Bright fluorescent hollow structural nanoparticles prepared from amphiphilic molecules with C2v symmetry†
Rong-Hua
Kang
,
Mei-Ling
Zheng
*,
Wei-Qiang
Chen
,
Zhen-Sheng
Zhao
and
Xuan-Ming
Duan
*
Laboratory of Organic NanoPhotonics and Key Laboratory of Functional Crystals and Laser Technology, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: xmduan@mail.ipc.ac.cn; zhengmeiling@mail.ipc.ac.cn; Fax: +86 10 8254 3597; Tel: +86 10 8254 3596
First published on 6th September 2012
Abstract
Bright fluorescent hollow structural nanoparticles were prepared from 3,6-bis-[2-(4-carboxylic acid)phenylethynyl]-9-pentyl carbazole (I), an amphiphilic molecule with C2v symmetry, by supramolecular interaction including hydrogen bonding, van de Waals interaction and π–π stacking. The nanoparticles with the average diameter of 88 nm exhibited a fluorescence quantum yield of 0.79.
The self-assembly of non-lipid amphiphilic molecules, which carry both hydrophilic and hydrophobic parts within one structure, has stimulated interest in the material science and basic research fields due to the possibility of forming a variety of nanostructures with unique physical and chemical properties.1 Among these nanostructures, hollow structural nanoparticles, enclosed spherical bilayer assemblies, have attracted great attention from a fundamental perspective as well as for their potential applications in drug or gene delivery, nanotechnology, and as model systems of biomembranes.2 Hollow structural nanoparticles constructed with copolymers and classical head-group surfactants and phospholipids have been intensively investigated.3 Few small synthetic molecules are known that spontaneously self-assemble into nanoparticles without the addition of surfactants and phospholipids. Recent exceptions include facially amphiphilic segmented dendrimers,4a functionalized calixarenes,4b,c cyclodexdrins,4d fullerenes,4e,f cucurbit[6]urils,4go-phenylene vinylene derivative,4hortho-phenylene ethyneylene macrocycle,4i guanosine derivatives,4j aromatic hydrazide4k and amide.4l Nanoparticles from functionalized small molecules have critical potential for applications such as nano-scopic intelligent materials and photochemical switching, since they can respond to external stimuli.4c,4f,5 Luminescent nanoparticles play important roles in the application of sensors, remote light-stimuli drug release systems, and nonlinear optical materials.6 Growing molecular architectures that can spontaneously form nanoparticles with strong fluorescence remains an important goal for better understanding the molecular design and exploiting the potential applications.
Here we report that an amphiphilic molecule, 3,6-bis-[2-(4-carboxylic acid)phenylethynyl]-9-pentyl carbazole (I in Scheme 1), forms bright fluorescent nanoparticles in aqueous media. Molecule I, a central carbazole linking two phenyl rings through acetylene bonds, possesses a unique rigid π-conjugated structure with C2v symmetry. The terminal benzoic acid forms the hydrophilic part and the rigid conjugated system with pentyl group forms the hydrophobic part. In aqueous media, the carboxylic acid moieties of molecule I provide the possibility to form dual hydrogen bonding between the molecules and self-assemble into a zig-zag alignment.7 Furthermore, the π–π stacking of conjugated phenyethynyl-carbazole and van de Waals interaction of alkyl chains facilitate the zig-zag alignment self-assembling into hollow nanostructures. Therefore, the supramolecular effects including the formation of hydrogen bonding and the π–π stacking of the molecules promote the self-assembly of compound I to form fluorescent nanoparticles (Fig. 1).
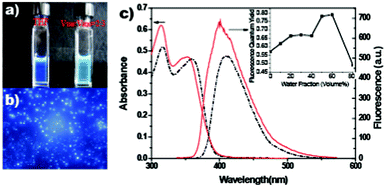 | ||
Fig. 1 (a) A fluorescence photograph of compound I in THF and THF/H2O (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 2 VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) mixed solvent. (b) Fluorescence microscopy of compound I in THF/H2O (VTHF 3) mixed solvent. (b) Fluorescence microscopy of compound I in THF/H2O (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 2 VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) mixed solvent. (c) The UV-Vis absorption and fluorescence spectra of compound I in THF (black dash–dot line) and in THF/H2O (VTHF 3) mixed solvent. (c) The UV-Vis absorption and fluorescence spectra of compound I in THF (black dash–dot line) and in THF/H2O (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 2 VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) mixed solvent (red line). Inset is the dependence of the fluorescence quantum yield of I on the different ratios of THF/H2O. 3) mixed solvent (red line). Inset is the dependence of the fluorescence quantum yield of I on the different ratios of THF/H2O. | ||
 | ||
| Scheme 1 A schematic illustration of the self-organisation process. | ||
C 2v symmetrical amphiphilic compound I was prepared via a two-step synthesis starting from 3,6-diethynyl-9-pentylcarbazole, which was synthesized from the commercial carbazole.8 The resulting diethyne was reacted with a little excess amount of ethyl 4-bromobenzoate via a Pd-catalysed Sonogashira cross-coupling reaction followed by esterification to achieve C2v amphiphilic compound I, which has a total yield of 68% (Scheme S1, ESI†).
