DOI:
10.1039/C2RA21130K
(Paper)
RSC Adv., 2012,
2, 11552-11556
New reports on anti-bacterial and anti-candidal activities of fatty acid methyl esters (FAME) obtained from Scenedesmus bijugatus var. bicellularis biomass
Received
6th June 2012
, Accepted 26th September 2012
First published on 2nd October 2012
Abstract
The present study evaluates the efficiency of fatty acid methyl esters (FAMEs) obtained from microalgal (Scenedesmus bijugatus var bicellularis) biomass as an antimicrobial agent against Staphylococcus aureus, Escherichia coli and Candida albicans. The FAME profiles were determined through Gas Chromatography (GC) with a Flame Ionization detector (FID). The FAMEs showed inhibitory activity against all three microorganisms and thereby exhibited both anti-bacterial and anti-candidal activity. GC-FID analysis reveals about 30 different FAMEs. Out of these, various pharmacologically active FAMEs like stearic acid methyl ester (C18:0) (0.6% w/w), oleic acid methyl ester (C18:1) (1% w/w), linoleic acid methyl ester (C18:2) (1.40% w/w), linolenic acid methyl ester (C18:3) (6.26%), eicosapentanoic acid methyl ester (C20:5) (1.13% w/w), erucic acid methyl ester (C22:1) (1.03% w/w) and docosahexenoic acid methyl ester (C22:6) (2.27% w/w) were detected, which accounted for the bioactivity. These results clearly indicate that the FAMEs of S. bijugatus var. bicellularis have strong antimicrobial properties and could thus be used as an effective source against microbial diseases.
1. Introduction
In the present scenario, microalgal oil and fatty acids have received global attention and interest. Microalgae have long been known for their lipids, where in relative terms they have a wide range of applications that may include biodiesel feed stock,1 aquaculture and mariculture feed, and be involved directly or indirectly in the human diet.2 Given that the applications of microalgal fatty acids are extensively studied, it is surprising that very few or almost no investigations were made into their antimicrobial activity. However, reports are available on the antimicrobial activity of fatty acids from plant leaves,3–6 seeds and roots7,8 and fish oil.9 Anti-inflammatory effects of fatty acids were also recently reported.10 This motivated us to test the nature of the microalgal FAMEs for their antimicrobial properties.
With this background, the present study aimed to supplement available data concerning the anti-candidal and anti-bacterial activity of FAMEs obtained from a microalga, Scenedesmus bijugatus var. bicellularis, green microalga of the phylum Chlorophyta, order Chlorococcales and family Scenedesmaceae. Through this study the anti-bacterial and anti-candidal activities were reported for the first time in the fatty acid methyl esters (FAMEs) obtained from S. bijugatus var. bicellularis.
2. Materials and methods
2.1. Algal culture
Scenedesmus bijugatus var. bicellularis was originally isolated from a freshwater source in Sooriyur, Tiruchirappalli, India (Latitude: 9° 51′ 30′′–10° 48′ 08′′ North Longitude: 78° 25′ 40′′–79° 16′ 22′′ East) and maintained in the Germplasm Culture Collection Centre, Department of Microbiology, Bharathidasan University, Tiruchirappalli, India. The culturing was carried out in glass tanks with Bold's basal medium at 25 ± 1 °C with 35 μmol m−2 s−1 light intensity under 12/12 light-dark cycles.11
2.3. Anti-bacterial assay
In vitro anti-bacterial activity was determined by a well diffusion method using Mueller Hinton agar and Mueller Hinton broth. Overnight (24 h) grown broth cultures of multi drug resistant pathogens such as Escherichia coli (gram negative) and Staphylococcus aureus (gram positive) (obtained from Doctor's Diagnostic Centre, Tiruchirappalli, India) were swabbed over freshly prepared MHA plates and allowed to dry for 5 min. Five wells were made using a sterile cork borer, and about 35 μl of the sample (cell free supernatant, lipid, FAME, ethyl acetate and methanol) were added, with ethyl acetate and methanol as solvent controls. The plates were incubated at 37 °C for 24 h. Before the analysis, bacterial cultures were tested against standard antibiotics.
2.4. Anti-candidal assay
In vitro anti-candidal activity was determined by using Sabouraud's Dextrose agar and broth. Here overnight grown (24 h) Candida albicans broth culture (obtained from Doctor's Diagnostic Center, Tiruchirappalli, India) was used and the well diffusion method was carried out as described above. After inoculation, the plates were incubated at room temperature for 48 h.
