction time of 30 min, the average diameter of silver nanoparticles decreased from 5.0 ± 0.6 nm to 4.8 ± 0.3 nm (Fig. S2 and Fig. S5 in ESI†, Table 1). The nucleation/diffusional growth model mentioned above indicates that the average diameter of silver nanoparticles can be regulated by varying reaction time due to the exponential growth. Alternatively, the average diameter of silver nanoparticles can be finely adjusted by varying reaction temperature because of the relationship between average diameter and reaction temperature.
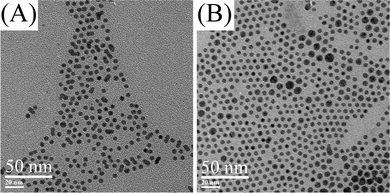 | ||
| Fig. 5 TEM images of silver nanoparticles. (A) 10 mM in oleic acid, 160 °C, 30 min. (B) 10 mM in oleic acid, 170 °C, 30 min. | ||
The relationship between the average diameter of silver nanoparticles and the reaction time was further investigated to find out when the size growth terminates. Fig. 6 shows TEM images of silver nanoparticles at 160 °C for 1 h and 170 °C for 2 h with 10 mM of silver oleate in oleic acid. The average diameter of silver nanoparticles slightly increased from 4.8 ± 0.3 nm to 4.9 ± 0.3 nm (Fig. S4 and Fig. S6 in the ESI†, Table 1) when the reaction time increased from 30 min to 1 h at 160 °C. However, the average diameters are almost the same (Fig. S5 and Fig. S7 in ESI†, Table 1) even with reaction time increased from 30 min to 2 h at 170 °C. These data indicate that the size growth of silver nanoparticles has already terminated at 30 min at high reaction temperature (170 °C) and almost finished at 30 min at slightly lower reaction temperature (150 and 160 °C).
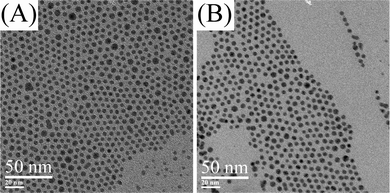 | ||
| Fig. 6 TEM images of silver nanoparticles. (A) 10 mM in oleic acid, 160 °C, 1 h. (B) 10 mM in oleic acid, 170 °C, 2 h. | ||
The average diameter and morphology of the silver nanoparticles are related to the reaction conditions, such as reaction temperature, reaction time and concentration of silver oleate in oleic acid. The average diameter of silver nanoparticles is mainly dependent on reaction time (exponential growth with time). Alternatively, reaction temperature can also influence the average diameter of silver nanoparticles by changing the solubility of the nanoparticles. At high temperature, more seed particles are formed by the nucleation of silver atoms at the initial stage of reaction. A higher concentration of silver seed particles causes smaller maximum size at the same precursor concentration. Furthermore, the morphology of silver nanoparticles is dependent on reaction temperature and reaction time. The shape of the silver nanoparticles is irregular (Fig. 4A, Fig. S1 in ESI†) and some rodlike structures occur spontaneously (Fig. 5A, Fig. S4 in ESI†) before the seed particles grow to the maximum size. The dissolution of silver oleate and deposition of silver atoms on seed particles are faster at higher temperature according to reaction kinetics, so silver nanoparticles need less time to reach their maximum size at higher temperature (as shown above). From the comparison between Fig. 4C, Fig. S4 and Fig. 5B, Fig. S5 in the ESI†, we can see that the percentage of silver nanoparticles with a diameter above 6.0 nm increases at higher temperature. However, from the comparison between Fig. 5B, Fig. S5 and Fig. 6B, Fig. S7 in the ESI†, we can see that the percentage of silver nanoparticles with a diameter above 6.0 nm decreases with increasing reaction time (aging time). The nanoparticles synthesized by green methods in previous studies are polydisperse (polydispersity > 10%),24 while the nanoparticles obtained from our green synthetic strategy are monodisperse (polydispersity < 10%). The concentration of silver oleate in oleic acid plays a critical role to obtain the monodisperse silver nanoparticles. We obtained the monodisperse silver nanoparticles at a low concentration of precursor (10 mM of silver oleate in oleic acid); while the silver nanoparticles produced at high concentration of precursor (100 mM of silver oleate in oleic acid) are polydisperse (Fig. S8 and Fig. S9 in ESI†). The peak position of the surface plasmon resonance is correlated with the size and uniformity of silver nanoparticles. When the silver nanoparticles became polydisperse, the peak position changed to 420 nm, a red shift of ∼10 nm as compared with that for the monodisperse silver nanoparticles (Table 1). The large scale production of monodisperse silver nanoparticles is limited by the low concentration of precursor, but at a low concentration of precursor the production can be scaled up by increasing the volume of silver oleate solution in oleic acid. The average diameter and diameter distribution of silver nanoparticles on a large scale remained the same as on a small scale.