Compound I formed aggregates in aqueous media, which led to a big difference in the steady state fluorescence spectra. With the excitation at 365 nm, compound I in a mixed THF/H2O (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 2
VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solvent exhibited enhanced fluorescence emission compared with that in THF at the same molecular concentration of 1 × 10−4 M (Fig. 1a). When irradiated with laser light, obvious scattering light was observed from compound I in the mixed THF/H2O (VTHF
3) solvent exhibited enhanced fluorescence emission compared with that in THF at the same molecular concentration of 1 × 10−4 M (Fig. 1a). When irradiated with laser light, obvious scattering light was observed from compound I in the mixed THF/H2O (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 2
VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solvent, which indicated that compound I aggregated in the aqueous medium. These aggregates exhibited strong fluorescence as depicted in Fig. 1b. UV-Vis absorption spectra of compound I in THF exhibited strong absorption at 316 nm (ε = 52
3) solvent, which indicated that compound I aggregated in the aqueous medium. These aggregates exhibited strong fluorescence as depicted in Fig. 1b. UV-Vis absorption spectra of compound I in THF exhibited strong absorption at 316 nm (ε = 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and 361 nm (ε = 46
000 M−1 cm−1) and 361 nm (ε = 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1). When the ratio of water was increased to 60%, the strong absorption band at 316 nm blue shifted to 314.5 nm with a molar coefficient of 62
200 M−1 cm−1). When the ratio of water was increased to 60%, the strong absorption band at 316 nm blue shifted to 314.5 nm with a molar coefficient of 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1, which was about 20% increased, and the band at 361 nm blue sifted to 353 nm (ε = 47
000 M−1 cm−1, which was about 20% increased, and the band at 361 nm blue sifted to 353 nm (ε = 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1) (Fig. 1c). With the excitation at 316 nm, the emission band of I in THF appeared at 410 nm, which became a sharp peak and blue shifted to 400 nm for that in the mixed THF/H2O (VTHF
200 M−1 cm−1) (Fig. 1c). With the excitation at 316 nm, the emission band of I in THF appeared at 410 nm, which became a sharp peak and blue shifted to 400 nm for that in the mixed THF/H2O (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 2
VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solvent with a shoulder peak at 410 nm. The results indicated that in mixed THF/H2O (VTHF
3) solvent with a shoulder peak at 410 nm. The results indicated that in mixed THF/H2O (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 2
VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solvent, compound I exhibited two emission bands: emissions from the aggregates and the molecular form. The fluorescence quantum yield was measured by using Quinine sulfate in 1.0 N aqueous H2SO4 solution (Φ = 0.54) as a reference standard. When excited at 314 nm, the fluorescence intensity of compound I in mixed THF/H2O solvent is strongly dependent on the amount of water (inset in Fig. 1c). The fluorescence quantum yield increased by 39% from 0.57 to 0.79 when the amount of water was increased from 0 to 60%. The increased fluorescence would contribute to the aggregation-induced emission enhancement.10 It was reasonable that the blue shift of the fluorescence emission for this kind of molecule with π-conjugated chromophores was attributed to the H-aggregation.10c Compound I possesses carboxylic acid moieties, an alkyl chain and a large π-conjugated structure, which tend to form dual hydrogen bonding, van de Waals interactions and π–π stacking interactions. Molecular interaction limits the twist and rotation of molecules. Therefore, the rotation and vibration of molecules are restricted in the aggregated state, leading to a fluorescence emission enhancement.6c,d
3) solvent, compound I exhibited two emission bands: emissions from the aggregates and the molecular form. The fluorescence quantum yield was measured by using Quinine sulfate in 1.0 N aqueous H2SO4 solution (Φ = 0.54) as a reference standard. When excited at 314 nm, the fluorescence intensity of compound I in mixed THF/H2O solvent is strongly dependent on the amount of water (inset in Fig. 1c). The fluorescence quantum yield increased by 39% from 0.57 to 0.79 when the amount of water was increased from 0 to 60%. The increased fluorescence would contribute to the aggregation-induced emission enhancement.10 It was reasonable that the blue shift of the fluorescence emission for this kind of molecule with π-conjugated chromophores was attributed to the H-aggregation.10c Compound I possesses carboxylic acid moieties, an alkyl chain and a large π-conjugated structure, which tend to form dual hydrogen bonding, van de Waals interactions and π–π stacking interactions. Molecular interaction limits the twist and rotation of molecules. Therefore, the rotation and vibration of molecules are restricted in the aggregated state, leading to a fluorescence emission enhancement.6c,d
