DOI:
10.1039/C2RA21468G
(Paper)
RSC Adv., 2012,
2, 9398-9402
Synthesis of 2,3-dihydro-4-pyranones from epoxides via intermolecular [4+2] cycloaddition reaction†
Received
4th June 2012
, Accepted 10th August 2012
First published on 13th August 2012
Abstract
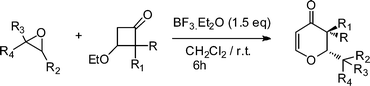
An efficient synthesis of 2,3-dihydro-4-pyranones from epoxides and 3-alkoxycyclobutanones via an intermolecular [4+2] cycloaddition reaction, mediated by boron trifluoride etherate has been developed. The reaction is diastereoselective and gives trans diastereomers in excess.
Introduction
2,3-Dihydro-4H-pyran-4-one is a key synthon for the synthesis of important biologically active molecules.1 It is also used for the synthesis of carbohydrates2 and highly functionalized aromatic rings.3 Thus, the synthesis of 2,3-dihydro-4H-pyran-4-one has strongly inspired the synthetic organic community to develop many new strategies, viz., hetero-Diels–Alder reaction,4 cyclization of 5-hydroxy-1,3-diketones,5 oxidative cyclization of β-hydroxyenones with palladium(II),6 addition of different nucleophiles to unsaturated lactones7 and others.8 However, most of the existing methods are associated either with tedious starting material preparations or with multistep synthesis.4,9 Furthermore, in most of the cases, the reaction ends up with a diastereomeric mixture of cis and trans 2,3-dihydro-4H-pyran-4-ones2a,4a–d,9a. Matsuo and coworkers have reported an alternative method, which involves a [4+2] cycloaddition reaction between 3-alkoxycyclobutanone and carbonyl compounds.10 The synthesis of 2,3-dihydro-4H-pyran-4-one bearing a monoalkyl group at their C-3 position is more difficult and only a few methods have been reported so far.4a,10b,11 On the other hand, epoxides are considered as one of the most useful and versatile intermediates in organic synthesis due to their high reactivity.12 One of the most important reaction is their rearrangement to a carbonyl equivalent under Lewis acid conditions.13 Recently, we have synthesized 4-aryltetrahydropyrans from epoxides and homoallylic alcohols mediated by boron trifluoride etherate.14 Herein, we report a diastereoselective synthesis of trans 2,3-dihydro-4H-pyran-4-one bearing mono and dialkyl as well as monoaryl groups at the C-3 position starting from 3-alkoxycyclobutanones and epoxides mediated by boron trifluoride etherate.
Results and discussion
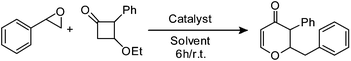
In an initial investigation styrene oxide was treated with 3-ethoxy-2-phenylcyclobutanone in the presence of boron trifluoride etherate at room temperature and 2-benzyl-2,3-dihydro-3-phenylpyran-4-one was obtained in 76% yield, with a cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) trans ratio of 1
trans ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. In order to improve the yield and diastereosectivity, the reaction was screened with other Lewis acids such as TiCl4, In(OTf)3, TMSOTf and the Brønsted acid TsOH as shown in Table 1. These results showed that BF3·Et2O was the most efficient Lewis acid. No reaction was observed when TiCl4 or TMSOTf was used. Brønsted acid TsOH was also found to be ineffective. The effect of solvent on diastereoselectivity was also studied. The results are summarized in Table 1. Among these dichloromethane was found to be the best solvent, whereas toluene and tetrahydrofuran were found to be less effective. Therefore, BF3·Et2O in CH2Cl2 was considered as the best combination for both the yields and stereoselectivity. The yield and the diastereoselectivity were found to be same after 12 h.
8. In order to improve the yield and diastereosectivity, the reaction was screened with other Lewis acids such as TiCl4, In(OTf)3, TMSOTf and the Brønsted acid TsOH as shown in Table 1. These results showed that BF3·Et2O was the most efficient Lewis acid. No reaction was observed when TiCl4 or TMSOTf was used. Brønsted acid TsOH was also found to be ineffective. The effect of solvent on diastereoselectivity was also studied. The results are summarized in Table 1. Among these dichloromethane was found to be the best solvent, whereas toluene and tetrahydrofuran were found to be less effective. Therefore, BF3·Et2O in CH2Cl2 was considered as the best combination for both the yields and stereoselectivity. The yield and the diastereoselectivity were found to be same after 12 h.
Table 1 Optimization with different Lewis acids and Brønsted acids
| Entry |
Catalyst (equiv) |
Solvent |
Cis:transa |
Yield (%)b |
|
Ratio is determined from 1H NMR.
Yield refers to isolated yield.
Not detected.
Reaction is continued for 12 h.
|
| 1 |
BF3·Et2O (1.5) |
CH2Cl2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
76 |
| 2 |
TiCI4 (1.0) |
CH2Cl2 |
— |
n.d.c |
| 3 |
In(OTf)3 (0.2) |
CH2Cl2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
28 |
| 4 |
TMSOTf (1.0) |
CH2Cl2 |
— |
n.d.c |
| 5 |
TsOH (1.0) |
CH2Cl2 |
— |
n.d.c |
| 6 |
BF3·Et2O (1.5) |
Toluene |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
42 |
| 7 |
BF3·Et2O (1.5) |
THF |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
35 |
| 8 |
BF3·Et2O (1.5) |
CH2Cl2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
76d |
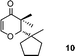
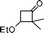
With the optimal conditions in hand, we examined the scope of the reaction by using variety of epoxides (Table 2). As shown in Table 2, both alkyl and aryl epoxides worked well. In general, good yields were obtained when using 1,1-disubstituted alkyl epoxides, whereas unsaturated alkyl epoxide (entry 4) gave the desired product with a low yield. On the other hand, monosubstituted alkyl epoxides (entry 11) are unreactive. This is attributed to the lower stability of the carbocation 21 (Scheme 2), formed from monosubstituted epoxides compared to 1,1-disubstituted, vinylic epoxides and styrene oxides, where carbocation 21 is better stabilized due to the tertiary, allylic and benzylic centres, respectively. The epoxide, 1-methyl-7-oxabicyclo[4.1.0]heptane (entry 10) gave the product 10 with a ring contraction in cyclohexyl ring with 35% yield. This is due to the fact that after ring opening, 1-methyl-7-oxabicyclo[4.1.0]heptane produces two products, ketone 19 and aldehyde 20 with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Scheme 1).15 The aldehyde 20 only takes part in the reaction to form the dihydropyranone 10. Similarly 2-methyl-3-phenyloxirane (entry 12) was also not effective for this reaction. In this case, the epoxide after rearrangement produces 1-phenylpropane-2-one, which being a ketone does not take part in the reaction.
