Micelle promoted supramolecular carbohydrate scaffold-catalyzed multicomponent synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and amidoalkyl naphthols derivatives in aqueous medium†
Atul
Kumar
*,
Maneesh Kumar
Gupta
and
Mukesh
Kumar
Medicinal and Process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow, India. E-mail: dratulsax@gmail.com; Fax: 91-522-26234051; Tel: 91-522-2612411
First published on 20th June 2012
Abstract
Micelle promoted natural carbohydrate scaffold catalyzed synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and amidoalkyl naphthol derivatives have been developed via a multicomponent one pot reaction viz 2-naphthol/1-naphthol, aromatic/heteroaromatic aldehyde and urea/thiourea/amide in water. The advantages of this method are efficient catalysis, excellent cost effectiveness, simple work-up and recyclability of catalyst.
In recent years, multicomponent reactions (MCRs)1 have attracted much interest and have been used to synthesize highly functionalized and complex molecules in a single synthetic operation. It is an innovative strategy for synthetic organic chemists to create combinatorial libraries of diverse organic molecules which are pharmacologically important and it has provided efficient lead scaffolds in modern drug discovery. In addition, the advantages of multicomponent reactions are high atom-economy, high selectivity, structural diversity and simpler procedure.
Recently reactions under green chemistry conditions,2 mainly in water,3 have fascinated the major scientific discipline because of their cost-effectiveness, safe, less toxic and environmentally benign characteristics. Therefore, use of water has led to development of cleaner and more efficient chemical synthesis, which is why it is often referred to as an ideal solvent. However reactions performed in water were avoided due to the insolubility of most of the reactants. This problem now has been covered by the use surfactants, which have the potential to facilitate reactions by solubilizing the reactant via micelle formation.4
In some cases, even surfactants are not sufficient to perform the reaction in water therefore the use of other catalysts (metal/non-metal) are needed to obtain the desired products.5 Therefore exploration of a new eco-friendly catalyst is required for environmentally benign synthetic methods for multicomponent reactions.
Cellulose and starch are the two most abundant natural carbohydrates (NCar), and are used as solid supports for catalysts because they are highly cost-effective, biodegradable, easy to handle, eco-friendly and easily available from renewable resources. Both (cellulose and starch) were transformed in to their sulfuric acid derivatives, (Fig. 1) such as cellulose sulfuric acid (CellSA) and starch sulfuric acid (StarSA),6 because most of the sulfonated active site-containing solid catalysts have shown promising results.
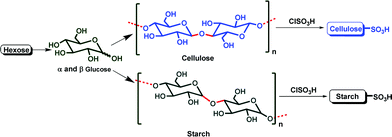 | ||
| Fig. 1 Natural carbohydrate catalysts. | ||
Cellulose sulfuric acid (CellSA)7 has shown excellent catalytic properties because it is highly stable at high temperature and has strong active sites from the sulfo functional groups. However it also shows better reusability compared to starch sulfuric acid (StarSA) due to its poor solubility.
Oxazinone derivatives8 are an important class of heterocyclic compounds containing many biological activities. However naphthoxazinones, on the other hand, have been reported as antibacterial agents.9 Szatmari et al.10 showed the synthesis of naphthoxazinones via condensation of aminoalkylnaphthols with phosgene in the presence of triethylamine whereas Cimarelli and coworkers11 used diimidazole instead of phosgene. Recently some other research groups have reported the synthesis of naphthoxazinones by the condensation of β-naphthol, aromatic aldehydes, and urea using various catalysts.12 Similarly, 1-amidoalkyl-2-naphthol derivatives are also considered as an important class of compound because of their promising biological and pharmacological activities.13 Moreover, the hydrolyzed product of 1-amidoalkyl-2-naphthol i.e., 1-aminoalkyl naphthol also shows hypotensive and bradycardiac effects. Therefore several alternatives and efficient methods have been reported for the synthesis of 1-amidoalkyl-2-naphthol derivatives.14 However, some protocols suffer from certain drawbacks such as high reaction temperature, the use of carcinogenic solvents, low yields and prolonged reaction time. Reusability and recovery of the catalyst is also a major problem. Therefore it is desirable to develop a green and eco-friendly protocol for the preparation of amidoalkyl naphthols. To the best of our knowledge, no reports are available for the synthesis of naphthoxazinones and 1-amidoalkyl-2-naphthol in water.