2.5. Gas chromatographic (GC) analysis
The FAME samples were analyzed by gas chromatography (Shimadzu, GC2014, Japan) with a flame ionization detector (FID).12 One microliter of each sample was injected in the FAMEWAX column (Restek, USA) (30 m × 32 mmID × 25μm film thickness). The temperature program was as follows: Initial 140 °C with 5 min hold; ramp 2 °C min−1 to 230 °C with a 5 min hold. Column flow was set at 22.2 ml min−1. Instrument conditions were as follows: Carrier gas nitrogen; FID set at 260 °C, split ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Run time for a single sample was 55 min. Triplicates of each sample were done and FAME identification was run by comparison with standard certificate, Supelco FAME mix C4 –C24 (Bellefonte, PA, USA). Each sample was run under GC in triplicate.
1. Run time for a single sample was 55 min. Triplicates of each sample were done and FAME identification was run by comparison with standard certificate, Supelco FAME mix C4 –C24 (Bellefonte, PA, USA). Each sample was run under GC in triplicate.
3. Results
S. bijugatus var. bicellularis (Fig. 1) was found to accumulate 42.2% of lipids over their dry cell weight. The total lipid upon transesterification yielded 26.7% FAME, as illustrated in Table 1.
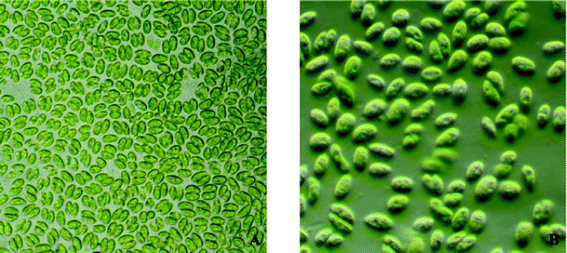 |
| | Fig. 1 Microphotographs of Scenedesmus bijugatus var. bicellularis (A) under light microscope 620×, (B) under phase contrast microscope 840×. | |
Table 1 Fatty acid and FAME content of Scenedesmus bijugatus var. bicellularis
| AFDWa of cell harvest (g) |
Fatty acid content (g) |
Fatty acid (wt%) |
FAME content (g) |
FAME content (wt%) |
|
AFDW: ash free dry weight.
|
| 2.87 |
1.21 |
42.2 |
0.77 |
18.8 |
The FAME extracts possessed both anti-bacterial and anti-candidal activity by forming a zone of inhibition. Their activity was a maximum against multi drug resistant pathogens, S. aureus (21 mm diameter) (Fig. 2) followed by E. coli (12 mm diameter) (Fig. 3), while C. albicans also showed high susceptibility (18 mm diameter) (Fig. 4) towards the FAME (Table 2). In contrast, no or very little zone of inhibition was observed in the case of fatty acids, while the cell free supernatant, methanol and hexane showed no activity at all. E. coli showed a resistant pattern to eight antibiotics among the antibiotics tested, whereas S. aureus showed resistance to six antibiotics (Table 3).