1 (Scheme 1).15 The aldehyde 20 only takes part in the reaction to form the dihydropyranone 10. Similarly 2-methyl-3-phenyloxirane (entry 12) was also not effective for this reaction. In this case, the epoxide after rearrangement produces 1-phenylpropane-2-one, which being a ketone does not take part in the reaction.
| Entry |
Epoxide |
Cyclobutanone |
Product |
Cis:transa |
Yield %b |
Ratios are determined from crude 1H NMR.
Yields refer to isolated yield. Compounds are characterized by IR, 1H, 13C NMR and Mass spectroscopy. Only trans isomer is shown.
The compound is obtained as an inseparable diastereomeric mixture at the benzylic position with a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the ration was determined by 1H NMR spectroscopy. 1, and the ration was determined by 1H NMR spectroscopy.
|
| 1 |
|
|
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
76 |
| 2 |
|
|
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
68 |
| 3 |
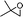
|
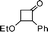
|
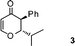
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
84 |
| 4 |
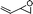
|
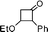
|
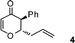
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
60 |
| 5 |
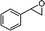
|
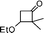
|
|
— |
81 |
| 6 |
|
|
|
— |
65 |
| 7 |
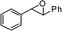
|
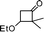
|
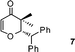
|
— |
71 |
| 8 |
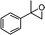
|
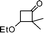
|

|
— |
63c |
| 9 |
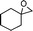
|
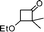
|
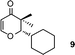
|
— |
78 |
| 10 |

|

|
|
— |
35 |
| 11 |
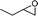
|
|
|
— |
0 |
| 12 |
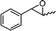
|
|
|
— |
0 |
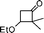
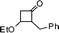
| 13 |
|
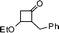
|
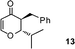
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
72 |
| 14 |
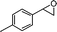
|
|
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
64 |
| 15 |
|
|
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
80 |
| 16 |
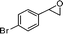
|
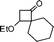
|
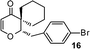
|
— |
58 |
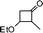
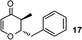
| 17 |
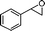
|
|
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
68 |
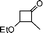
| 18 |
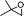
|
|
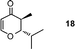
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
79 |
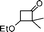
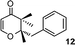
Simultaneously, we explored the reaction with various cyclobutanone derivatives such as 3-ethoxy-2-phenylcyclobutanone, 3-ethoxy-2,2-dimethylcyclo-butanone, 2-benzyl-3-ethoxycyclobutanone, 3-ethoxyspiro[3.5]nonan-1-one and 3-ethoxy-2-methylcyclo-butanone. It was observed that the reaction furnished moderate to good yields in all the cases as outlined in the Table 2. The 2,2-disubstituted cyclobutanones gave a single product, whereas, the cyclobutanones possessing monosubstitution at the C-2 position gave a mixture of diastereomers with the trans isomer as major product, which was determined from the coupling constants of 1H NMR spectroscopy. Thus, the coupling constants of C-3H for compounds cis-1 and trans-1 were found to be 4.4 and 11.6 Hz, respectively (Fig. 1).
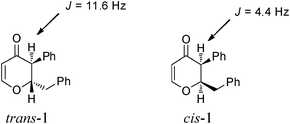 |
| | Fig. 1 Coupling constants of cis and trans isomer of 1. | |
The mechanism of the reaction can be explained as follows. The epoxide under Lewis acidic conditions opens up to a carbocation 21, which rearranges to the corresponding aldehyde 22. Similarly in presence of Lewis acid, 3-alkoxycyclobutanones undergoe regioselective cleavage of the highly substituted C2–C3 bond via bicyclobutonium ion 23 to give a more stable enolate, which exists as a zwitterionic intermediate 24. The intermediate 24 eliminates a proton to generate diene 25, similar to Danishefsky's diene, which reacts with aldehyde 22 in a step wise fashion to give trans intermediate 26 as a major and cis27 as a minor one via a six membered, more favoured chair transition state [A] and a less favoured [B], respectively. The intermediates 26 and 27 after cyclization and subsequent elimination of ethanol affords the desired 2,3-dihydropyranones 30 and 31, respectively (Scheme 2). This stepwise mechanism is in accordance with the mechanism proposed by Danishefsky group,16 which supports the formation of the trans product as the major one.