As a part of our continual work towards the progress of environmentally benign synthetic methods for multicomponent reactions (MCRs) to synthesize various biologically important heterocyclic compounds,15 we wish to report here a highly efficient synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and amidoalkyl naphthol derivatives using cellulose sulfuric acid as a solid catalyst and SDS as a surfactant in water from 2-naphthol/1-naphthol, aromatic/heteroaromatic aldehyde and urea/thiourea/amide (Scheme 1).
![Synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and amidoalkyl naphthol derivatives catalyzed by micelle promoted cellulose sulphuric acid in water.](/image/article/2012/RA/c2ra20848b/c2ra20848b-s1.gif) | ||
| Scheme 1 Synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and amidoalkyl naphthol derivatives catalyzed by micelle promoted cellulose sulphuric acid in water. | ||
Our initial efforts were focused on the actual effectiveness of an efficient catalyst for the three-component reaction in water. For this investigation the reaction of 2-naphthol, benzaldehyde and urea, taken as a model reaction, without any catalyst in water at room temperature, resulted in no product after 24 h. We further carried out the reaction with heating for 24 h but only a trace amount of product was obtained. However, the use of surfactant (SDS) also showed no effect on the reaction. Therefore, we used TsOH as a Brønsted acid with surfactant (SDS) to catalyse the reaction mixture. It was found that the use of TsOH resulted in the formation of the desired product in 39% yield. In search of an efficient catalyst we further screened various Brønsted/Lewis acids in the model three component reaction in water. Other Brønsted acids (MSA, acetic acid, TFA) did not give satisfactory results and poor yields were obtained. However, the use of metal Lewis acids (Table 1) was also found to be inefficient to synthesize the desired product in good yield. As an exception copper sulphate was found to be modest in catalyzing the reaction but took a longer time.
| Entry | Catalyst (mol%) | Time (h) | Yield (%)i |
|---|---|---|---|
| a The reaction was conducted with benzaldehyde (2 mmol), 2-naphthol (2 mmol), urea (3 mmol), SDS (20 mol%) and catalyst (20 mol%) in water (5 mL) at 80 °C. b Without SDS at room temp. c Without SDS at reflux. d With SDS. e 0.03 g of CellSA. f 0.04 g of CellSA. g 0.05 g of CellSA. h 0.05 g of StarSA were used. i Isolated yield. | |||
| 1 | — | 24b | — |
| 2 | — | 24c | Trace |
| 3 | — | 20d | 22 |
| 1 | TsOH | 8 | 39 |
| 2 | MSA | 8 | 40 |
| 3 | Acetic acid | 8 | 35 |
| 4 | TFA | 8 | 38 |
| 5 | Zinc acetate | 14 | 28 |
| 6 | Zinc triflet | 14 | 18 |
| 7 | Copper triflet | 14 | 18 |
| 8 | CuBr | 20 | 28 |
| 9 | CuSO4 | 18 | 58 |
| 10 | FeCl3 | 18 | 21 |
| 11 | FeSO4 | 18 | 21 |
| 12 | ZrCl4 | 16 | 30 |
| 13 | NiCl4 | 18 | 47 |
| 14 | Nickel acetate | 18 | 48 |
| 15 | CellSAe | 6 | 71 |
| 16 | CellSAf | 4 | 85 |
| 17 | CellSAg | 2.5 | 92 |
| 18 | StarSAh | 4 | 80 |
In view of our previous experience in sculpting various biologically active motifs using natural supramolecular carbohydrates (NSCar), we turned our attention towards cellulose sulfuric acid (CellSA). It was observed that when cellulose sulfuric acid was used as the catalyst both yield and reaction time were improved. In order to evaluate the catalyst loading, the reaction was carried out using 0.03, 0.04, and 0.05 g CellSA at 80 °C in water. It was found that 0.05 g CellSA is sufficient to give the desired product in excellent yield with an enhanced rate. The other example of NSCar, starch sulfuric acid (StarSA), was found to be less effective than CellSA on the grounds of catalyst loading.