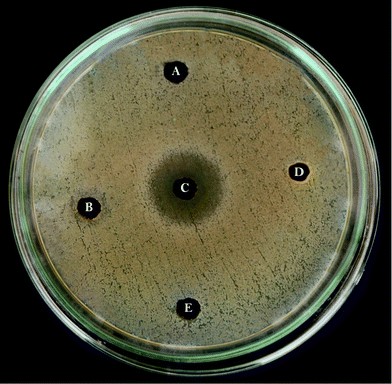 |
| | Fig. 2 Anti-bacterial assay: well diffusion method with Staphylococcus aureus against (A) cell free supernatant, (B) lipid, (C) FAME, (D) ethyl acetate, (E) methanol. | |
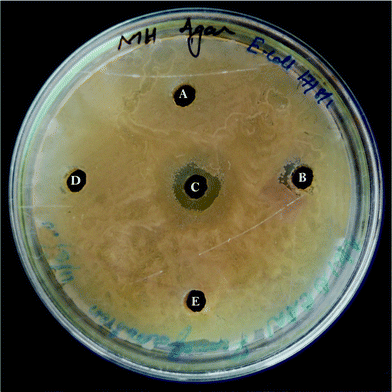 |
| | Fig. 3 Anti-bacterial assay: well diffusion method with Escherichia coli against (A) cell free supernatant, (B) lipid, (C) FAME, (D) ethyl acetate, (E) methanol. | |
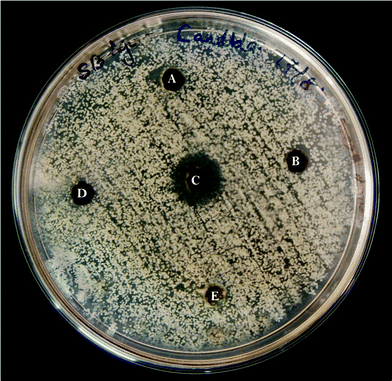 |
| | Fig. 4 Anti-candidal assay: well diffusion method with Candida albicans against (A) cell free supernatant, (B) lipid, (C) FAME, (D) ethyl acetate, (E) methanol. | |
Table 2 Antimicrobial activity of FAME extracts of Scenedesmus bijugatus var. bicellularis
| Pathogens tested |
Zone of inhibition (mm in dm) |
|
Staphylococcus aureus
|
21.0 ± 0.2 |
|
Escherichia coli
|
12.2 ± 0.1 |
|
Candida albicans
|
18.1 ± 0.2 |
Table 3 Antibiogram pattern of tested bacterial pathogens
| S. No |
Test organism |
Antibiotic used |
| Amikacin |
Methicillin |
Novobiocin |
Vancomycin |
Rifambicin |
Ampicillin |
Clindamycin |
Piperacillin |
Levoflaxacin |
Fusidic acid |
Oxacillin |
| + Resistant to antibiotics. − Sensitive to antibiotics. |
| 1. |
E. coli
|
+ |
− |
+ |
− |
+ |
+ |
− |
+ |
+ |
+ |
+ |
| 2. |
S. aureus
|
+ |
− |
− |
+ |
+ |
− |
+ |
+ |
− |
+ |
− |
The compositions of FAME were determined through GC-FID (Table 4) with the help of FAME-MIX standard (C4–C24) (Supelco Analytical, USA). The major compounds that were identified are cis-10-heptadecanoic acid methyl ester (C17:1) (5.54 wt%), linolelaidic acid methyl ester (C18:2) (4.63 wt%), linolenic acid methyl ester (C18:3) (6.26%), and cis-11,14-eicosadienoic acid methyl ester (C20:2) (8.95 wt%). Apart from these major FAMEs, upon keen observation various pharmacologically active FAMEs like stearic acid methyl ester (C18:0) (5.54 wt%), oleic acid methyl ester (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (1 wt%), linoleic acid methyl ester (C18
1) (1 wt%), linoleic acid methyl ester (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (1.40 wt%), eicosapentanoic acid methyl ester (C20
2) (1.40 wt%), eicosapentanoic acid methyl ester (C20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (1.13 wt%), erucic acid methyl ester (C22
5) (1.13 wt%), erucic acid methyl ester (C22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (1.03 wt%) and docosahexenoic acid methyl ester (C22
1) (1.03 wt%) and docosahexenoic acid methyl ester (C22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) (2.27 wt%) were also determined at comparatively lower concentrations.
6) (2.27 wt%) were also determined at comparatively lower concentrations.