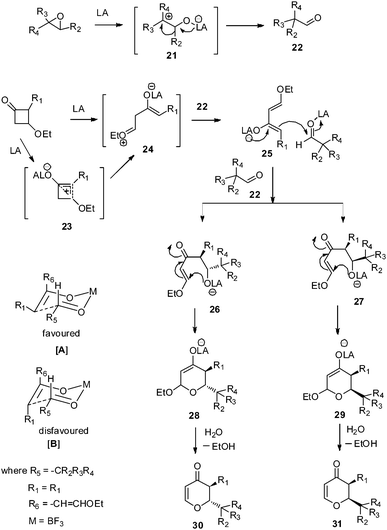 |
| | Scheme 2 Proposed mechanism of the reaction. | |
Conclusion
In conclusion we have developed a versatile methodology for the synthesis of 2,3-dihydro-4H-pyran-4-ones using epoxides and 3-alkoxycyclobutanones via an intermolecular [4+2] cycloaddition reaction in moderate to good yields. One of the important features of the reaction is that 2,3-dihydro-4H-pyran-4-ones with a benzylic group in the 2-position can be obtained. The method provides an alternative to aldehydes for the synthesis of 2,3-dihydro-4H-pyran-4-ones where the aldehyde cannot be accessed directly.
Experimental
General methods
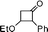
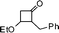
All the reagents are commercially obtained. BF3·Et2O was distilled over CaH2 prior to use. 1H NMR spectra were recorded in CDCl3 on a Varian AS 400 (400 MHz) spectrometer using TMS as internal standard. The 13C spectra were obtained on a Varian AS 400 spectrometer operating at 100 MHz. IR spectra were recorded on a Nicolet Impact 410 FT-IR spectrometer. HRMS spectra were recorded using ESI mode. Elemental analysis was performed on Perkin Elmer 2400 series II CHNS analyzer. Melting points were measured in open capillary tubes and are uncorrected. All 3-ethoxy cyclobutanones are prepared by following literature methods.10a,17
General procedure for the synthesis of 2,3-dihydro-4-pyranones (1-18).
To a mixture of epoxide (1.0 equiv.) and 3-ethoxy cyclobutanone (1.2 equiv) in dichloromethane (3 mL) was added freshly distilled boron trifluoride etherate (1.5 equiv). The reaction mixture was stirred at room temperature for the specified time. The progress of the reaction was monitored by TLC with ethyl acetate and hexane as eluents. After completion of the reaction, the reaction mixture was treated with aqueous sodium bicarbonate and the product was extracted with dichloromethane and then the organic layer washed with brine. The organic layer was dried over (Na2SO4) and evaporated to leave the crude product which was purified by column chromatography over silica gel to give the title compounds.
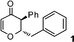
2-Benzyl-2,3-dihydro-3-phenylpyran-4-one (1).
To a mixture of 2-phenyl-oxirane (60 mg, 0.5 mmol) and 3-ethoxy-2-phenyl cyclobutanone (57 mg, 0.6 mmol) in dichloromethane (3 mL) was added freshly distilled boron trifluoride etherate (107 mg, 0.75 mmol). The reaction mixture was stirred at room temperature for the specified time. The progress of the reaction was monitored by TLC with ethyl acetate and hexane as eluents. After completion of the reaction, the reaction mixture was treated with aqueous sodium bicarbonate and the product was extracted with dichloromethane and then the organic layer washed with brine. The organic layer was dried over (Na2SO4) and evaporated to leave the crude product which was purified by column chromatography over silica gel to give 2-benzyl-2,3-dihydro-3-phenylpyran-4-one (100 mg, 76%) as a pale yellow liquid; 1H NMR (400 MHz, CDCl3) δ 2.89 (2H, d, J = 6.0 Hz), 3.65 (1H, d, J = 11.6 Hz), 4.85 (1H, ddd, J = 11.6, 6.4 and 6.0 Hz), 5.55 (1H, d, J = 6.0 Hz), 7.11–7.18 (4H, m), 7.24–7.35 (5H, m), 7.36–7.41 (2H, m); 13C NMR (100 MHz, CDCl3) δ 38.9, 56.3, 84.0, 107.2, 127.0, 128.0, 128.6, 129.2, 129.3, 129.8, 135.8, 136.7, 162.5, 193.0; IR (KBr, neat) 3030, 2924, 1675, 1600, 1495, 1277, 1181, 1037, 751, 670 cm−1. HRMS (ESI) cald. for C18H16O2 (M+H)+ 265.1223. Found 265.1234.
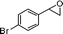
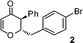
2-(4-Bromobenzyl)-2,3-dihydro-3-phenylpyran-4-one (2).
Pale yellow liquid (116 mg, 68%). 1H NMR (400 MHz, CDCl3) δ 2.83 (2H, d, J = 6.0 Hz), 3.65 (1H, d, J = 12.0 Hz), 4.85 (1H, ddd, J = 12.0, 6.4 and 5.6 Hz), 5.56 (1H, d, J = 6.0 Hz), 6.99 (2H, d, J = 8.4 Hz), 7.14 (2H, d, J = 8.0 Hz), 7.30–7.43 (6H, m); 13C NMR (100 MHz, CDCl3) δ 38.4, 56.3, 83.6, 107.4, 121.0, 128.1, 129.2, 129.3, 131.5, 131.6, 135.5, 135.6, 162.4, 192.8; IR (KBr, neat) 2924, 1676, 1600, 1489, 1405, 1275, 1037, 1012, 802, 701 cm−1. HRMS (ESI) cald. for C18H15BrO2 (M+H)+ 343.0328. Found 343.0334.
2,3-Dihydro-2-isopropyl-3-phenylpyran-4-one (3).
Pale yellow liquid, (90 mg, 84%). 1H NMR (400 MHz, CDCl3) δ 0.96 (3H, d, J = 6.8 Hz), 1.01 (3H, d, J = 6.8 Hz), 1.56–1.65 (1H, m), 3.75 (1H, d, J = 12.4 Hz), 4.43 (1H, dd, J = 12.4 and 2.8 Hz), 5.55 (1H d, J = 6.0 Hz), 7.13 (2H, d, J = 8.4 Hz), 7.28–7.38 (3H, m), 7.47 (1H, d, J = 6.0 Hz); 13C NMR (100 MHz, CDCl3) δ 15.1, 19.8, 28.9, 54.7, 87.6, 107.0, 127.7, 129.1, 129.3, 135.7, 163.3, 194.1; IR (KBr, neat) 2966, 2875, 1675, 1600, 1466, 1406, 1276, 1035, 748, 701 cm−1. HRMS (ESI) cald. for C14H16O2 (M+H)+ 217.1223. Found 217.1226.