The morphology of the aggregates of compound I in the mixed THF/H2O solvent was investigated by scanning electron microscopy (SEM). The SEM images provided evidence for the formation of the spherical structures. As depicted in Fig. 2a, compound I in the mixed THF/H2O (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 2
VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solvent formed spherical nanostructures. The average size of the self-assembled spherical nano-aggregates was about 88 nm (Fig. 2b). The dynamic light scattering (DLS) measurement showed that the average size of the spherical nano-aggregates reached 320 nm, in which the large nanoparticles contributed to this larger size compared to that of the SEM result (Fig. S1, ESI†). These spherical structures became flat when the sample was kept for a longer time under vacuum before SEM measurement (Fig. S2, ESI†). This demonstrated that the aggregates were of hollow spherical structure and the solvent inside the nano-aggregates was removed in a vacuum. Transmission electron microscopy (TEM) images provided further evidence for their hollow nature (Fig. 2d), as revealed by the contrast between the peripheral and central areas of the spherical aggregates, which is typically produced by the projection of hollow spheres.9 It was estimated that the wall thickness of the nanoparticles was approximately 6 nm. The results suggest that the wall of the fluorescent nanoparticles has an interdigitated bilayer structure (Scheme 1), although other stacking patterns can not be excluded.
3) solvent formed spherical nanostructures. The average size of the self-assembled spherical nano-aggregates was about 88 nm (Fig. 2b). The dynamic light scattering (DLS) measurement showed that the average size of the spherical nano-aggregates reached 320 nm, in which the large nanoparticles contributed to this larger size compared to that of the SEM result (Fig. S1, ESI†). These spherical structures became flat when the sample was kept for a longer time under vacuum before SEM measurement (Fig. S2, ESI†). This demonstrated that the aggregates were of hollow spherical structure and the solvent inside the nano-aggregates was removed in a vacuum. Transmission electron microscopy (TEM) images provided further evidence for their hollow nature (Fig. 2d), as revealed by the contrast between the peripheral and central areas of the spherical aggregates, which is typically produced by the projection of hollow spheres.9 It was estimated that the wall thickness of the nanoparticles was approximately 6 nm. The results suggest that the wall of the fluorescent nanoparticles has an interdigitated bilayer structure (Scheme 1), although other stacking patterns can not be excluded.
 | ||
Fig. 2 (a) An SEM image of compound I in THF/H2O (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 2 VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) mixed solvent at a concentration of 1 × 10−4 M. (b) The size distribution of the aggregates. (c) A TEM image and (d) a magnified TEM image of the fluorescent nanoparticles. 3) mixed solvent at a concentration of 1 × 10−4 M. (b) The size distribution of the aggregates. (c) A TEM image and (d) a magnified TEM image of the fluorescent nanoparticles. | ||
Additionally, the morphologies and diameters of the nanoparticles measured by SEM are correlated with the ratio of THF/H2O. The aggregates were sheet-like pieces in THF, and became irregular and bonded together when the fraction of water was increased to 30%. If the fraction of water was increased to 80%, spherical nanostructures with a large size range were observed. With the increasing ratio of water, the morphologies of the nanoparticles became more regular and the mean of diameters increased (Fig. S2, ESI†). The results demonstrated that compound I self-assembled into fluorescent hollow structural nanoparticles in the mixed THF/H2O solvent through intermolecular hydrogen bonds and π–π stacking interactions.
The nanoparticles obtained are very stable at room temperature, and can be also destroyed by the addition of THF and recovered by the injection of water. We have also investigated the UV-Vis absorption spectra and fluorescence spectra of the nanoparticles in THF/H2O (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 2
VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) at different temperatures and found there was little change (Fig. S3, ESI†). It is notable that hydrogen bonds are sensitive to temperature,10 however, the nanoparticles in this research were proved to be thermostable. Thus, we can confirm that π–π stacking and van de Waals interaction of the molecules were important forces as well as the intermolecular hydrogen bonds in the process of self-assembly.