Once we found an efficient catalyst to synthesize the desired product, we further studied the effect of surfactant on the cellulose sulfuric acid catalyzed condensation reaction of 2-naphthol, benzaldehyde and urea in water, and the results are summarized in Table 2. Non-ionic surfactants (Triton X-100, Tween-20, Tween-80, and Triton CF-10) were not effective and reduced the yield. This is because of a lowering of the critical micelle concentration (CMC) value with the increase in the temperature. Whereas, scandium tris-(dodecyl sulfate) Sc(DS)3 also failed to synthesize the desired product in good yield. CTAB, TBAB and TBAF were employed as surfactants for the formation of desired product in water. Unfortunately, moderate yields were obtained. Therefore, SDS was found to be the best surfactant with CellSA for the synthesis of 4a in water.
| S.N. | Surfactant | Time (h) | Yieldb (%) |
|---|---|---|---|
| a The reaction was conducted with benzaldehyde (2 mmol), naphthol (2 mmol), urea (3 mmol), 0.05 g of cellulose sulphuric acid (CellSA) and surfactant (20 mol%) in water (5 mL) at 80 °C. b Isolated yield. | |||
| 1 | Triton X-100 | 2.5 | 30 |
| 2 | Tween-20 | 2.5 | 32 |
| 3 | Tween-80 | 2.5 | 32 |
| 4 | Triton CF-10 | 2.5 | 35 |
| 7 | Sc(DS)3 | 2.5 | 50 |
| 8 | CTAB | 2.5 | 60 |
| 9 | TBAB | 2.5 | 57 |
| 10 | TBAF | 2.5 | 58 |
| 12 | SDS | 2.5 | 92 |
Thereafter, a series of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and amidoalkyl naphthol derivatives were prepared with various aromatic aldehydes containing electron-withdrawing and electron-donating substituents, urea/thiourea/amide and naphthol (α and β) under the optimized reaction conditions. These results are listed in Table 3.
| Entry | Aldehyde R | Amide R1 | Naphthol | Productb(4) | Time (h) | Yieldc (%) |
|---|---|---|---|---|---|---|
| a The reaction was conducted with benzaldehyde (2 mmol), naphthol (2 mmol), urea/thiourea/amide (3 mmol), 0.05 g of cellulose sulphuric acid (CellSA) and SDS (20 mol%) in water (5 mL) at 80 °C temperature for a given time. b All products were characterized by 1H, 13C NMR, IR and mass spectroscopy. c Isolated yield. | ||||||
| 1 |
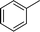
|
NH2 |
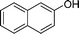
|
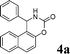 4a
4a
|
2.5 | 92 |
| 2 |
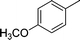
|
NH2 |
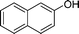
|
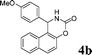 4b
4b
|
3 | 85 |
| 3 |
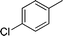
|
NH2 |
|
 4c
4c
|
2.5 | 88 |
| 4 |
|
NH2 |
|
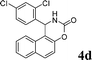 4d
4d
|
2.0 | 90 |
| 5 |
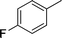
|
NH2 |
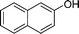
|
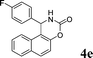 4e
4e
|
2.0 | 90 |
| 6 |
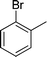
|
NH2 |
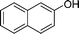
|
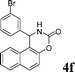 4f
4f
|
2.5 | 89 |
| 7 |
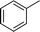
|
NH2 |
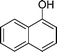
|
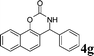 4g
4g
|
3.0 | 82 |
| 8 |
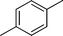
|
NH2 |
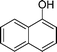
|
 4h
4h
|
3.0 | 84 |
| 9 |
|
NH2 |
|
 4i
4i
|
3.5 | 80 |
| 10 |
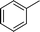
|
NH2 |
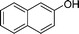
|
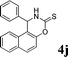 4j
4j
|
2.0 | 84 |
| 11 |
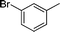
|
NH2 |
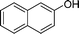