2-Allyl-2,3-dihydro-3-phenylpyran-4-one (4).
Pale yellow liquid, (64 mg, 60%). 1H NMR (400 MHz, CDCl3) δ 2.25–2.40 (2H, m), 3.69 (3H, d, J = 12.0 Hz), 4.66 (1H, ddd, J = 12.0, 6.8 and 4.0 Hz), 5.05 (1H, dd, J = 15.6 and 4.8 Hz), 5.15 (1H, dd, J = 10.4 Hz), 5.57 (1H, d, J = 6.0 Hz), 5.77–5.88 (1H, m), 7.13 (2H, d, J = 8.4 Hz), 7.27–7.38 (3H, m), 7.44 (1H, d, J = 6.0 Hz); 13C NMR (100 MHz, CDCl3) δ 36.8, 56.0, 82.7, 107.2, 119.0, 127.8, 129.0, 129.3, 132.4, 135.5, 162.5, 193.1; IR (KBr, neat) 2926, 1676, 1601, 1496, 1406, 1281, 1034, 801, 701 cm−1. HRMS (ESI) cald. for C14H14O2 (M+H)+ 215.1067. Found 215.1077.
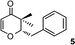
2-Benzyl-2,3-dihydro-3,3-dimethylpyran-4-one (5).
Colourless liquid, (86 mg, 81%). 1H NMR (400 MHz, CDCl3) δ 1.14 (3H, S), 1.23 (3H, s), 2.91–3.02 (2H, m), 4.24 (1H, dd, J = 9.6 and 3.6 Hz), 5.34 (1H, d, J = 6.0 Hz), 7.23–7.28 (3H, m), 7.31–7.36 (3H, m); 13C NMR (100 MHz, CDCl3) δ 18.2, 20.1, 35.0, 44.6, 87.5, 105.4, 126.8, 128.6, 129.4, 138.0, 161.8, 198.4; IR (KBr, neat) 2971, 2873, 1673, 1602, 1496, 1403, 1270, 1058, 1040, 751 cm−1. HRMS (ESI) cald. for C14H16O2 (M+H)+ 217.1223. Found 217.1232.
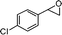
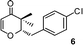
2-(4-Chlorobenzyl)-2,3-dihydro-3,3-dimethylpyran-4-one (6).
Colourless liquid, (81 mg, 65%). 1H NMR (400 MHz, CDCl3) δ 1.13 (3H, S), 1.22 (3H, s), 2.87–3.00 (2H, m), 4.19 (1H, dd, J = 10.0 and 2.8 Hz), 5.35 (1H, d, J = 5.6 Hz), 7.18 (2H, d, J = 8.4 Hz), 7.23 (1H, d, J = 6.0 Hz), 7.30 (2H, d, J = 8.4 Hz); 13C NMR (100 MHz, CDCl3) δ 18.2, 20.1, 34.3, 44.5, 87.2, 105.5, 128.8, 130.8, 132.7, 136.4, 161.6, 198.1; IR (KBr, neat) 2971, 2873, 1673, 1603, 1493, 1401, 1270, 1095, 1041, 815, 796 cm−1. HRMS (ESI) cald. for C14H15ClO2 (M+H)+ 251.0833. Found 251.0845.
2-Benzhydryl-2,3-dihydro-3,3-dimethylpyran-4-one (7).
Pale yellow liquid, (103 mg, 71%). 1H NMR (400 MHz, CDCl3) δ 0.81 (3H, S), 1.06 (3H, s), 4.38 (1H, d, J = 6.0 Hz), 4.83 (1H, d, J = 6.0 Hz), 5.34 (1H, d, J = 5.2 Hz), 7.19–7.32 (7H, m), 7.36 (2H, d, J = 7.2 Hz), 7.43 (2H, d, J = 8.0 Hz); 13C NMR (100 MHz, CDCl3) δ 19.2, 20.6, 45.7, 51.9, 88.0, 105.5, 126.9, 127.9, 128.6, 128.8, 129.3, 129.6, 141.0, 142.6, 161.9, 198.6; IR (KBr, neat) 2967, 2926, 1673, 1601, 1495, 1452, 1276, 1058, 1040, 816, 704 cm−1. HRMS (ESI) cald. for C20H20O2 (M+H)+ 293.1536. Found 293.1549.
2,3-Dihydro-3,3-dimethyl-2-(1-phenylethyl)pyran-4-one (diastereomeric mixture with a ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) (8).
Colourless liquid, (72 mg, 63%). 1H NMR (400 MHz, CDCl3) δ 0.92 (3H, S, minor), 0.99 (3H, s, major), 1.08 (s, 3H, minor), 1.12 (3H, s, 3H, major), 1.41 (3H, d, J = 6.8 Hz, major), 1.47 (3H, d, J = 7.2 Hz, minor), 3.13–3.17 (1H, m, minor), 3.18–3.26 (1H, m, major), 4.15 (1H, d, J = 2.8 Hz, minor), 4.28 (1H, d, J = 4.8 Hz, major), 5.30 (1H, d, J = 6.0 Hz, minor), 5.35 (1H, d, J = 5.6 Hz, major), 7.20–7.42 (5H m), 7.48–7.51 (1H, m); 13C NMR (100 MHz, CDCl3) δ 17.9, 18.7, 19.1, 19.3, 21.2, 22.0, 40.2, 40.4, 45.4, 45.6, 89.9, 90.2, 104.6, 105.3, 126.8, 126.9, 127.6, 127.8, 128.5, 128.8, 143.0, 145.8, 159.3, 162.0, 198.6, 198.8; IR (KBr, neat) 2979, 2933, 1675, 1603, 1454, 1404, 1271, 1158, 1034, 814, 702 cm−1. HRMS (ESI) cald. for C15H18O2 (M+H)+ 231.1380. Found 231.1389.