Table 4 Fatty acid methyl ester composition of Scenedesmus bijugatus var. bicellularis
| S. No |
Fatty acid methyl ester |
Content (wt%) (mean ± S.D.) |
| 1. |
6:0 |
Caproic |
0.07 ± 0.01 |
| 2. |
8:0 |
Caprylic |
0.21 ± 0.01 |
| 3. |
10:0 |
Capric |
0.35 ± 0.02 |
| 4. |
12:0 |
Lauric |
0.46 ± 0.01 |
| 5. |
13:0 |
Tridecanoic |
0.39 ± 0.02 |
| 6. |
14:0 |
Myristic |
0.37 ± 0.03 |
| 7. |
14:1 |
Myristoleic |
0.22 ± 0.02 |
| 8. |
15:0 |
Pentadecanoic |
0.38 ± 0.01 |
| 9. |
15:1 |
cis-10-Pentadecenoic |
0.41 ± 0.01 |
| 10. |
16:0 |
Palmitic |
0.89 ± 0.01 |
| 11. |
16:1 |
Palmitoleic |
0.23 ± 0.02 |
| 12. |
17:0 |
Heptadecanoic |
0.46 ± 0.04 |
| 13. |
17:1 |
cis-10-Heptadecanoic |
0.61 ± 0.03 |
| 14. |
18:0 |
Stearic |
5.54 ± 0.03 |
| 15. |
18:1 |
Elaidic |
0.99 ± 0.01 |
| 16. |
18:1 |
Oleic |
0.98 ± 0.01 |
| 17. |
18:2 |
Linolelaidic |
4.63 ± 0.02 |
| 18. |
18:2 |
Linoleic |
1.40 ± 0.03 |
| 19. |
18:3 |
Linolenic |
6.26 ± 0.05 |
| 20. |
20:0 |
Arachidic |
2.84 ± 0.04 |
| 21. |
20:1 |
cis-11-Eicosenoic |
3.37 ± 0.04 |
| 22. |
20:2 |
cis-11,14-Eicosadienoic |
8.96 ± 0.02 |
| 23. |
20:3 |
cis-11,14,17-Eicosatrienoic |
1.70 ± 0.01 |
| 24. |
20:5 |
cis-5,8,11,14,17-Eicosapentaenoic |
1.13 ± 0.02 |
| 25. |
21:0 |
Heneicosanoic |
0.76 ± 0.01 |
| 26. |
22:0 |
Behenic |
0.80 ± 0.01 |
| 27. |
22:1 |
Erucic |
1.03 ± 0.01 |
| 28. |
22:6 |
cis-4,7,10,13,16,19-Ocosahexaenoic |
2.27 ± 0.03 |
| 29. |
24:0 |
Lignoceric |
0.72 ± 0.01 |
| 30. |
24:1 |
Nervonic |
0.67 ± 0.01 |
4. Discussion
In spite of extensive studies on the applications of microalgal lipids, the present study discovers the anti-bacterial and anti-candidal activity of the FAME. As a special case, instead of preparing the isolates from the microbial culture collection centers, we actually isolated the cultures directly from the environmental source, which may have some novel and unstudied potential.
In this investigation, for the first time the anti-bacterial and anti-candidal activity of FAMEs from S. bijugatus var. bicellularis was determined. The fatty acid methyl ester showed activity against both the gram positive (S. aureus) and gram negative pathogen (E. coli) along with the yeast (C. albicans), which was in agreement with the statement given by Huang and Ebersole (2010) that the action of FAMEs were not limited to the cell wall variations. Here S. aureus and E. coli were selectively chosen based on their increasing multi drug resistant property, which is a serious clinical problem.9
Through GC-FID analysis, about 30 different fatty acid methyl esters were identified, among which linoleic acid methyl ester (C18:2), linolenic acid methyl ester (C18:3), stearic acid methyl ester (C18:0), oleic acid methyl ester (C18:1), docosahexenoic acid methyl ester (C22:6), eicosapentanoic acid methyl ester (C20:5) and erucic acid methyl ester (C22:1) gain high significance due to their strong antimicrobial activity.8,10 This group of FAMEs was known to inhibit the fatty acid biosynthetic pathway by inhibiting FabI gene expression.13 Fatty acid methyl esters, especially, were found to exhibit strong anti-bacterial activity when compared to the lipids or free fatty acids. This is a peculiar behavior influenced through the modification of carbonyl species in the fatty acids.9 Nevertheless, the modifications were effected through the process of transesterification, which may increase their biological activity.14
5. Conclusion
The present investigation demonstrates that the FAME of S. bijugatus var bicellularis exhibits the most promising anti-bacterial and antifungal activity. The GC-FID data reveals the possible source of activity. Through this, the possibility to bring up the most functional and active FAMEs from microalgae is promising. This report is rushed to demonstrate the technical feasibility of treating the multidrug resistant pathogens with a new source of antimicrobial products. The valuable outputs of the present study may have a strong insight into the field of natural drug discovery.
Abbreviation List
| FAME | Fatty acid methyl ester |
Acknowledgements
The financial support from the Department of Biotechnology (DBT), Government of India is gratefully acknowledged (Project reference: BT/PR/11316/PBD/26/164/2008) and Council for Scientific and Industrial Research (CSIR), Government of India for Senior Research Fellowship (09/475/0167/2012-EMR-I).