|
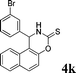 4k
4k
|
1.5 | 89 |
| 12 |
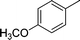
|
NH2 |
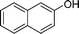
|
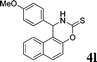 4l
4l
|
2.5 | 80 |
| 13 |
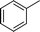
|
Ph |
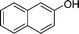
|
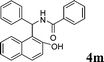 4m
4m
|
1.0 | 91 |
| 14 |
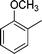
|
Ph |
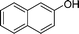
|
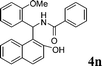 4n
4n
|
1.5 | 89 |
| 15 |
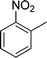
|
Ph |
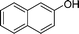
|
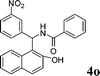 4o
4o
|
1.0 | 87 |
| 16 |
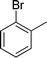
|
Ph |
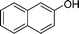
|
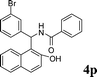 4p
4p
|
1.0 | 88 |
| 17 |
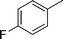
|
Ph |
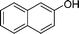
|
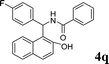 4q
4q
|
1.0 | 86 |
| 18 |
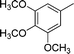
|
Ph |
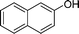
|
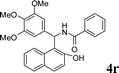 4r
4r
|
2.5 | 81 |
| 19 |
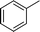
|
CH3 |
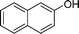
|
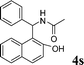 4s
4s
|
1.5 | 90 |
| 20 |
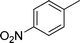
|
CH3 |
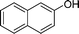
|
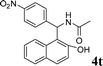 4t
4t
|
1.0 | 86 |
| 21 |
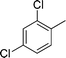
|
CH3 |
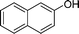
|
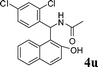 4u
4u
|
1.0 | 89 |
| 22 |
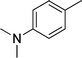
|
CH3 |
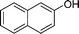
|
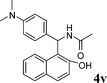 4v
4v
|
2.5 | 82 |
| 23 |
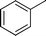
|
CH2Cl |
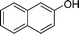
|
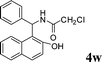 4w
4w
|
1.0 | 90 |
| 24 |
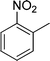
|
CH2Cl |
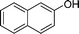
|
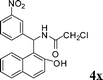 4x
4x
|
1.0 | 88 |
| 25 |
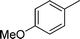
|
CH2Cl |
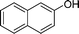
|
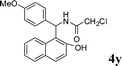 4y
4y
|
1.5 | 87 |
| 26 |
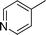
|
CH3 |
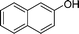
|
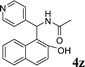 4z
4z
|
2.5 | 88 |
| 27 |
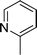
|
CH2Cl |
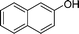
|
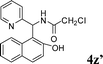 4z′
4z′
|
2.5 | 87 |
| 28 |
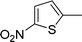
|
Ph |
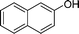
|
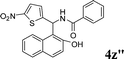 4z′′
4z′′
|
1.5 | 83 |
One of the advantages of cellulose sulphuric acid as a catalyst is its recyclability. Once the reaction was complete (monitored by TLC), the reaction mixture was extracted with ethyl acetate and the solid product filtered off (cellulose sulphuric acid) and further washed with ethyl acetate (3 times). After drying, it was reprocessed for consecutive reactions (at least 3–4 runs) with only a slight loss of catalytic activity (Fig. 2). It was characterized by FT-IR spectroscopy: two characteristic peaks at 1217 and 1043 cm−1 assigned to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, are observed16 and a broad feature is seen at 3267 cm−1 belonging to the S–OH groups. (Fig. 3)