2) (8).
Colourless liquid, (72 mg, 63%). 1H NMR (400 MHz, CDCl3) δ 0.92 (3H, S, minor), 0.99 (3H, s, major), 1.08 (s, 3H, minor), 1.12 (3H, s, 3H, major), 1.41 (3H, d, J = 6.8 Hz, major), 1.47 (3H, d, J = 7.2 Hz, minor), 3.13–3.17 (1H, m, minor), 3.18–3.26 (1H, m, major), 4.15 (1H, d, J = 2.8 Hz, minor), 4.28 (1H, d, J = 4.8 Hz, major), 5.30 (1H, d, J = 6.0 Hz, minor), 5.35 (1H, d, J = 5.6 Hz, major), 7.20–7.42 (5H m), 7.48–7.51 (1H, m); 13C NMR (100 MHz, CDCl3) δ 17.9, 18.7, 19.1, 19.3, 21.2, 22.0, 40.2, 40.4, 45.4, 45.6, 89.9, 90.2, 104.6, 105.3, 126.8, 126.9, 127.6, 127.8, 128.5, 128.8, 143.0, 145.8, 159.3, 162.0, 198.6, 198.8; IR (KBr, neat) 2979, 2933, 1675, 1603, 1454, 1404, 1271, 1158, 1034, 814, 702 cm−1. HRMS (ESI) cald. for C15H18O2 (M+H)+ 231.1380. Found 231.1389.
2-Cyclohexyl-2,3-dihydro-3,3-dimethylpyran-4-one (9).
Colourless liquid, (81 mg, 78%). 1H NMR (400 MHz, CDCl3) δ 1.09 (3H, S), 1.18 (3H, s), 1.20–1.50 (4H, m), 1.55–1.70 (3H, m), 1.71–1.80 (4H, m), 3.88 (1H, d, J = 2.8 Hz), 5.28 (1H, d, J = 6.0 Hz), 7.31 (1H, d, J = 6.0 Hz); 13C NMR (100 MHz, CDCl3) δ 19.1, 22.3, 26.2, 26.5, 26.7, 28.2, 32.6, 39.0, 44.7, 91.1, 104.9, 162.2, 198.9; IR (KBr, neat) 2930, 2854, 1675, 1603, 1450, 1408, 1276, 1159, 1041, 814 cm−1. HRMS (ESI) cald. for C13H20O2 (M+H)+ 209.1536. Found 209.1546.
2,3-Dihydro-3,3-dimethyl-2-(1-methyl cyclopentyl)pyran-4-one (10).
Pale yellow liquid, (37 mg, 35%). 1H NMR (400 MHz, CDCl3) δ 1.07 (3H, s), 1.16 (3H, s), 1.24 (3H, s), 1.49–1.53 (2H, m), 1.58–1.70 (6H, m), 3.82 (1H, s), 5.31 (1H, d, J = 5.6 Hz), 7.35 (1H, d, J = 5.6 Hz); 13C NMR (100 MHz, CDCl3) δ 20.3, 21.9, 22.5, 22.6, 24.6, 39.0, 40.4, 46.0, 48.7, 95.0, 104.8, 162.6, 199.5; IR (KBr, neat) 2929, 2873, 1676, 1604, 1460, 1407, 1271, 1239, 1157, 1037, 814 cm−1. Analysis calculated for C13H20O2: C, 74.96; H, 9.68. Found C, 75.05; H, 9.51.
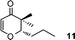
3-Benzyl-2,3-dihydro-2-isopropylpyran-4-one (13).
Pale yellow liquid, (83 mg, 72%). 1H NMR (400 MHz, CDCl3) δ 0.82 (3H, d, J = 6.8 Hz), 0.88 (3H, d, J = 6.8 Hz), 2.07–2.17 (1H, m), 2.68–2.75 (1H, m), 2.90 (1H, dd, J = 13.6 and 8.8 Hz), 3.04 (1H, dd, J = 13.6 and 5.2 Hz), 3.89 (1H, t, J = 7.2 Hz), 5.39 (1H, d, J = 5.6 Hz), 7.16–7.24 (3H, m), 7.25–7.32 (3H, m); 13C NMR (100 MHz, CDCl3) δ 17.7, 18.9, 28.2, 34.7, 49.1, 85.8, 105.9, 126.7, 128.6, 129.3, 138.4, 161.5, 194.4; IR (KBr, neat) 2965, 2931, 1673, 1602, 1496, 1409, 1278, 1033, 800, 701 cm−1. HRMS (ESI) cald. for C15H18O2 (M+H)+ 231.1380. Found 231.1390.
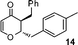
3-Benzyl-2-(4-methylbenzyl)-2,3-dihydropyran-4-one (14).
Pale yellow liquid, (93 mg, 64%). 1H NMR (400 MHz, CDCl3) δ 2.31 (3H, s), 2.60 (1H, dd, J = 13.2 and 6.0 Hz), 2.86 (1H, dd, J = 13.2 and 5.2 Hz), 2.98–3.04 (3H, m), 4.45 (1H, ddd, J = 13.6, 8.4 and 5.2 Hz), 5.45 (1H, d, J = 5.6 Hz), 6.92 (2H, d, J = 8.0 Hz), 7.06 (2H, d, J = 8.0 Hz), 7.14 (2H, d, J = 8.4 Hz), 7.20–7.30 (4H, m); 13C NMR (100 MHz, CDCl3) δ 21.2, 34.9, 37.0, 50.4, 81.4, 106.0, 126.8, 128.8, 129.2, 129.3, 129.4, 133.4, 136.6, 137.9, 161.0, 194.1; IR (KBr, neat) 2924, 2855, 1673, 1600, 1495, 1407, 1269, 1039, 810, 717 cm−1. HRMS (ESI) cald. for C20H20O2 (M+H)+ 293.1536. Found 293.1546.
