A cyclam-type “turn on” fluorescent sensor selective for mercury ions in aqueous media†
Styliani
Voutsadaki
,
George K.
Tsikalas
,
Emmanuel
Klontzas
,
George E.
Froudakis
,
Spiros A.
Pergantis
,
Konstantinos D.
Demadis
and
Haralambos E.
Katerinopoulos
*
Department of Chemistry, University of Crete, Voutes Campus, Crete, 71003 Heraklion, Greece. E-mail: kater@chemistry.uoc.gr
First published on 16th October 2012
Abstract
The synthesis and spectral profile of a cyclam-type “turn on” fluorescent sensor selective for Hg2+ ions in aqueous media is described. Its properties are compared to those of a second probe with an N-deprotected cyclam system. The vast difference in ion selectivity between the two sensors reveals the influence of functional group modifications on the selectivity of fluorescent ion probes.
Due to its 5d10 6s0 electronic configuration, the Hg2+ ion belongs to the so-called “silent ions”, since it lacks an intrinsic spectroscopic or magnetic signal. One of the most reliable means of detection of these ions is based on the use of fluorescent sensors, compounds bearing both an ionophore and a chromophore moiety. These probes may coordinate selectively to the target ion and respond to changes in ion levels with changes in their fluorescence profile, which can be translated to accurate measurements of ion concentrations. The detection and monitoring of mercury ions in environmental and biological systems present a great challenge for the scientific community, triggering a large number of fluorescent probe-related investigations that have been reviewed recently.1,2
In this report we present a continuation of our studies3 on the use of macrocyclic systems as ligands for the mercury ion, and in particular the synthesis and fluorescence profile of 1,4,8,11-tetraazacyclotetradecane-type (cyclam-type) fluorescent Hg2+ probes. The ability of the cyclam system to form complexes with Hg2+ has long been known,4 and some Hg2+ fluorescent probes bearing the cyclam ionophore have been reported recently.5 In a recent report we indicated that the presence of a thiocarbonyl linker attached to monoaza-15-crown-5 ionophores enhances the ion selectivity in favour of mercury ions.3 We therefore designed and synthesized a sensor containing the cyclam moiety coupled to an aminocoumarin fluorophore via a thiourea linker.
The synthesis of 4-methyl-7-[(2-thioxoethenylidene)amino]-1H-isochromen-1-one (1) has been described previously.3 It should be noted that the excess of thiophosgene used in the reaction must be fully removed (caution!), after the completion of the reaction, to avoid reaction with the aza-macrocycle in the next step. Tri-BOC cyclam 2 was prepared by slow addition of three equivalents of di-tert-butyl dicarbonate (BOC anhydride) to a solution of cyclam at −30 °C (Scheme 1).6 This procedure gave an 87% yield of compound 2 that was easily separated from traces of tetra- or disubstituted analogs by flash chromatography. Nucleophilic attack of the ionophore 2 at intermediate 1 was carried out very rapidly at room temperature, and almost quantitatively. Unfortunately, any attempts to remove the protective groups in 3 were fruitless, yielding an inseparable mixture of partially deprotected compounds. Congener 4, containing the tri-N-trifluoroacetyl protected cyclam,7 had the same fate, where a host of deprotection methods (Na2CO3, KOH, Ba(OH)2, NaBH4, NH3, MeONa, t-BuONa)8–13 yielded only decomposition products (see ESI†).
 | ||
| Scheme 1 Synthesis of probe 3. | ||
Finally, sensor 5 was prepared by the slow addition of unprotected cyclam to 1 at −50 °C (Scheme 2).