References
- Y. Chisti, Biotechnol. Adv., 2007, 25(3), 294 CrossRef CAS.
- L. A. Meireles, A. C. Guedes and F. X. Malcata, J. Agric. Food Chem., 2003, 51(8), 2237 CrossRef CAS.
- G. Agoramoorthy, M. Chandrasekaran, V. Venkatesalu and M. J. Hsu, Braz. J. Microbiol., 2007, 38, 739 CrossRef.
- M. U. Rasheed and N. Thajuddin, Asian Pacific J. of Tropi. Med., 2011, 133 CAS.
- M. Chandrasekaran, A. Senthilkumar and V. Venkatesalu, Eur. Rew. Med. Pharmacol. Sci., 2011, 15, 775 CAS.
- L. A. R. S. Lima, S. Johann, P. S. Cisalpino, L. P. S. Pimenta and M. A. D. Boaventura, Rev. Soc. Bras. Med. Trop., 2011, 44(6), 777 CrossRef.
- M. Khoobchandani, B. K. Ojeswi, N. Ganesh, M. M. Srivastava, S. Gabbanini and R. Matera, Food Chem., 2010, 120, 217 CrossRef CAS.
- T. Kilic, T. Dirmenci, F. Satil, G. Bilsel, T. Kocagoz and M. Altun, Chem. Nat. Compd., 2005, 41(3), 276 CrossRef CAS.
- C .B. Huang and J. L. Ebersole, Mol. Oral Microbiol., 2010, 25, 75 CrossRef CAS.
- T. T. H. Hanh, D. T. T. Hang, C. V. Minh and N. T. Dat, Asian Pac. J. Trop. Med., 2011, 760 CrossRef CAS.
- A. Ilavarasi, D. Mubarak Ali, R. Praveenkumar, E. Baldev and N. Thajuddin, Biotechnology, 2011, 10(6), 360 Search PubMed.
- R. Praveenkumar, K. Shameera, G. Mahalakshmi, M. A. Akbarsha and N. Thajuddin, Biomass Bioenergy, 2012, 37, 60 CrossRef CAS.
- C. J. Zheng, J. S. Yooa, T. G. Leeb, H. Y. Choc, Y. H. Kimd and W. Gon, FEBS Lett., 2005, 579, 5157 CrossRef CAS.
- J. J. Kabara, J. Am. Oil Chem. Soc., 1984, 61(2), 397 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and mixed vigorously in a shaker for 20 min. The organic phase was washed with 10 ml water, vortexed and centrifuged at 2000 rpm for 5 min. The upper aqueous phase was removed and the lower organic phase was then rinsed twice with 15 ml methanol and water (1
1) and mixed vigorously in a shaker for 20 min. The organic phase was washed with 10 ml water, vortexed and centrifuged at 2000 rpm for 5 min. The upper aqueous phase was removed and the lower organic phase was then rinsed twice with 15 ml methanol and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Finally the extract was collected from the solvent phase, evaporated in a rotary vacuum evaporator and quantified gravimetrically.
1). Finally the extract was collected from the solvent phase, evaporated in a rotary vacuum evaporator and quantified gravimetrically.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Run time for a single sample was 55 min. Triplicates of each sample were done and FAME identification was run by comparison with standard certificate, Supelco FAME mix C4 –C24 (Bellefonte, PA, USA). Each sample was run under GC in triplicate.
1. Run time for a single sample was 55 min. Triplicates of each sample were done and FAME identification was run by comparison with standard certificate, Supelco FAME mix C4 –C24 (Bellefonte, PA, USA). Each sample was run under GC in triplicate.




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (1 wt%), linoleic acid methyl ester (C18
1) (1 wt%), linoleic acid methyl ester (C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (1.40 wt%), eicosapentanoic acid methyl ester (C20
2) (1.40 wt%), eicosapentanoic acid methyl ester (C20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (1.13 wt%), erucic acid methyl ester (C22
5) (1.13 wt%), erucic acid methyl ester (C22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (1.03 wt%) and docosahexenoic acid methyl ester (C22
1) (1.03 wt%) and docosahexenoic acid methyl ester (C22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) (2.27 wt%) were also determined at comparatively lower concentrations.
6) (2.27 wt%) were also determined at comparatively lower concentrations.