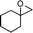
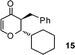
3-Benzyl-2-cyclohexyl-2,3-dihydropyran-4-one (15).
Pale yellow liquid, (108 mg, 80%). 1H NMR (400 MHz, CDCl3) δ 0.83–1.25 (6H, m), 1.42–1.80 (5H, m), 2.73 (1H, ddd, J = 13.6, 9.2 and 4.8 Hz), 2.83 (1H, dd, J = 13.6 and 9.6 Hz), 3.07 (1H, dd, J = 13.6 and 4.4 Hz), 3.95 (1H, dd, J = 7.2 and 4.8 Hz), 5.39 (1H, d, J = 6.0 Hz), 7.18–7.24 (3H, m), 7.25–7.32 (3H, m); 13C NMR (100 MHz, CDCl3) δ 25.6, 25.8, 26.1, 28.2, 28.9, 34.9, 37.5, 48.6, 85.1, 105.7, 126.6, 128.6, 129.2, 138.3, 161.5, 194.6; IR (KBr, neat) 2929, 2853, 1672, 1600, 1452, 1410, 1274, 1033, 797, 700 cm−1. HRMS (ESI) cald. for C18H22O2 (M+H)+ 271.1693. Found 271.1703.
1-(4-Bromobenzyl)-2-oxaspiro[5.5]undec-3-en-5-one (16).
Colourless solid, mp 106–108 °C (from MeOH) (97 mg, 58%). 1H NMR (400 MHz, CDCl3) δ 1.38–1.52 (4H, m), 1.58–1.64 (2H, m), 1.68–1.78 (2H, m), 1.98–2.05 (2H, m), 2.79 (1H, dd, J = 14.4 and 2.8 Hz), 2.96 (1H, dd, J = 14.4 and 11.2 Hz), 4.61 (1H, dd, J = 11.2 and 3.2 Hz), 5.34 (1H, d, J = 6.0 Hz), 7.03 (1H, d, J = 6.0 Hz), 7.06 (2H, d, J = 8.4 Hz), 7.44 (2H, d, J = 8.4 Hz); 13C NMR (100 MHz, CDCl3) δ 21.7, 22.0, 25.9, 27.6, 32.2, 32.6, 47.5, 85.4, 105.9, 120.7, 131.0, 131.8, 137.3, 158.5, 198.4; IR (KBr, neat) 2928, 2856, 1672, 1604, 1490, 1402, 1254, 1011, 792 cm−1. HRMS (ESI) cald. for C17H19BrO2 (M+H)+ 335.0641. Found 335.0654.
2-Benzyl-3-methyl-2,3-dihydro-pyran-4-one (17).
Pale yellow liquid (69 mg, 68%). 1H NMR (400 MHz, CDCl3) δ 1.22 (3H, d, J = 7.2 Hz), 2.39–2.50 (1H, m), 2.98 (1H, dd, J = 14.4 and 8.0 Hz), 3.16 (1H, dd, J = 14.4 and 4.0 Hz), 4.34 (1H, ddd, J = 11.6, 8.0 and 4.0 Hz), 5.37 (1H, d, J = 6.0 Hz), 7.19–7.35 (6H, m); 13C NMR (100 MHz, CDCl3) δ 11.4, 38.5, 43.6, 84.4, 106.0, 126.9, 128.5, 129.7, 136.5, 162.1, 194.8; IR (KBr, neat) 2924, 1675, 1599, 1405, 1254, 1025, 750, 699 cm−1. HRMS (ESI) cald. for C13H14O2 (M+H)+ 203.1067. Found 203.1062.
2-Isopropyl-3-methyl-2,3-dihydro-pyran-4-one (18).
Colourless liquid (60 mg, 79%). 1H NMR (400 MHz, CDCl3) δ 0.97 (3H, d, J = 6.8 Hz), 1.09 (3H, d, J = 6.4 Hz), 1.10 (3H, d, J = 6.4 Hz), 1.89–2.03 (1H, m), 2.50–2.58 (1H, m), 2.92 (1H, dd, J = 12.0 and 3.2 Hz), 5.37 (1H, d, J = 6.0 Hz), 7.35 (1H, d, J = 6.0 Hz); 13C NMR (100 MHz, CDCl3) δ 10.2, 14.8, 19.6, 28.7, 41.9, 88.10, 105.9, 162.7, 195.6; IR (KBr, neat) 2969, 2878, 1676, 1602, 1407, 1298, 1048, 1025, 815 cm−1. HRMS (ESI) cald. for C9H14O2 (M+H)+ 155.1067. Found 155.1070.
Acknowledgements
The authors are grateful to the Council of Scientific and Industrial Research (CSIR) New Delhi for financial support [Grant No. 01(2332)/09/EMR-II]. K. Indukuri acknowledges IIT Guwahati for a fellowship.