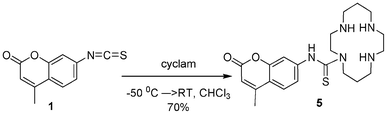 | ||
| Scheme 2 Synthesis of unprotected cyclam 5. | ||
The fluorescence profile of sensor 3 was studied in the presence of increasing Hg2+ concentrations (Fig. 1). Both excitation and emission spectra displayed an increase in signal intensity following an increase in the mercury ion concentration (Table 1). The λmax in the excitation spectra was detected at 338 nm, whereas the corresponding value for the emission spectra was λmax = 407 nm. No shift in either maxima was observed, indicating that the compound functions as a PET (photo-induced electron transfer) probe rather than a PCT (photo-induced charge transfer) one.1 The dissociation constant (Kd), calculated according to Tsien's algorithm,15 was 35.48 μM. The detection limit was approximately 21 μM (see ESI†).
| Indicator | Free | Hg2+-bound | K d (μM) | ||||
|---|---|---|---|---|---|---|---|
| λ exc (nm) | λ emis (nm) | Φ | λ exc (nm) | λ emis (nm) | Φ 14 | ||
| a Experiments performed in aqueous media, at RT and pH = 7.2. Quantum yields were calculated as described in ref. 14 using 10 μM solutions of dyes and 120 μM Hg2+ ion concentrations. | |||||||
| 3 | 338 | 407 | 0.09 | 341 | 407 | 0.56 | 35.48 |
| 5 | 347 | 441 | ND | 349 | 441 | ND | 28.08 |
 | ||
| Fig. 1 Excitation and emission spectra of 10 μM of dye 3 in solutions of increasing Hg2+ concentration. The excitation and emission wavelengths were set at 338 and 407 nm, respectively. Experiments were performed in aqueous media, at RT and pH = 7.2. | ||
The selectivity of sensor 3 for Hg2+ was confirmed by a series of ion competition experiments, depicted in Fig. 2. As shown by the first column (“free”), the fluorescence ratio [F(407) − Fo(407)]/Fo(407) of the free probe is zero, whereas a significant increase in the fluorescence ratio follows the addition of 120 μM of mercury (second column: “Hg”). Apparently the interaction of Hg2+ with the heteroatoms in 3 cancels the PET effect. The addition of an equivalent amount of zinc ions to the free probe solution (third column: “Zn”) did cause a negligible increase in the fluorescence ratio. Addition of mercury ions to the latter solution caused an immediate increase in fluorescence intensity level, which reached that of column 2 (“Hg”). Similar experiments with a host of ions usually included in competition studies indicated that mercury ions do bind to 3 with the same affinity, independent to the presence of zinc or other competitive ions.
![Ion competition study for dye 3. First measurement (“free”): fluorescence ratio for the free probe (zero). Second measurement (“Hg”): increase in fluorescence ratio [F(407) − Fo(407)]/Fo(407) in the presence of 120 μM Hg2+. Third measurement (“Zn”): fluorescence ratio in the presence of 120 μM Zn2+ (almost zero), and fourth measurement (“Zn Hg”): increase in fluorescence ratio in the presence of both 120 μM Zn2+ and 120 μM Hg2+. Likewise, the rest of the measurement sets indicate the fluorescence ratio of the dye in the presence of 120 μM of a metal ion vs. the ratio after addition of 1 equiv. of Hg2+ to the sample. Fluorescence ratio measured for: free dye, Hg2+, Zn2+, Cd2+, Pb2+, Ca2+, Ni2+, Co2+, Cu2+, and Na2+.](/image/article/2012/RA/c2ra20971c/c2ra20971c-f2.gif) | ||
| Fig. 2 Ion competition study for dye 3. First measurement (“free”): fluorescence ratio for the free probe (zero). Second measurement (“Hg”): increase in fluorescence ratio [F(407) − Fo(407)]/Fo(407) in the presence of 120 μM Hg2+. Third measurement (“Zn”): fluorescence ratio in the presence of 120 μM Zn2+ (almost zero), and fourth measurement (“Zn Hg”): increase in fluorescence ratio in the presence of both 120 μM Zn2+ and 120 μM Hg2+. Likewise, the rest of the measurement sets indicate the fluorescence ratio of the dye in the presence of 120 μM of a metal ion vs. the ratio after addition of 1 equiv. of Hg2+ to the sample. Fluorescence ratio measured for: free dye, Hg2+, Zn2+, Cd2+, Pb2+, Ca2+, Ni2+, Co2+, Cu2+, and Na2+. | ||