Silver nanoparticles are expected to be SERS-active by using graphene36 as the analyte. Chemical derived graphene was prepared according to our previous studies6 and used as the analyte to investigate the SERS properties of silver nanoparticles synthesized at 150 °C, 1 h and 10 mM of silver oleate in oleic acid. Fig. 7 shows the Raman spectra of graphene on a silicon wafer with and without an active layer of silver nanoparticles. The Raman spectrum of graphene exhibits two characteristic peaks, corresponding to the D-band at ∼1360 cm−1 and G-band at ∼1585 cm−1. The D-band is a breathing mode of k-point phonons of A1g symmetry, which is attributed to local defects and disorders, particularly the defects located at the edges of graphene and graphite platelets, while the G-band corresponds to the E2g phonon of sp2 bonds of carbon atoms and is mainly sensitive to the configuration of sp2 sites.37 The intensities of the D-band and G-band with an active layer of silver nanoparticles increased fifteen-fold and twenty-fold, respectively, in comparison to those without an active layer of silver nanoparticles on the silicon wafer. The enhancement factors of the D-band and G-band are not the same, and the cause of unequal enhancement factors is unclear. The sharp Raman peak at 518 cm−1 is corresponding to the Raman-active mode in bulk silicon occurring at the center of the Brillouin zone.38 The Raman peak intensity of the silicon wafer with an active layer of silver nanoparticles also increased three-fold in comparison to that without an active layer of silver nanoparticles.
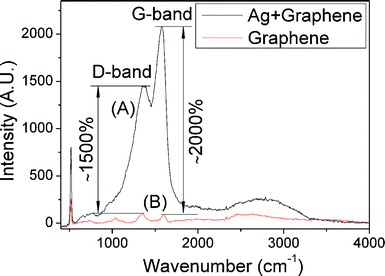 | ||
| Fig. 7 Raman spectra for graphene on a silicon wafer with (A) and without (B) an active layer of silver nanoparticles. | ||
It is generally agreed that long-range electromagnetic and short-range chemical mechanisms are thought to be the origin of the SERS effect. The electromagnetic mechanism is based on the amplified electromagnetic field generated upon optical excitation of the localized surface plasmon resonance of nanoscale surface roughness features involving physical interactions in the 10–100 nm range, while the chemical mechanism is associated with the electronic coupling due to the formation of charge-transfer complexes between the analyte and metal surface through chemical interaction on a atomic-scale range. The charge-transfer complexes can not form between graphene and silver nanoparticles because the surface of the silver nanoparticles is modified by oleic acid. So, the SERS effect of our samples is the result of intense localized fields at the junction of silver nanoparticles. The electromagnetic SERS effect relies on interparticle coupling. The highly localized electromagnetic fields at the junction of silver nanoparticles affect the incident light, so the interparticle junction points are hot sites for electromagnetic SERS. As shown in the TEM images, silver nanoparticles tend to aggregate together, so they possess a high density of hot sites. The distances between the centers of two adjacent particles and the particles diameter are important parameters, as shown in previous studies.39 To further investigate the SERS effect of the silver nanoparticles synthesized at 150 °C, 1 h and 10 mM of silver oleate in oleic acid, they were directly placed on a paper-like graphene film prepared according to our previous study.6 As a comparison to those of a bare paper-like graphene film, the intensities of the D-band and G-band of graphene films coated with an active layer of silver nanoparticles increased by roughly six-fold and seven-fold, respectively, (Fig. S10 in ESI†). The result provides a piece of further evidence to support the SERS activity of our silver nanoparticles.