O stretching vibration, are observed16 and a broad feature is seen at 3267 cm−1 belonging to the S–OH groups. (Fig. 3)
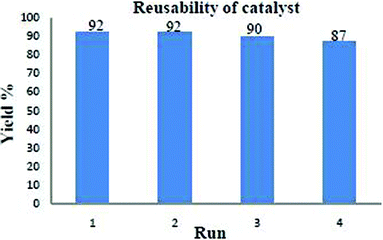 | ||
| Fig. 2 Reusability of catalyst in the case of 4a. | ||
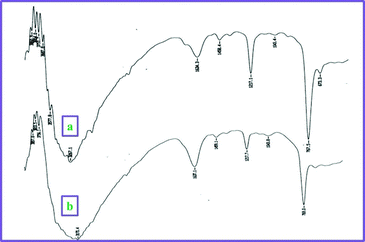 | ||
| Fig. 3 FT-IR spectra comparison of (a) fresh CellSA, (b) CellSA used 4 times. | ||
In 1997 Kappe17 reported the mechanism of the Biginelli reaction via the formation of an N-acyliminium ion intermediate with urea and aldehyde. This was again supported by Zhou and co-workers18 and De Souza et al.19 with the help of computational analysis (Gaussian' 03). Keeping these report in mind we hypothesized the mechanism of our reaction as shown in Scheme 2. We suppose that the reaction may start with the formation of an N-acylimine intermediate (5) with aldehyde and urea/thiourea/amide instead of ortho-quinonemethides (o-QMs).14e,g The formation of 5 may be stabilized by micelle4a,b followed by the activation of naphthol by the conjugate base of cellulose sulphuric acid thus favoring nucleophilic attack to provide the intermediate 6. The aromatization, due to α-proton elimination, leads to the formation of 4l-z′′ whereas, in the case of the urea/thiourea analogue of 6, compounds 4a–m were obtained due to the elimination of NH3.
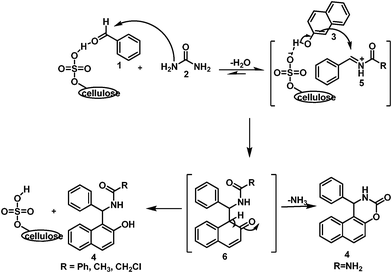 | ||
| Scheme 2 The plausible mechanism of a micelle-promoted cellulose sulphuric acid catalyzed multicomponent reaction. | ||
Conclusions
In conclusion, we have developed an easy, efficient and eco-friendly procedure for the one-pot synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and amidoalkylnaphthols via a multicomponenet reaction of aldehydes, urea/thiourea/amides with 1/2-naphthol using SDS promoted cellulose sulfuric acid, a recyclable solid-supported acid catalyst in water. This novel methodology allows for the first time preparation of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and amidoalkyl naphthols in water. We plan to keep exploring the scope and synthetic application of this methodology in our laboratory.Experimental
Typical experimental procedure for the synthesis of 4
In a typical experiment, the aldehyde (2 mmol), urea/thiourea/amide (3 mmol), 1 or 2-naphthol (2 mmol), cellulose sulfuric acid (0.05 g), and SDS (20 mol%) were taken in 5 mL water. The reaction mixture was vigorously stirred at 80 °C till the completion of the reaction (monitored by TLC). After completion the reaction mixture was extracted with ethyl acetate and the solid catalyst filtered off to be reused, and the organic phase dried over sodium sulphate and evaporated under vacuum to give the crude product, which was purified by column chromatography using ethyl acetate–hexane (silica gel, 230–400 mesh) as an eluent or by recrystallization from ethanol to afford the corresponding product.Acknowledgements
M.K.G. and M.K. acknowledges CSIR-UGC, New Delhi for the award of a SRF. The authors also acknowledge SAIF-CDRI for providing spectral and analytical data. CDRI communication no. 8284.References
- for recent reviews, see: (a) A. Domling, Chem. Rev., 2006, 106, 17–89 CrossRef; (b) A. C. C. Cariou, G. J. Clarkson and M. Shipman, J. Org. Chem., 2008, 73, 9762 CrossRef; (c) M. Umkeherer, C. Kalinski, J. Kolb and C. Burdack, Tetrahedron Lett., 2006, 47, 2391 CrossRef; (d) A. Alizadeh, F. Mobahedi and A. Esmaili, Tetrahedron Lett., 2006, 47, 4469 CrossRef CAS; (e) A. Domling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef CAS.
