Synthesis of hierarchical anatase TiO2 nanostructures with tunable morphology and enhanced photocatalytic activity†
Raed
Rahal
,
Atul
Wankhade
,
Dongkyu
Cha
,
Aziz
Fihri
,
Samy
Ould-Chikh
,
Umesh
Patil
and
Vivek
Polshettiwar
*
Nanocatalysis Laboratory (NanoCat), Catalysis Center, King Abdullah University of Science and Technology (KAUST), Thuwal, KSA. E-mail: vivek.pol@kaust.edu.sa; Fax: +966) 2 802-1253; Tel: (+966) 2 808-0302
First published on 13th June 2012
Abstract
A facile one-pot method to prepare three-dimensional hierarchical nanostructures of titania with good control over their morphologies without the use of hydrofluoric acid is developed. The reaction is performed under microwave irradiation conditions in pure water, and enables enhanced photocatalytic activity. This study indicates that photocatalytic activity depends not only on the surface area but also on the morphology of the titania.
Titanium oxide is one of the most widely studied semiconductors and has numerous applications in various areas such as photocatalysis,1 photovoltaics,2 lithium ion batteries,3 water splitting,4 and surface coating5etc. These applications depend not only on their particle size, crystal phase, and crystallinity but also on their morphologies.6 A number of recent reports demonstrated the importance of tailoring the morphologies of TiO2 into shapes such as hollow boxes,7 hollow microspheres,8–11 ellipsoidal nanostructures,12 layered nanostructures,13–15 asymmetric nano-crystals,16 and hierarchical flower like microspheres.17–24 Despite this abundant literature on the synthesis of hierarchical nano-structures, there are several drawbacks, such as use of highly corrosive and environmentally unfriendly hydrofluoric acid (HF) and less control over morphologies. Therefore, there is an urgent need to develop a comparatively green and sustainable method for producing hierarchical TiO2 nanostructures with easy control over the morphology.
Following our initial success in the hierarchical self-assembly of nanomaterials under green and sustainable conditions,25–31 here we report a simple and facile one-pot method to prepare three-dimensional (3D) hierarchical nanostructures of titania (Fig. 1) with good control over their morphologies. The synthesis was achieved without using corrosive HF, under microwave (MW) irradiation conditions. The prepared anatase TiO2 structures exhibited high photocatalytic activity for the oxidation of formic acid and degradation of Rhodamine B dye.
 | ||
| Fig. 1 SEM images of the as-synthesized anatase TiO2. The images in the insets show close-up views of the nano-particles. | ||
In a typical synthesis, cetyltrimethylammonium bromide (CTAB, 4 g, 11 mmol) and urea (2.4 g, 40 mmol) were dissolved in 200 mL of water. After stirring for 30 min, cyclohexane (200 mL) and 1-pentanol (12 mL) were added and stirred for another 30 min. Then solid TiF4 (5.94 g, 48 mmol) was added to this solution and the resulting mixture was stirred for 1 h at room temperature. The reaction solution was then transferred to a Teflon-sealed microwave reactor and was exposed to microwave irradiation (800 W maximum power) at 120 °C for different time periods. After cooling the mixture to room temperature, the precipitated product was isolated by centrifugation, washed thoroughly with distilled water and ethanol before being air dried.
Fig. 2 shows scanning electron microscopy (SEM) images of the titania nanostructures obtained after irradiation by microwaves at 120 °C for 5 min. The images (Fig. 2A, 2B) indicate that the as-synthesized material consists of aggregated flower shape nanoparticles with diameters ranging from 200 to 300 nm. Close inspection of these particles (Fig. 2C, 2D) reveal that they are composed of a large number of layers interconnected with each other and self-organized in three dimensions to form flowery structure. These TiO2 nanostructures will be referred as TiO2–I hereafter.
 | ||
| Fig. 2 SEM images of the anatase TiO2–I nano-structures obtained after microwave irradiation at 120 °C for 5 min. | ||
Further structural characterization of the synthesized TiO2–I using high-resolution transmission electron microscopy (HRTEM) (Fig. 3) revealed that these particles are made of numerous bundles of multiple rolled layers or sheets forming a organized 3D spheres. The FET patterns (Fig. 3 C, 3D-insets) confirms the crystalline nature of the material.