Conclusions
An environmentally benign approach was developed for the synthesis of monodisperse silver nanoparticles by the thermal reduction of a pre-synthesized silver oleate precursor in oleic acid. Silver nanoparticles with an average diameter in the range from 4.0 nm to 5.0 nm and with the standard deviation as low as 0.3 nm (polydispersity: 5.5%) were prepared without a size-sorting procedure. Only three renewable reagents are required to obtain the monodisperse silver nanoparticles, indicating the advantages of simplicity and environmental friendliness. The average diameter of these nanoparticles can be greatly adjusted by varying reaction time and finely regulated by varying reaction temperature at the same precursor concentration. The size and morphology difference of silver nanoparticles synthesized under different conditions can be interpreted by the nucleation/diffusional growth model. A SERS active coating made of silver nanoparticles was fabricated to enhance the Raman signal of graphene. The Raman signal of graphene with an active layer increased by fifteen-fold in comparison to that without an active layer. The SERS activity of silver nanoparticles is the result of intense localized fields at the junction (hot sites) of silver nanoparticles and can be explained using interparticle coupling induced Raman enhancement.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51076094, No. 51176113). The authors thank Instrumental Analysis center of SJTU for Raman spectra measurements.References
- K. Shimizu, K. Sawabe and A. Satsuma, Catal. Sci. Technol., 2011, 1, 331–341 CAS.
- Y. Shiraishi and N. Toshima, J. Mol. Catal. A: Chem., 1999, 141, 187–192 CrossRef CAS.
- N. H. Mack, J. A. Bailey, S. K. Doorn, C.-A. Chen, H.-M. Gau, P. Xu, D. J. Williams, E. A. Akhadov and H.-L. Wang, Langmuir, 2011, 27, 4979–4985 CrossRef CAS.
- M. D. Musick, C. D. Keating, L. A. Lyon, S. L. Botsko, D. J. Peña, W. D. Holliway, T. M. McEvoy, J. N. Richardson and M. J. Natan, Chem. Mater., 2000, 12, 2869–2881 CrossRef CAS.
- S. Choi, R. M. Dickson and J. Yu, Chem. Soc. Rev., 2012, 41, 1867–1891 RSC.
- Z. Xu, H. Gao and H. Guoxin, Carbon, 2011, 49, 4731–4738 CrossRef CAS.
- Y. Lu and W. Chen, J. Power Sources, 2012, 197, 107–110 CrossRef CAS.
- G. Wee, W. F. Mak, N. Phonthammachai, A. Kiebele, M. V. Reddy, B. V. R. Chowdari, G. Gruner, M. Srinivasan and S. G. Mhaisalkar, J. Electrochem. Soc., 2010, 157, A179–A184 CrossRef CAS.
- M. Popa, T. Pradell, D. Crespo and J. M. Calderón-Moreno, Colloids Surf., A, 2007, 303, 184–190 CrossRef CAS.
- P. C. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391–3395 CrossRef CAS.
- X. Z. Lin, X. Teng and H. Yang, Langmuir, 2003, 19, 10081–10085 CrossRef CAS.
- H. Hiramatsu and F. E. Osterloh, Chem. Mater., 2004, 16, 2509–2511 CrossRef CAS.
- A. Taleb, C. Petit and M. P. Pileni, Chem. Mater., 1997, 9, 950–959 CrossRef CAS.
- A. PanáČek, L. Kvítek, R. Prucek, M. Kolář, R. VeČeřová, N. Pizúrová, V. K. Sharma, T. J. NevěČná and R. Zbořil, J. Phys. Chem. B, 2006, 110, 16248–16253 CrossRef.
- A. M. E. Badawy, T. P. Luxton, R. G. Silva, K. G. Scheckel, M. T. Suidan and T. M. Tolaymat, Environ. Sci. Technol., 2010, 44, 1260–1266 CrossRef.