3) at different temperatures and found there was little change (Fig. S3, ESI†). It is notable that hydrogen bonds are sensitive to temperature,10 however, the nanoparticles in this research were proved to be thermostable. Thus, we can confirm that π–π stacking and van de Waals interaction of the molecules were important forces as well as the intermolecular hydrogen bonds in the process of self-assembly.
Conclusions
In conclusion, we successfully prepared fluorescent hollow structural nanoparticles on the submicrometer scale in THF aqueous media using a C2v symmetrical amphiphilic molecule, containing terminal benzoic acid moiety and pentyl group, which can form dual hydrogen bonding, van de Waals interactions and π–π interactions. In the mixed THF/H2O (VTHF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 2
VH2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solvent, the nanoparticles prepared from compound I showed strong fluorescence emission, and their fluorescence quantum yield reached 0.79. SEM and TEM demonstrated that these assemblies contained a bilayer and had an average diameter of around 88 nm. This study would provide prospects for developing fluorescent nanoparticles and exploiting their potential application in photochemistry.
3) solvent, the nanoparticles prepared from compound I showed strong fluorescence emission, and their fluorescence quantum yield reached 0.79. SEM and TEM demonstrated that these assemblies contained a bilayer and had an average diameter of around 88 nm. This study would provide prospects for developing fluorescent nanoparticles and exploiting their potential application in photochemistry.
Acknowledgements
The authors are grateful to the National Basic Research Program of China (2010CB934103), International Cooperation Program of MOST (2010DFA01180) and the National Natural Science Foundation of China (Grant No. 50973126) for financial support.References
- (a) D. Y. Chen and M. Jiang, Acc. Chem. Res., 2005, 38, 494–502 CrossRef CAS; (b) D. Y. Yan and Y. F. Zhou, Science, 2004, 303, 65–67 CrossRef CAS; (c) P. Jonkheijm and E. W. Meijer, Science, 2006, 313, 80–83 CrossRef CAS; (d) D. N. Reinhoudt and M. Crego-Calama, Science, 2002, 295, 2403–2407 CrossRef CAS.
- (a) E. Soussan, S. Cassel, M. Blanzat and I. Rico-Lattes, Angew. Chem., Int. Ed., 2009, 48, 274–288 CrossRef CAS; (b) S. H. Seo, J. Y. Chang and G. N. Tew, Angew. Chem., Int. Ed., 2006, 45, 7526–7530 CrossRef CAS; (c) T. M. Allen and P. R. Cullis, Science, 2004, 303, 1818–1822 CrossRef CAS; (d) A. Mueller and D. F. O’Brien, Chem. Rev., 2002, 102, 727–758 CrossRef CAS; (e) R. Blumenthal, M. J. Clague, S. R. Durell and R. M. Epand, Chem. Rev., 2003, 103, 53–70 CrossRef CAS.
- (a) S. Chen, B. Yang, C. Guo, J. H. Ma, L. R. Yang, X. F. Liang, C. Hua and H. Z. Liu, J. Phys. Chem. B, 2008, 112, 15659–15665 CrossRef CAS; (b) E. P. Holowka, D. J. Pochan and T. J. Deming, J. Am. Chem. Soc., 2005, 127, 12423–124289 CrossRef CAS; (c) T. Zehl, M. Wahab, R. Schmidt, P. Schiller and H. J. Mögel, J. Mol. Liq., 2009, 147, 178–181 CrossRef CAS; (d) V. Chan and K. T. Wan, Langmuir, 2002, 18, 3134–3141 CrossRef CAS.