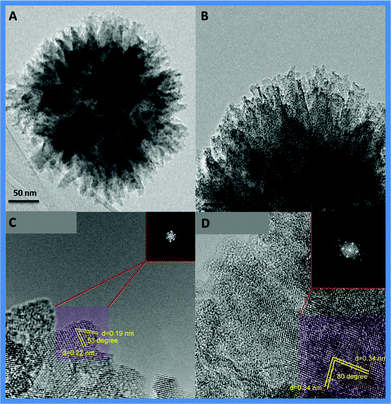 | ||
| Fig. 3 HRTEM micrographs of the TiO2–I at low and high magnifications, insets are local FFT of the selected areas. | ||
The phase purity and crystallinity of TiO2–I was examined by X-Ray diffraction (XRD) (Fig. 4A). The pattern could be assigned to a pure tetragonal anatase phase with a high crystallization degree (JCPDS No. 21-1272; space group I41/amd; a = 3.785 Å, c = 9.513 Å).
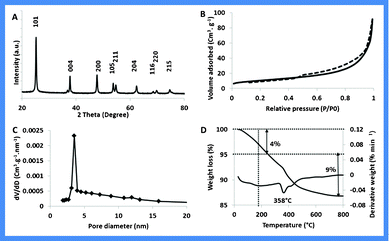 | ||
| Fig. 4 A) XRD pattern; B) N2 adsorption desorption isotherm; C) Pore size distribution plots and D) Thermogram of TiO2–I in air. | ||
The surface area and the porosity of the TiO2–I nanostructures were measured by nitrogen physisorption. The N2 physisorption isotherm of degassed TiO2–I sample (350 °C/6 h) (Fig. 4B) shows a type-IV isotherm with an H3 hysteresis typical of slit-shaped pores. The specific surface area of the sample calculated by the Brunauer–Emmett–Teller (BET) method was 47 m2 g−1 with a total pore volume of 0.32 cm3 g−1. Fig. 4C shows the pore size distribution plot obtained by using the Barrett–Joyner–Halenda (BJH) method.
Thermogravimetric analysis (Fig. 4D) was carried out in air using a differential thermal analyzer. TiO2–I showed a total weight loss of 13%, of which about 4% (between 25–220 °C) is contributed by moisture and residual solvents whereas the rest (200–600 °C) can be attributed to organic residues.
The formation mechanism of these structures is generally complex and difficult to predict.32,33 To gain insight into the formation mechanism, a series of experiments with different reaction times were carried out to monitor the evolution of the particle shapes. The shape and morphology of the particles obtained at specific interval of time were then examined by using the scanning electron microscopy.
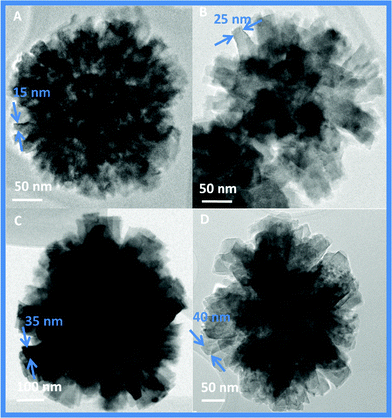
We found that the morphology of these hierarchical TiO2 nanostructures could easily be controlled by simply adjusting the reaction time or the temperature. The SEM and TEM images (Fig. 5A to 5D, Fig. 6A to 6D) were recorded for the samples, obtained by irradiating reaction mixture at 120 °C for 30 min, 1 h, 2 h and 3 h respectively.