To our surprise, the “deprotected” analog 5 exhibited no ion selectivity in similar experiments (see ESI†). Although its fluorescence profile in the presence of increasing Hg2+ concentrations was similar to that of 3, in experiments performed using a buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM KCl, pH = 7.5) and a final ion concentration of 50 μM, the sensor responded positively to the presence of a host of ions, including Zn2+, Cd2+, Hg2+, and Ni2+. This lack of selectivity may be due to the increased electron density of the secondary macrocycle nitrogen atoms in 5 compared to that of the amide nitrogen atoms in 3. In a recent report, Song et al.5 mentioned that the “dioxocyclam binding unit consisting of two amide and two amine groups seems to be somewhat inferior to that of the cyclam molecular framework” in terms of copper and mercury ion affinity. In this respect the free cyclam system might be stronger in binding, but less selective for the ions in study. There are a few examples of coordination of tertiary amide nitrogens in macrocycles. Sibbons et al. reported a “rare and unexpected coordination to Cu2+ by a tertiary amide”, the amide donor being a part of a 1,4,7,-triazacyclononane system.16 Schickaneder et al. reported X-ray structural data on the Cu2+ complex with a monoacylated cyclam. Coordination of the metal with the tertiary nitrogen of the BOC–alanine–cyclam system resulted in a full pyramidalization of the nitrogen, and the amide resonance being completely cancelled.17
In an attempt to find an explanation for the selectivity profile of the two probes, we performed first principles calculations in the DFT level of theory for the Hg2+–3 and Hg2+–5 complexes.‡
The structure obtained is shown in Fig. 3. In agreement with previously communicated data, mercury, being a thiophile, coordinates with the sulphur atom, interacting simultaneously with elements of the macrocycle system. Mercury selectivity in probe 3 is enhanced by the coordination of Hg2+ with one BOC carbonyl moiety oxygen, an interaction missing in the case of probe 5 (see ESI†). The mercury ion selectivity of 3 may be due to the weaker interaction of Hg2+ with the electron pairs of the amide/thioamide macrocyclic nitrogens, supplemented by additional interactions with the sulphur atom as well as the BOC oxygen. Binding of Hg2+ with 5 was found to be 33 kcal mol−1 stronger than that with 3; however, Zn2+ ion binding with 5 was also 31.4 kcal mol−1 stronger than that of mercury, a fact that supports the lack of Hg2+ selectivity observed in 5 (see ESI†).
 | ||
| Fig. 3 DFT geometry optimization for the 3–Hg2+ complex. Atoms are represented by colour; mercury: orange, sulphur: yellow, nitrogen: blue, oxygen: red, carbon: grey. Hydrogen atoms are not shown for simplicity. | ||
We used COSMO (COnductor-like Screening MOdel) as a continuum solvation model to screen the effect of electrostatics of the surrounding solvent on the stability of the 3–Hg2+ and 5–Hg2+ complexes in “aqueous solution”. No significant changes in the Hg–N bond distances were observed. A second set of DFT calculations on 3–Hg2+ and 5–Hg2+ were performed in “gas phase” in the presence of two molecules of water. The results indicated that in 5–Hg2+, one of the water molecules is coordinated with the mercury ion while the second one forms a hydrogen bond with a N–H hydrogen in the cyclam system. No significant differences were observed in Hg–N bond distances. In 3–Hg2+, both water molecules are coordinated with the mercury ion, and the Hg–N bond distances for two of the cyclam nitrogens increased significantly (see ESI†). These results support the hypothesis that in aqueous solution, water may compete with the ligand and, as a result, weaker ligand coordination would be expected.