- (a) A. Kumar and S. Sharma, Green Chem., 2011, 13, 2017–2020 RSC; (b) A. Kumar, G. Gupta and S. Srivastava, Green Chem., 2011, 13, 2459–2463 RSC; (c) A. Kumar, M. K. Gupta and M. Kumar, Green Chem., 2012, 14, 290–295 RSC; (d) A. Kumar, S. Sharma, R. A. Maurya and J. Sarkar, J. Comb. Chem., 2010, 12, 20–24 CrossRef CAS; (e) K. Tanaka, T. Sugino and F. Toda, Green Chem., 2000, 2, 303 RSC; (f) C. L. Raston and J. L. Scott, Green Chem., 2000, 2, 49 RSC.
- (a) A. Kumar, V. D. Tripathi and P. Kumar, Green Chem., 2011, 13, 51–54 RSC; (b) A. Kumar, S. Sharma and R. A. Maurya, Tetrahedron Lett., 2010, 51, 6224–6226 CrossRef CAS; (c) S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275–3279 CrossRef CAS; (d) C. D. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7816–7817 CrossRef.
- (a) A. Kumar, M. K. Gupta and M. Kumar, Tetrahedron Lett., 2010, 51, 1582–1584 CrossRef CAS; (b) A. Kumar, M. K. Gupta and M. Kumar, Tetrahedron Lett., 2011, 52, 4521–4525 CrossRef CAS; (c) A. Chatterjee, D. K. Maiti and P. K. Bhattacharya, Org. Lett., 2003, 5, 3967 CrossRef CAS; (d) A. Chatterjee, S. K. Hota, M. Banerjee and P. K. Bhattacharya, Tetrahedron Lett., 2010, 51, 6700–6703 CrossRef CAS.
- (a) S. Bhattacharya, A. Srivastava and S. Sengupta, Tetrahedron Lett., 2005, 46, 3557–3560 CrossRef CAS; (b) S. Kobayashi, T. Wakabayashi, S. Nagayama and O. H. Yamada, Tetrahedron Lett., 1997, 38, 4559–4562 CrossRef CAS; (c) A. Kumar, R. A. Maurya and D. Saxena, Mol. Diversity, 2010, 14, 331–341 CrossRef CAS; (d) A. Kumar and R. A. Maurya, Tetrahedron Lett., 2008, 49, 5471–5474 CrossRef CAS.
- General procedure for prepration of CellSA and StarSA is given in supporting information.
- (a) A. Kumar, G. Gupta and S. Srivastava, J. Comb. Chem., 2010, 12, 458–462 CrossRef CAS; (b) A. Kumar, S. Srivastava, G. Gupta, V. Chaturvedi, S. Sinha and R. Srivastava, ACS Comb. Sci., 2010, 13, 65–71 CrossRef; (c) A. Kumar, S. Srivastava and G. Gupta, Tetrahedron Lett., 2010, 51, 517–520 CrossRef CAS.
- (a) M. Patel, R. J. Jr. McHugh, B. C. Cordova, R. M. Klabe, S. Erickson-Viitanen, G. L. Trainor and S. S. Ko, Bioorg. Med. Chem. Lett., 1999, 9, 3221 CrossRef CAS; (b) L. Waxman and P. L. Darke, Antiviral Chem. Chemother., 2000, 11, 1 CAS; (c) A. S. Girgis, Pharmazie, 2000, 466 Search PubMed.
- N. Latif, N. Mishriky and F. M. Assad, Aust. J. Chem., 1982, 35, 1037–1043 CrossRef CAS.