 | ||
| Fig. 5 SEM images of hierarchical nanostructures obtained after microwave irradiation at 120 °C for A) 30 min; B) 1 h; C) 2 h and D) 3 h (TiO2–II). | ||
 | ||
| Fig. 6 TEM images of hierarchical nanostructures obtained after microwave irradiation at 120 °C for A) 30 min; B) 1 h; C) 2 h and D) 3 h (TiO2–II). | ||
As shown in Fig. 5A & 6A, only sphere with densely packed thick rods (15–20 nm) were obtained at the early stage (30 min) of the reaction. When the reaction time was prolonged to 1 h, these rods start to segregate into individual 35–40 nm thick rods (Fig. 5B & 6B). By increasing reaction time to 3 h, asterisk-like structures with stacked and thick tetragonal pyramidal nanorods originating from their centers are formed (referred as TiO2–II hereafter) with aggregate sizes of 500–600 nm (Fig. 5C, 5D & 6C, 6D). Thickening of the rods was also evident from the reduction in the BET surface area from 47 m2 g−1 to 20 m2 g−1 and also in the pore volume from 0.32 to 0.10 cm3 g−1 for TiO2–I and TiO2–II respectively. XRD patterns of these samples were similar and assigned to a pure tetragonal anatase phase with a high crystallization degree (Fig. S1, ESI†).
To pursue the growth mechanism of as-synthesized TiO2 with different shapes and morphologies, temperature-dependent experiments were also conducted and the obtained materials were examined by SEM. The reaction at 100 °C resulted in a similar asterisk-like morphology (Fig. 7A, 7B) with particles of 500–700 nm size (TiO2–III). The thickness of the rods was not homogeneous, and the SEM images showed sharpness on its edges. The surface area of TiO2–III was found to be 16 m2 g−1 (Figure S2C, ESI†). The reaction at 80 °C, resulted in another nano-flower like morphology (TiO2–IV), formed by interwoven sharp edged nano-rods (Fig. 7C, 7D). The specific surface area increased to 32 m2 g−1 (Figure S3D, ESI†). These results indicate that the sharpness of as-synthesized TiO2 rods increases at lower temperature, which is probably due to the lower production of fluoride (F−) ions in the reaction system released by the hydrolysis of TiF4. Hence, the adsorbed F− ions are not able to stabilize the external crystal planes of these materials due to their excessively high surface energy. The corresponding X-Ray patterns of TiO2–III and –IV nanostructures were similar and can also be assigned to a pure tetragonal anatase phase with a high crystallization degree (Figure S2C and S3C, ESI†).
 | ||
| Fig. 7 SEM images of TiO2 nanostructures obtained after microwave irradiation for 3 h (A, B) at 100 °C (TiO2–III); (C–D) at 80 °C (TiO2–IV). | ||
Apart from the reaction time and temperature, the roles of the template (CTAB), solvents (cyclohexane, 1-pentanol) and urea were also evaluated for the morphology control. In a control experiment without the template, no hierarchical TiO2 nanostructures (Figure S4A, ESI†) were formed, indicating the important role of a structure-directing agent. However, organic solvents and urea had no effect on the morphology and hierarchical structures were obtained even when pure water was used as solvent (TiO2–V Figure S4B, ESI†). This makes the overall process comparatively green and sustainable. Microwave irradiation appears to play a key role in the morphology control of TiO2 nanostructures,34–36 as large agglomerated particles were obtained when the experiments were carried out in an autoclave, with ageing times of 5, 10 and 18 h, at the same temperature (Figure S5, ESI†). These results clearly show the advantages of our developed synthesis process, which allows control over the morphology by simply tuning the reaction conditions such as the time and the temperature.
It has recently been observed that the morphologies of nanomaterials play key roles in their photocatalytic properties.6–24 We studied the photocatalytic activity of various synthesized TiO2 nanostructures (TiO2–I to IV) for the degradation of formic acid and Rhodamine B dye under UV light irradiation and also compared with Degussa P25. The photodegradation experiments were performed in a small photo reactor containing 40 mL of a formic acid solution (50 ppm) or Rhodamine B solution (50 ppm) and 40 mg of catalyst. A 300W Xe lamp was used as a source of UV light. Prior to irradiation, the suspension was stirred in the dark for 1 h to allow it reach a complete adsorption–desorption equilibrium. After a periodic interval of UV exposure, adequate aliquots of the sample were withdrawn and analyzed using HPLC (for formic acid) and using UV-Visible spectrometer (for Rhodamine dye).