- (a) M. Yang, W. Wang, F. Yuan, X. W. Zhang, J. Y. Li, F. X. Liang, B. L. He, B. Minch and G. Wegner, J. Am. Chem. Soc., 2005, 127, 15107–15111 CrossRef CAS; (b) Y. Tanaka, M. Miyachi and Y. Kobuke, Angew. Chem., Int. Ed., 1999, 38, 504–506 CrossRef CAS; (c) M. Lee, S. J. Lee and Li. H. Jiang, J. Am. Chem. Soc., 2004, 126, 12724–12725 CrossRef CAS; (d) B. J. Ravoo and R. Darcy, Angew. Chem., Int. Ed., 2000, 39, 4324–4326 CrossRef CAS; (e) S. Zhou, C. Burger, B. Chu, M. Sawamura, N. Nagahama, M. Toganoh, U. E. Hackler, H. Isobe and E. Nakamura, Science, 2001, 291, 1944–1947 CrossRef CAS; (f) R. Charvet, D. L. Jiang and T. Aida, Chem. Commun., 2004, 2664–2665 RSC; (g) H. K. Lee, K. M. Park, Y. J. Jeon, D. Kom, D. Y. Oh, H. S. Kim, C. K. Park and K. Kim, J. Am. Chem. Soc., 2005, 127, 5006–5007 CrossRef CAS; (h) F. J. M. Hoeben, I. O. Shklyarevskiy, M. J. Pouderoijen, H. Engelkamp, L. Schenning, P. C. M. Christianen, J. C. Maan and E. W. Meijer, Angew. Chem., Int. Ed., 2006, 45, 1232–1236 CrossRef CAS; (i) S. H. Seo, J. Y. Chang and G. N. Tew, Angew. Chem., Int. Ed., 2006, 45, 7526–7530 CrossRef CAS; (j) I. Yoshikawa, J. Sawayama and K. Araki, Angew. Chem., Int. Ed., 2008, 47, 1038–1041 CrossRef CAS; (k) W. Cai, G. T. Wang, Y. X. Xu, X. K. Jiang and Z. T. Li, J. Am. Chem. Soc., 2008, 130, 6936–6937 CrossRef CAS; (l) Y. X. Xu, G. T. Wang, X. Zhao, X. K. Jiang and Z. T. Li, Langmuir, 2009, 25, 2684–2688 CrossRef CAS.
- (a) S. Y. Yu, T. Azzam, I. Rouiller and A. Eisenberg, J. Am. Chem. Soc., 2009, 131, 10557–10566 CrossRef CAS; (b) C. Wang, Y. Guo, Y. Wang, H. Xu and X. Zhang, Chem. Commun., 2009, 5380–5382 RSC.
- (a) D. A. Joe, S. Stadlbauer and B. König, Chem.–Eur. J., 2009, 15, 7404–7412 CrossRef; (b) D. V. Volodkin, A. G. Skirtach and H. Mohwald, Angew. Chem., Int. Ed., 2009, 48, 1807–1809 CrossRef CAS; (c) M.-L. Zheng, W.-Q. Chen, K. Fujita, X.-M. Duan and S. Kawata, Nanoscale, 2010, 2, 913–916 RSC; (d) M.-L. Zheng, K. Fujita, W.-Q. Chen, X.-M. Duan and S. Kawata, J. Phys. Chem. C, 2011, 115, 8988–8993 CrossRef CAS.
- T. L. Zhou, F. Li, Y. Fan, W. F. Song, X. Y. Mu, H. Y. Zhang and Y. Wang, Chem. Commun., 2009, 3199–3201 RSC.
- (a) J. Gu, Y.-L. Wang, W.-Q. Chen, X.-Z. Dong, X.-M. Duan and S. Kawata, New J. Chem., 2007, 31, 63–68 RSC; (b) J.-F. Xing, W.-Q. Chen, J. Gu, X.-Z. Dong, N. Takeyasu, T. Tanaka, X.-M. Duan and S. Kawatac, J. Mater. Chem., 2007, 17, 1433–1438 RSC; (c) J. Gu, W.-Q. Chen, T. Wada, D. Hashizume and X.-M. Duan, CrystEngComm, 2007, 9, 541–544 RSC.
- (a) A. Ajayaghosh, P. Chithra and P. Varghese, Angew. Chem., Int. Ed., 2007, 46, 203–233 Search PubMed; (b) D. Xie, M. Jiang, G. Z. Zhang and D. Y. Chen, Chem.–Eur. J., 2008, 13, 3346–3353 CrossRef.
- (a) J. D. Luo, Z. L. Xie, L. Cheng, H. Y. Chen, C. F. Qiu, H. S. Kwok, X. W. Zhan, Y. Q. Liu, D. B. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC; (b) Q. Zeng, Z. Li, Y.Q. Dong, C. A. Di, A. J. Qin, Y. N. Hong, L. Ji, Z. C. Zhu, G. Yu, Q. Q. Li, Z. A. Li, Y. Q. Liu, J. G. Qin and B. Z. Tang, Chem. Commun., 2007, 70–72 RSC; (c) S. Li, L. He, F. Xiong, Y. Li and G. Yang, J. Phys. Chem. B, 2004, 108, 10887–10892 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and measurements, synthesis and characterization of the compounds, DLS experiments, SEM images and the influence of the temperature. See DOI: 10.1039/c2ra21394j |
| This journal is © The Royal Society of Chemistry 2012 |