References
-
(a) S. Danishefsky, N. Kato, D. Askin and J. F. Kerwin, J. Am. Chem. Soc., 1982, 104, 360 CrossRef CAS;
(b) S. Danishefsky, J. F. Kerwin Jr. and S. Kobayashi, J. Am. Chem. Soc., 1982, 104, 358 CrossRef CAS;
(c) J. S. Danishefsky, W. H. Pearson, D. F. Harvey, C. J. Maring and J. P. Springer, J. Am. Chem. Soc., 1985, 107, 1256 CrossRef;
(d) J. S. Danishefsky, D. C. Myles and D. F. Harvey, J. Am. Chem. Soc., 1987, 109, 862 CrossRef;
(e) J. S. Danishefsky, S. DeNinno and P. Lartey, J. Am. Chem. Soc., 1987, 109, 2082 CrossRef;
(f) D. Meng, E. J. Sorensen, P. Bertinato and J. S. Danishefsky, J. Org. Chem., 1996, 61, 7998 CrossRef CAS;
(g) S. R. Chemler, U. Iserloh and J. S. Danishefsky, Org. Lett., 2001, 3, 2949 CrossRef CAS;
(h) S. Raghavan and P. K. Samanta, Org. Lett., 2012, 14, 2346 CrossRef CAS;
(i) J. M. Cox and J. D. Rainier, Org. Lett., 2001, 3, 2919 CrossRef CAS;
(j) J. D. Rainier, S. P. Allwein and J. M. Cox, Org. Lett., 2000, 2, 231 CrossRef CAS;
(k) A. B. Smith, R. J. Fox and T. M. Razler, Acc. Chem. Res., 2008, 41, 675 CrossRef CAS.
-
(a) S. Danishefsky and M. Bednarski, J. Am. Chem. Soc., 1986, 108, 7060 CrossRef;
(b) S. J. Denishefsky, W. H. Pearson and B. E. Segmuller, J. Am. Chem. Soc., 1985, 107, 1280 CrossRef;
(c) S. J. Denishefsky and M. P. DeNinno, Angew. Chem., Int. Ed. Engl., 1987, 26, 15 CrossRef;
(d) S. J. Denishefsky and M. T. Bilodeau, Angew. Chem., Int. Ed. Engl., 1996, 35, 1380 CrossRef.
- D. Obrecht, C. Zumbrunn and K. Müller, J. Org. Chem., 1999, 64, 6182 CrossRef CAS.
-
(a) K. Maruoka, T. Itoh, T. Shirasaka and H. Yamamoto, J. Am. Chem. Soc., 1988, 110, 310 CrossRef CAS;
(b) P. A. Evans and J. D. Nelson, J. Org. Chem., 1996, 61, 7600 CrossRef CAS;
(c) K. Maruoka and H. Yamamoto, J. Am. Chem. Soc., 1989, 111, 789 CrossRef CAS;
(d) Y. Motoyama, Y. Koga and H. Nishiyama, Tetrahedron, 2001, 57, 853 CrossRef CAS;
(e) J. L. Ruano, M. A. Fernández-Ibáñez and M. C. Maestro, J. Org. Chem., 2006, 71, 7683 CrossRef;
(f) L. Lin, Y. Kuang, X. Liu and X. Feng, Org. Lett., 2011, 13, 3868 CrossRef CAS.
-
(a) P. C. Andrews, W. J. Gee, P. C. Junk and H. Krautscheid, Org. Biomol. Chem., 2010, 8, 698 RSC;
(b) H. Wang, B. J. Shuhler and M. Xian, J. Org. Chem., 2007, 72, 4280 CrossRef CAS;
(c) R. A. Khera, M. Hussain, R. Ahmed, A. Saeed, A. Villinger, C. Fischer and P. Langer, J. Fluorine Chem., 2010, 131, 892 CrossRef CAS.
- M. Reiter, S. Ropp and V. Gouverneur, Org. Lett., 2004, 6, 91 CrossRef CAS.
- J. O. Winkler and K. Oh, Org. Lett., 2005, 7, 2421 CrossRef CAS.
-
(a) G. G. Zipp, M. A. Hilfikker and S. G. Nelson, Org. Lett., 2002, 4, 1823 CrossRef CAS;
(b) B. Gao, Z. Yu, Z. Fu and X. Feng, Tetrahedron Lett., 2006, 47, 1537 CrossRef CAS.
-
(a) S. Danishefsky, E. R. Larson and D. Askin, J. Am. Chem. Soc., 1982, 104, 6457 CrossRef CAS;
(b) Y. Yamashita, S. Saito, H. Ishitani and S. Kobayashi, J. Am. Chem. Soc., 2003, 125, 3793 CrossRef CAS;
(c) Y. Yamashita, S. Saito, H. Ishitani and S. Kobayashi, Org. Lett., 2002, 4, 1221 CrossRef CAS.
-
(a) J.-I. Matsuo, S. Sasaki, H. Tanaka and H. Ishibashi, J. Am. Chem. Soc., 2008, 130, 11600 CrossRef CAS;
(b) S. Negishi, H. Ishibashi and J.-I. Matsuo, Org. Lett., 2010, 12, 4984 CrossRef CAS.
-
(a) S. Yao, M. Johannsen, H. Audrain, R. G. Hazell and K. A. Jørgensen, J. Am. Chem. Soc., 1998, 120, 8599 CrossRef CAS;
(b) M. Anada, T. Washio, N. Shimada, S. Kitagaki, M. Nakajima, M. Shiro and S. Hashimoto, Angew. Chem., Int. Ed., 2004, 43, 2665 CrossRef CAS.
-
(a) R. E. Parker and N. S. Isaacs, Chem. Rev., 1959, 59, 737 CrossRef CAS;
(b) A. S. Rao, S. K. Paknikar and J. G. Kirtane, Tetrahedron, 1983, 39, 2323 CrossRef CAS;
(c) J. G. Smith, Synthesis, 1984, 629 CrossRef CAS.
-
(a) B. C. Ranu and U. Jana, J. Org. Chem., 1998, 63, 8212 CrossRef CAS;
(b) J. Li and C.-J. Li, Tetrahedron Lett., 2001, 42, 793 CrossRef CAS.
- K. Indukuri, S. Bondalapati, T. Kotipalli, P. Gogoi and A. K. Saikia, Synlett, 2012, 23, 233 CAS.