- I. Szatmari, A. Hetenyi, L. Lazar and F. Fulop, J. Heterocycl. Chem., 2004, 41, 367–373 CrossRef CAS.
- C. Cimarelli, G. Palmieri and E. Volpini, Can. J. Chem., 2004, 82, 1314–1321 CrossRef CAS.
- (a) M. Dabiri, A. S. Delbari and A. Bazgir, Synlett, 2007, 5, 821–823 Search PubMed; (b) A. Hajra, D. Kundu and A. Majee, J. Heterocycl. Chem., 2009, 46, 1019–1022 CrossRef CAS; (c) A. Nizam and M. A. Päsha, Synth. Commun., 2010, 40, 2864–2868 CrossRef CAS.
- Y. Shen, C. T.n Tsai and C. L. Chen, Eur. J. Med. Chem., 1999, 34, 877 CrossRef.
- (a) S. Kantevari, S. V. N. Vuppalapati and L. Nagarapu, Catal. Commun., 2007, 8, 1857 CrossRef CAS; (b) M. M. Khodaei, A. R. Khosropour and H. Moghanian, Synlett, 2006, 916 CrossRef CAS; (c) B. Das, K. Laxminarayana, B. Ravikanth and R. Rao, J. Mol. Catal. A: Chem., 2007, 261, 180 CrossRef CAS; (d) H. R. Shaterian, H. Yarahmadi and M. Ghashang, Bioorg. Med. Chem. Lett., 2008, 18, 788 CrossRef CAS; (e) Q. Zhang, J. Luo and Y. Wei, Green Chem., 2010, 12, 2246–2254 RSC; (f) M. Lei, L. Ma and L. Hu, Tetrahedron Lett., 2009, 50, 6393–6397 CrossRef CAS; (g) G. C. Nandi, S. K. Samai, R. Kumar and M. S Singh, Tetrahedron Lett., 2009, 50, 7220–7222 CrossRef CAS.
- (a) A. Kumar, S. Sharma and R. A. Maurya, Tetrahedron Lett., 2009, 50, 5937–5940 CrossRef CAS; (b) A. Kumar and R. A. Maurya, Tetrahedron, 2008, 64, 3477–3488 CrossRef CAS; (c) A. Kumar, M. Kumar and M. K. Gupta, Tetrahedron Lett., 2009, 50, 7024–7027 CrossRef CAS; (d) A. Kumar, S. Sharma, V. D. Tripathi, R. A. Maurya, S. P. Srivastava, G. Bhatia, A. K. Tamrakar and A. K. Srivastava, Bioorg. Med. Chem., 2010, 18, 4138–4148 CrossRef CAS; (e) A. Kumar, R. A. Maurya, S. Sharma, P. Ahmad, A. B. Singh, A. K. Tamrakar and A. K. Srivastava, Bioorg. Med. Chem., 2009, 17, 5285–5292 CrossRef CAS; (f) A. Kumar, P. Ahmad, R. A. Maurya, A. B. Singh and A. K. Srivastava, Eur. J. Med. Chem., 2009, 44, 109–116 CrossRef CAS.
- (a) R. Langner and G. Zundel, J. Phys. Chem., 1995, 99, 12214 CrossRef CAS; (b) K. Miyatake, H. Iyotani, K. Yamamoto and E. Tsuchida, Macromolecules, 1996, 29, 6969 CrossRef CAS.
- C. O. Kappe, J. Org. Chem., 1997, 62, 7201–7204 CrossRef CAS.
- J. G. Ma, J. M. Zhang, H. H. Jiang, W. Y. Ma and J. H. Zhou, Chin. Chem. Lett., 2008, 19, 375–378 CrossRef CAS.
- R. O. M. A. De Souza, E. T. D. Penha, H. M. S. Milagre, S. J. Garden, P. M. Esteves, M. N. Eberlin and O. A. C. Antunes, Chem.–Eur. J., 2009, 15, 9799–9804 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20848b/ |
| This journal is © The Royal Society of Chemistry 2012 |