- K. A. Huynh and K. L. Chen, Environ. Sci. Technol., 2011, 45, 5564–5571 CrossRef CAS.
- I. Römer, T. A. White, M. Baalousha, K. Chipman, M. R. Viant and J. R. Lead, J. Chromatogr., A, 2011, 1218, 4226–4233 CrossRef.
- H. S. Shin, H. J. Yang, S. B. Kim and M. S. Lee, J. Colloid Interface Sci., 2004, 274, 89–94 CrossRef CAS.
- P. Jeevanandam, C. K. Srikanth and S. Dixit, Mater. Chem. Phys., 2010, 122, 402–407 CrossRef CAS.
- S. J. Lee, S. W. Han and K. Kim, Chem. Commun., 2002, 442–443 RSC.
- S. Navaladian, B. Viswanathan, R. Viswanath and T. Varadarajan, Nanoscale Res. Lett., 2007, 2, 44–48 CrossRef CAS.
- L. Zhang, Y. Shen, A. Xie, S. Li, B. Jin and Q. Zhang, J. Phys. Chem. B, 2006, 110, 6615–6620 CrossRef CAS.
- M. N. Nadagouda and R. S. Varma, Green Chem., 2006, 8, 516–518 RSC.
- P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940–13941 CrossRef CAS.
- A. Kumar, P. K. Vemula, P. M. Ajayan and G. John, Nat. Mater., 2008, 7, 236–241 CrossRef CAS.
- J. Park, K. An, Y. Hwang, J.-G. Park, H.-J. Noh, J.-Y. Kim, J.-H. Park, N.-M. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891–895 CrossRef CAS.
- M. V. Kovalenko, M. I. Bodnarchuk, R. T. Lechner, G. Hesser, F. Schäffler and W. Heiss, J. Am. Chem. Soc., 2007, 129, 6352–6353 CrossRef CAS.
- P. Li, Q. Peng and Y. Li, Chem.–Eur. J., 2011, 17, 941–946 CrossRef CAS.
- Y. Lu and J. D. Miller, J. Colloid Interface Sci., 2002, 256, 41–52 CrossRef CAS.
- F. Söderlind, H. Pedersen, R. M. Petoral Jr, P.-O. Käll and K. Uvdal, J. Colloid Interface Sci., 2005, 288, 140–148 CrossRef.
- L. M. Bronstein, X. Huang, J. Retrum, A. Schmucker, M. Pink, B. D. Stein and B. Dragnea, Chem. Mater., 2007, 19, 3624–3632 CrossRef CAS.
- V. K. LaMer and R. H. Dinegar, J. Am. Chem. Soc., 1950, 72, 4847–4854 CrossRef CAS.
- V. Privman, D. V. Goia, J. Park and E. Matijević, J. Colloid Interface Sci., 1999, 213, 36–45 CrossRef CAS.
- J. Park, V. Privman and E. Matijević, J. Phys. Chem. B, 2001, 105, 11630–11635 CrossRef CAS.
- D. T. Robb and V. Privman, Langmuir, 2007, 24, 26–35 CrossRef.
- C. Liu, G. Hu and H. Gao, J. Supercrit. Fluids, 2012, 63, 99–104 CrossRef CAS.
- B. Tang, H. Guoxin and H. Gao, Appl. Spectrosc. Rev., 2010, 45, 369–407 CrossRef CAS.
- C. M. Hessel, J. Wei, D. Reid, H. Fujii, M. C. Downer and B. A. Korgel, J. Phys. Chem. Lett., 2012, 3, 1089–1093 CrossRef CAS.
- F. J. García-Vidal and J. B. Pendry, Phys. Rev. Lett., 1996, 77, 1163–1166 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Additional TEM images and particle size distribution of silver nanoparticles synthesized in various conditions. SERS spectra of paper-like graphene film coated with an active layer of silver nanoparticles and bare paper-like graphene film. See DOI: 10.1039/c2ra21745g |
| This journal is © The Royal Society of Chemistry 2012 |