- Y. Sugihara, S. Iimura and J. Nakayama, Chem. Commun., 2002, 134 RSC.
- S. J. Danishefsky, E. Larson, D. Askin and N. Kato, J. Am. Chem. Soc., 1985, 107, 1246 CrossRef CAS.
- J.-I. Matsuo, R. Okuno, K. Takeuchi, M. Kawano and H. Ishibashi, Tetrahedron Lett., 2010, 51, 3736 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21468g |
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) trans ratio of 1
trans ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. In order to improve the yield and diastereosectivity, the reaction was screened with other Lewis acids such as TiCl4, In(OTf)3, TMSOTf and the Brønsted acid TsOH as shown in Table 1. These results showed that BF3·Et2O was the most efficient Lewis acid. No reaction was observed when TiCl4 or TMSOTf was used. Brønsted acid TsOH was also found to be ineffective. The effect of solvent on diastereoselectivity was also studied. The results are summarized in Table 1. Among these dichloromethane was found to be the best solvent, whereas toluene and tetrahydrofuran were found to be less effective. Therefore, BF3·Et2O in CH2Cl2 was considered as the best combination for both the yields and stereoselectivity. The yield and the diastereoselectivity were found to be same after 12 h.
8. In order to improve the yield and diastereosectivity, the reaction was screened with other Lewis acids such as TiCl4, In(OTf)3, TMSOTf and the Brønsted acid TsOH as shown in Table 1. These results showed that BF3·Et2O was the most efficient Lewis acid. No reaction was observed when TiCl4 or TMSOTf was used. Brønsted acid TsOH was also found to be ineffective. The effect of solvent on diastereoselectivity was also studied. The results are summarized in Table 1. Among these dichloromethane was found to be the best solvent, whereas toluene and tetrahydrofuran were found to be less effective. Therefore, BF3·Et2O in CH2Cl2 was considered as the best combination for both the yields and stereoselectivity. The yield and the diastereoselectivity were found to be same after 12 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Scheme 1).15 The aldehyde 20 only takes part in the reaction to form the dihydropyranone 10. Similarly 2-methyl-3-phenyloxirane (entry 12) was also not effective for this reaction. In this case, the epoxide after rearrangement produces 1-phenylpropane-2-one, which being a ketone does not take part in the reaction.
1 (Scheme 1).15 The aldehyde 20 only takes part in the reaction to form the dihydropyranone 10. Similarly 2-methyl-3-phenyloxirane (entry 12) was also not effective for this reaction. In this case, the epoxide after rearrangement produces 1-phenylpropane-2-one, which being a ketone does not take part in the reaction.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the ration was determined by 1H NMR spectroscopy.
1, and the ration was determined by 1H NMR spectroscopy.



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9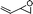


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8

















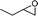





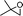


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17
17


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5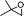


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7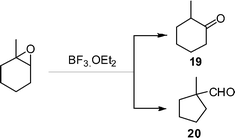


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) (8).
Colourless liquid, (72 mg, 63%). 1H NMR (400 MHz, CDCl3) δ 0.92 (3H, S, minor), 0.99 (3H, s, major), 1.08 (s, 3H, minor), 1.12 (3H, s, 3H, major), 1.41 (3H, d, J = 6.8 Hz, major), 1.47 (3H, d, J = 7.2 Hz, minor), 3.13–3.17 (1H, m, minor), 3.18–3.26 (1H, m, major), 4.15 (1H, d, J = 2.8 Hz, minor), 4.28 (1H, d, J = 4.8 Hz, major), 5.30 (1H, d, J = 6.0 Hz, minor), 5.35 (1H, d, J = 5.6 Hz, major), 7.20–7.42 (5H m), 7.48–7.51 (1H, m); 13C NMR (100 MHz, CDCl3) δ 17.9, 18.7, 19.1, 19.3, 21.2, 22.0, 40.2, 40.4, 45.4, 45.6, 89.9, 90.2, 104.6, 105.3, 126.8, 126.9, 127.6, 127.8, 128.5, 128.8, 143.0, 145.8, 159.3, 162.0, 198.6, 198.8; IR (KBr, neat) 2979, 2933, 1675, 1603, 1454, 1404, 1271, 1158, 1034, 814, 702 cm−1. HRMS (ESI) cald. for C15H18O2 (M+H)+ 231.1380. Found 231.1389.
2) (8).
Colourless liquid, (72 mg, 63%). 1H NMR (400 MHz, CDCl3) δ 0.92 (3H, S, minor), 0.99 (3H, s, major), 1.08 (s, 3H, minor), 1.12 (3H, s, 3H, major), 1.41 (3H, d, J = 6.8 Hz, major), 1.47 (3H, d, J = 7.2 Hz, minor), 3.13–3.17 (1H, m, minor), 3.18–3.26 (1H, m, major), 4.15 (1H, d, J = 2.8 Hz, minor), 4.28 (1H, d, J = 4.8 Hz, major), 5.30 (1H, d, J = 6.0 Hz, minor), 5.35 (1H, d, J = 5.6 Hz, major), 7.20–7.42 (5H m), 7.48–7.51 (1H, m); 13C NMR (100 MHz, CDCl3) δ 17.9, 18.7, 19.1, 19.3, 21.2, 22.0, 40.2, 40.4, 45.4, 45.6, 89.9, 90.2, 104.6, 105.3, 126.8, 126.9, 127.6, 127.8, 128.5, 128.8, 143.0, 145.8, 159.3, 162.0, 198.6, 198.8; IR (KBr, neat) 2979, 2933, 1675, 1603, 1454, 1404, 1271, 1158, 1034, 814, 702 cm−1. HRMS (ESI) cald. for C15H18O2 (M+H)+ 231.1380. Found 231.1389.