Electrospray mass spectrometry provided additional evidence for the formation of an [indicator 3 + Hg2+] complex. This was derived from the resulting mass spectrum in which ions having m/z 988 correspond to the mass of [indicator 3 + Hg2+ + 2Cl− − H+]−.
Fluorescence detection of ions in a biological environment using small-molecule sensors is still a difficult task. Only a small number of reports on fluorescent probes that have been used successfully for this purpose appear in the literature.18 We believe that the combination of properties such as water solubility, a clear and distinct “turn on” response to increasing concentrations of mercury ions in an aqueous environment, and the presence of a sulphur atom to enhance selectivity for the thiophile mercury, constitute a very attractive set of features for the cyclam-type sensor reported in this manuscript. Its properties are compared to those of a second probe with an N-deprotected cyclam system. The vast difference in selectivity between the two sensors reveals the influence of functional group modification on the ion selectivity of fluorescent ion probes.
Acknowledgements
This work is part of the 03ED375 research project, implemented within the framework of the “Reinforcement Programme of Human Research Manpower” (PENED) and co-financed by National and Community Funds (25% from the Greek Ministry of Development-GSRT and 75% from the E.U.-European Social Fund). We thank JEOL Inc. for DART-TOF mass spectra recording, and ProFi (ITE, Heraklion, Greece) for obtaining the HRMS.References
- E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443 CrossRef CAS.
- (a) I. W. Zhu, Y. Xu and X. Qian, Prog. Chem., 2007, 19, 1229 Search PubMed; (b) Leray and B. Valeur, Eur. J. Inorg. Chem., 2009, 3525 CrossRef CAS; (c) D. W. Domaille, E. L. Que and C. J. Chang, Nat. Chem. Biol., 2008, 4, 168 CrossRef CAS; (d) Y.-W. Lin, C.-C. Huang and H.-T. Chang, Analyst, 2011, 136, 863 RSC; (e) X. Qian, Y. Xiao, Y. Xu, X. Guo, J. Qian and W. Zhu, Chem. Commun., 2010, 46, 6418 RSC; (f) N. Kaur and S. Kumar, Tetrahedron, 2011, 67, 9233 CrossRef CAS; (g) M. Dutta and D. Das, Trends Anal. Chem., 2012, 32, 113 CrossRef CAS; (h) H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210 RSC.
- S. Voutsadaki, G. K. Tsikalas, E. Klontzas, G. E. Froudakis and H. E. Katerinopoulos, Chem. Commun., 2010, 46, 3292 RSC.
- R. L. Deming, A. L. Allred, A. R. Dahl, A. W. Herlinger and M. O. Kestner, J. Am. Chem. Soc., 1976, 98, 4132–4137 CrossRef CAS.
- (a) K.-C. Song, M. H. Kim, H. J. Kim and S.-K. Chang, Tetrahedron Lett., 2007, 48, 7464 CrossRef CAS; (b) M. P. Sang, H. K. Mi, J.-I. Choe and S.-K. Chang, J. Org. Chem., 2007, 72, 3550 CrossRef; (c) S.-Y. Moon, J. Y. Na, M. P. Sang and S.-K. Chang, J. Org. Chem., 2005, 70, 2394 CrossRef CAS; (d) N. J. Youn and S.-K. Chang, Tetrahedron Lett., 2005, 46, 125 CrossRef CAS.
- J. Kotek and P. Hermann, Ten-membered Rings or Larger with One or More Nitrogen Atoms, in Comprehensive Heterocyclic Chemistry III, ed. A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Elsevier, Oxford, 2008, vol. 14, pp. 613–666 Search PubMed.
- W. Yang, C. M. Giandomenico, M. Sartori and D. A. Moore, Tetrahedron Lett., 2003, 44, 2481 CrossRef CAS.
- F. Weygand and W. Swodenk, Chem. Ber., 1957, 90, 639 CrossRef CAS.
- F. Weygand and E. Frauendorfer, Chem. Ber., 1970, 103, 2437 CrossRef CAS.