For the photo-degradation of formic acid, as expected, the results demonstrated that the hierarchical TiO2–I which have the best surface area (47 m2 g−1) and the smallest size (250 nm) exhibited the highest activity in comparison to the other TiO2–II to IV samples, as well as Degussa P25. After 30 min, almost complete photo-degradation of formic acid was observed using TiO2–I (Fig. 8) as compared to 68% degradation using Degussa P25 (Fig. 8). The complete photo-degradation of formic acid was achieved only with TiO2–I while 28% of formic acid was not degraded by TiO2–II (20 m2 g−1) and more than 50% by TiO2–III (16 m2 g−1) and TiO2–IV (32 m2 g−1) (Fig. 8). Interestingly, although TiO2–IV has almost double the surface area (32 m2 g−1) than TiO2–III (16 m2 g−1), this does not translate into any enhancement in its photocatalytic activity. Also, the nano size of Degussa P25 over that of submicron TiO2 (300 nm) could not make it to perform better.
 | ||
| Fig. 8 Photocatalytic oxidation of formic acid under UV irradiation. | ||
Such enhancement of photocatalytic activity of TiO2–I nanostructures can be attributed to their three dimensional hierarchical morphologies which are regarded to have a greater number of active sites than 1D structures. Also a sufficient surface area can offer more active adsorption sites and photocatalytic reaction centers. Moreover, the porous structure is believed to favor light-harvesting and mass transport. All these factors enhance the rate of the photocatalytic reaction. These results indicate that the photocatalytic activity depends not only on the surface area but also on the morphology of the materials.
The catalytic activity of the as-synthesized TiO2 series was also evaluated for photo-degradation of Rhodamine B dye. The UV exposure of Rhodamine B solution for 1 h did not result in its discoloration in the absence of the photocatalyst. After the addition of the photocatalyst, (TiO2–I to V or standard anatase titania), the concentration of Rhodamine B decreases with increasing UV irradiation time (Fig. 9). The catalytic activity of TiO2–I surpasses that of the other synthesized variants and standard anatase titania. This further confirmed that the TiO2–I exhibits a higher overall photocatalytic efficiency.
 | ||
| Fig. 9 Photocatalytic degradation of Rhodamine B dye under UV irradiation. | ||
TiO2 nanostructures with a various well-defined morphologies were synthesized; which was achieved without using corrosive HF, under microwave irradiation conditions in pure water. The morphology and shape of the synthesized TiO2 nanostructures can be easily tuned by simply varying the heating time or temperature of reaction. The prepared anatase TiO2 structures exhibited high photocatalytic activity for the oxidation of formic acid and degradation of Rhodamine B dye. Results suggest that the photocatalytic activity depends not only on the surface area but also on the morphology of the materials.
References
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS.
- B. O'Regan and M. Gratzel, Nature, 1991, 353, 737 CrossRef CAS.
- A. R. Armstrong, G. Armstrong, J. Canales, R. Garcia and P. G. Bruce, Adv. Mater., 2005, 17, 862 CrossRef CAS.
- S. U. M. Khan, M. Al-Shahry and W. B. Jr. Ingler, Science, 2002, 297, 2243 CrossRef CAS.
- Y. Paz, Z. Luo, L. Rabenberg and A. Heller, J. Mater. Res., 1995, 10, 2842 CrossRef CAS.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638 CrossRef CAS.
- S. Xie, X. Han, Q. Kuang, J. Fu, L. Zhang, Z. Xie and L. Zheng, Chem. Commun., 2011, 47, 6722 RSC.
- S. Ding, Y. Wang, Z. Hong, X. Lue, D. Wan and F. Huang, Chem.–Eur. J., 2011, 17, 11535 CrossRef CAS.
- J. H. Pan, G. Han, R. Zhou and X. S.Zhao, Chem. Commun., 2011, 47, 6942 RSC.
- H. Yu, J. Yu, B. Cheng and S. Liu, Nanotechnology, 2007, 18, 065604 CrossRef.
- S. Liu, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2010, 132, 11914 CrossRef CAS.
- J. S. Chen, C. Chen, J. Liu, R. Xu, S. Z. Qiao and X. W. Lou, Chem. Commun., 2011, 47, 2631 RSC.
- H. Yu, B. Tian and J. Zhang, Chem.–Eur. J., 2011, 17, 5499 CrossRef CAS.
