Bisactinyl halogenated complexes: relativistic density functional theory calculation and experimental synthesis†
Abstract
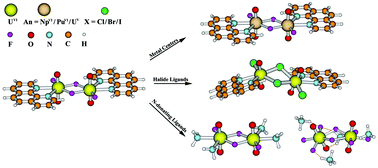
Relativistic density functional theory was used to explore a series of bisactinyl complexes, [(phen)(AnVIO2)(μ2-F)(F)]2 (An = U (1), Np (2) and Pu (3); phen = ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O indicate the partial triple bonding character, and agree with the trends in the An
O indicate the partial triple bonding character, and agree with the trends in the An![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrational frequencies. The free energies of formation reactions of 1–11 calculated in the aqueous solution demonstrate that 1, 8 and 11 are thermodynamically stable. In this work, we have successfully synthesized 1 and [Me2NH2]4[(F)2(UVIO2)(μ2-F)(F)]2 (12), which is represented by theoretical model complex 11. Their characterizations of the single crystal X-ray diffraction and infrared are consistent with the calculated results; the measured fine-structured fluorescent emissions were assigned as transitions from the U
O stretching vibrational frequencies. The free energies of formation reactions of 1–11 calculated in the aqueous solution demonstrate that 1, 8 and 11 are thermodynamically stable. In this work, we have successfully synthesized 1 and [Me2NH2]4[(F)2(UVIO2)(μ2-F)(F)]2 (12), which is represented by theoretical model complex 11. Their characterizations of the single crystal X-ray diffraction and infrared are consistent with the calculated results; the measured fine-structured fluorescent emissions were assigned as transitions from the U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonding to the U (f) orbital by analyzing the electronic structure and
O bonding to the U (f) orbital by analyzing the electronic structure and

 Please wait while we load your content...
Please wait while we load your content...