- J. A. Halfen and V. G. Young, Jr., Chem. Commun., 2003, 2894 RSC.
- R. J. Bergeron and J. S. McManis, J. Org. Chem., 1988, 53, 3108 CrossRef CAS.
- C. Li and W.-T. Wong, Tetrahedron, 2004, 60, 5595 CrossRef CAS.
- T. G. Greene and P. G. M. Wuts, Protective Groups in Organic Synthesis, Wiley-Interscience, New York, 2nd edn, 1991, pp. 353–354 Search PubMed.
- Reported quantum yields are based on quinine sulfate Φ = 0.57 in 0.1 M H2SO4. W. H. Melhuish, J. Phys. Chem., 1961, 65, 229 CrossRef CAS.
- G. Grynkiewicz, M. Poenie and R. Y. Tsien, J. Biol. Chem., 1985, 260, 3350 Search PubMed.
- K. F. Sibbons, M. Al-Hashimi, M. Motevalli, J. Wolowska and M. Watkinson, Dalton Trans., 2004, 3163 RSC.
- C. Schickaneder, F. W. Heinemann and R. Alsfasser, Eur. J. Inorg. Chem., 2006, 2357 CrossRef CAS.
- K. M. Dean, Y. Qin and A. E. Palmer, Biochim. Biophys. Acta, Mol. Cell Res., 2012, 1823, 1406 CrossRef CAS.
- J. J. Stewart, J. Mol. Model., 2007, 13, 1173 CrossRef CAS.
- J. J. Stewart, J. Mol. Model., 2008, 14, 499–535 CrossRef CAS.
- (a) J. J. P. Stewart, MOPAC2009 Search PubMed; (b) J. J. P. Stewart, Stewart Computational Chemistry Search PubMed , Version 9.259W, web: http://OpenMOPAC.net.
- F. Weigend, M. Haser, H. Patzelt and R. Ahlrichs, Chem. Phys. Lett., 1998, 294, 143 CrossRef CAS.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS.
- J. P. Perdew, Phys. Rev. B, 1986, 33, 8822 CrossRef.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC.
- A. Schäfer, H. Horn and R. Ahlrichs, J. Chem. Phys., 1992, 97, 2571 CrossRef.
- R. Ahlrichs, M. Bär, M. Häser, H. Horn and C. Kölmel, Chem. Phys. Lett., 1989, 162, 165 CrossRef CAS.
- Turbomole Ver. 5.9, http://www.turbomole.com. Accessed on 7/5/2012.
- A. Klamt and G. Schuurmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental section, 1H and 13C NMR, and all fluorescence studies not shown in the text. See DOI: 10.1039/c2ra20971c |
| ‡ As a first step we used quantum chemistry semi-empirical methods in order to locate some energetically preferred configurations of the Hg-complex. These methods had the ability to find these configurations in a qualitative manner in much shorter computational times than any ab initio method. These calculations were done at the PM619,20 semi-empirical level with the MOPAC200921 package (gas phase model). The molecular configurations derived from semi-empirical calculations were further explored by performing more sophisticated quantum chemistry calculations within the framework of Density Functional Theory (DFT). Density Functional Theory in the Resolution of Identity (RI) approximation22 was applied in our calculations. The B-P8622,23 exchange–correlation functional along with the def2-SVP24,25 basis set (with the corresponding auxiliary basis set for the RI approximation27) were used. Default effective core potentials (ECPs) were included for Hg. All structures were optimized without any symmetry constraints and the optimized minimum-energy structures were verified as stationary points on the potential energy surface by performing numerical harmonic vibrational frequency calculations. DFT calculations were performed with the TURBOMOLE program.28 COSMO29 was used as a continuum solvation model to screen the effect of electrostatics of the surrounding solvent on the stability of the formed complexes, as it is implemented in TURBOMOLE. The Hg+2 complexes were fully optimized without any symmetry restrictions by the RI-B-P-86/def2-SVP method. |
| This journal is © The Royal Society of Chemistry 2012 |