- W. Q. Fang, J. Z. Zhou, J. Liu, Z. G. Chen, C. Yang, C. H. Sun, G. R. Qian, J. Zou, S. Z. Qiao and H. G. Yang, Chem.–Eur. J., 2011, 17, 1423 CrossRef CAS.
- J. S. Chen, Y. L. Tan, C. M. Li, Y. L. Cheah, D. Luan, S. Madhavi, F. Y. C. Boey, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 6124 CrossRef CAS.
- H. B. Wu, J. S. Chen, X. W. Lou and H. H. Hng, Nanoscale, 2011, 3, 4082 RSC.
- T. Zhu, J. Li and Q. Wu, ACS Appl. Mater. Interfaces, 2011, 3, 3448 CAS.
- H. Zhang, Y. Wang, P. Liu, Y. Han, X. Yao, J. Zou, H. Cheng and H. Zhao, ACS Appl. Mater. Interfaces, 2011, 3, 2472 CAS.
- Z. Sun, J. H. Kim, Y. Zhao, F. Bijarbooneh, V. Malgras, Y. Lee, Y.-M. Kang and S. X. Dou, J. Am. Chem. Soc., 2011, 133, 19314 CrossRef CAS.
- J. Zhu, S.-H. Wang, Z.-F. Bian, C.-L. Cai and H.-X. Li, Res. Chem. Intermed., 2009, 35, 769 CrossRef CAS.
- Q. Xiang, J. Yu and M. Jaroniec, Chem. Commun., 2011, 47, 4532 RSC.
- M. Liu, L. Piao, W. Lu, S. Ju, L. Zhao, C. Zhou, H. Li and W. Wang, Nanoscale, 2010, 2, 1115 RSC.
- J. S. Chen and X. W. Lou, Chem. Sci., 2011, 2, 2219 RSC.
- F. Sun, W. Zhou, G. Tian, K. Oan, X. Miao, Y. Li, G. Zhang, T. Li and H. Fu, ChemCatChem, 2012, 4, 844 CrossRef CAS.
- A. Fihri, R. Sougrat, R. R. Baby, R. Rahal, D. Cha, M. Hedhili, M. Bouhrara, H. N. Alshareef and V. Polshettiwar, ChemSusChem, 2012, 5, 1241 CrossRef CAS.
- V. Polshettiwar, J. Thivolle-Cazat, M. Taoufik, F. Stoffelbach, S. Norsic and J. M. Basset, Angew. Chem., Int. Ed., 2011, 50, 2747 CrossRef CAS.
- V. Polshettiwar, D. Cha, X. Zhang and J. M. Basset, Angew. Chem., Int. Ed., 2010, 49, 9652 CrossRef CAS.
- V. Polshettiwar, B. Baruwati and R. S. Varma, ACS Nano, 2009, 3, 728 CrossRef CAS.
- V. Polshettiwar, M. N. Nadaguada and R. S. Varma, Chem. Commun., 2008, 47, 6318 RSC.
- P. A. Mangrulkar, M. M. Joshi, S. N. Tijare, V. Polshettiwar, N. K. Labhsetwar and S. S. Rayalu, Int. J. Hydrogen Energy, 2012, 37, 10462 CrossRef CAS.
- M. N. Nadaguada, V. Polshettiwar and R. S. Varma, J. Mater. Chem., 2009, 19, 2026 RSC.
- Nanochemistry: A chemical approach to nanomaterials (Eds: G. A. Ozin, A. C. Arsenault, L. Cademartiri), Royal Society of Chemistry, 2nd edition, 2008 Search PubMed.
- Nanostructures and nanomaterials (Eds: G. Cao, Y. Wang), World Scientific Publishing, 2nd edition, 2011 Search PubMed.
- V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743 RSC.
- M. Baghbanzadeh, L. Carbone, P. D. Cozzoli and C. O. Kappe, Angew. Chem., Int. Ed., 2011, 50, 11312 CrossRef CAS.
- Aqueous microwave assisted chemistry: Synthesis and catalysis(Eds: V. Polshettiwar, R. S. Varma) 2010, Royal Society of Chemistry Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra21104a/ |
| This journal is © The Royal Society of Chemistry 2012 |
