DOI:
10.1039/C2RA20798B
(Paper)
RSC Adv., 2012,
2, 6209-6215
Bistriphenylamine-based organic sensitizers with high molar extinction coefficients for dye-sensitized solar cells
Received
27th April 2012
, Accepted 30th April 2012
First published on 11th June 2012
Abstract
A series of novel bistriphenylamine-based organic sensitizers (coded as RC-dyes) with high molar extinction coefficients (>5.0 × 104 M−1 cm−1) have been synthesized for dye-sensitized solar cells (DSSCs). By changing the length of the π-conjugation bridge thiophene from zero to two between the donor and the acceptor part, the range of absorption and its corresponding photon-to-current conversion was modulated gradually to the low energy region in the visible spectrum. Based on the electrochemical and theoretical investigations on the electronic and geometrical structure, RC dyes were shown to be a promising candidate for efficient metal-free organic sensitizers with strong light absorption. Among RC dyes (11∼13) tested, the highest power conversion efficiency of 5.67% was obtained from RC-13 with a short circuit current of 10.78 mA cm−2, an open circuit voltage of 0.71 V, and a fill factor of 0.74 under standard AM 1.5 measurement conditions.
1. Introduction
Since being reported by Grätzel and O’Regan in 1991, dye sensitized solar cells (DSSCs) based on mesoporous metal oxide films have received great attention due to their high photon-to-current conversion efficiency, obtainable at a low fabrication cost.1,2 With great effort by many investigators, the overall power conversion efficiency (η) of DSSCs could be improved up to about 12.5% from porphyrin dyes2a while metal-free organic dyes have recorded ∼10% as the best one reported so far,2b with advancement in knowledge of the operating mechanism and a variety of component materials.2 As for a light-harvesting chromophore in DSSCs, various ruthenium-based sensitizers have been developed that are stable and outstandingly efficient, thus leading to a reference dye in the research of DSSCs, such as the N719 family for evaluating the performances of newly reported dyes.3 Although the ruthenium-based sensitizers were very impressive, the metal-free organic dyes, generally composed of a donor-π-acceptor (D–π–A) configuration, have been conceived and synthesized as a promising alternative, due to their high molar absorption coefficients and convenient structural modifications, with no concern for using precious metals.4 In this D–π–A configuration, an efficient electron transfer from donor to acceptor can happen upon photo-excitation of the sensitizer, and then the negative charges accumulated on the acceptor can be injected into the conduction band (CB) of the metal oxide. Among various kinds of functional groups tested as donors and acceptors in organic sensitizers, triphenylamine and cyanoacrylic acid have been proven to be the most promising candidates, respectively;4 excellent electron-donating power and the aggregation-resistant nonplanar molecular configuration of triphenylamine appear to be essential for a promising donor in many organic sensitizers.5 Cyanoacrylic acid has also been adopted as an efficient acceptor in most cases, due to its strong electron-withdrawing properties and anchoring capabilities on the TiO2 surface. Thiophene derivatives were inserted as a π-conjugated bridge between the donor and the acceptor in most organic dyes. In attempting to make highly efficient DSSCs based on ideal organic sensitizers, one of the most important prerequisites is the design of an organic sensitizer with a high molar extinction coefficent for strong absorption of incident light.4a Recently, several organic sensitizers with high molar extinction coefficients have been reported as an important step toward the ideal chromophore.6 To enhance the molar extinction coefficient of the sensitizer, it is important to choose a proper component with high absorptivity by considering a highly planar molecular conformation for increased electron delocalization. Herein, a series of novel bistriphenylamine-based sensitizers, RC-11, RC-12 and RC-13 with high molar extinction coefficients (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000∼70
000∼70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) have been synthesized and then tested as a sensitizer in DSSCs. The chemical structures of sensitizers used in this study are shown in Fig. 1.
000) have been synthesized and then tested as a sensitizer in DSSCs. The chemical structures of sensitizers used in this study are shown in Fig. 1.
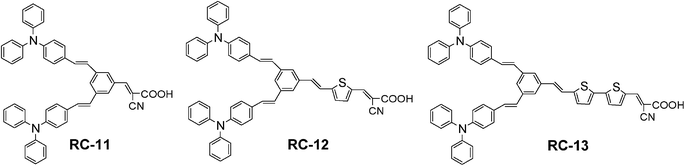 |
| | Fig. 1 Chemical structures of RC-11, RC-12 and RC-13. | |
2. Experimental
2.1. Synthesis and characterization of RC-dyes
3,5-Dibromobenzaldehyde, 5-bromothiophene-2-carbaldehyde, and 5-bromo-2,2′-bithiophene-5′-carbaldehyde were obtained from TCI. Cyanoacetic acid and piperidine were used as received from Aldrich. All reagents and solvents were purchased from Aldrich. The solvents were purified by the standard method. N-Phenyl-N-(4-vinylbenzene)benzenamine (1) was synthesized according to the literature procedures.71H and 13C NMR spectra were recorded on a Varian VNMRS 600 spectrometer. UV-vis spectra were recorded on a Perkin-Elmer Lambda 900 UV-vis spectrometer, while photoluminescence emissions (PL) were measured on a Perkin-Elmer LS 55 spectrometer. Differential pulse voltammetry (DPV) measurements were performed in DMF containing 0.1 M TBAPF6 and each dye (about 0.03 mM) using an EG&G 263 potentiostat/galvanostat in a three-electrode configuration. A platinum disk electrode and a platinum gauze were used as the working and the counter electrodes, respectively. A silver (Ag) wire was used as a pseudo-reference electrode with ferrocene internal standard.
3,5-Bis(4-(diphenylamino)styryl)benzaldehyde (2).
1 (5.00 g, 18.62 mmol), 3,5-dibromobenzaldehyde (2.21 g, 8.37 mmol), palladium acetate (0.11 g, 0.49 mmol), potassium carbonate (2.31 g, 16.71 mmol), and tetra-n-butylammoniumbromide (2.71 g, 8.37 mmol) were mixed with 20 ml dry DMF. After degassing for 30 min with Ar, the mixture was heated under Ar at 90 °C for 2 days. The resultant black mixture was diluted with chloroform and washed with 1 M HCl and brine. After drying over MgSO4, the remaining liquid was concentrated and purified by column chromatography (ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to produce 3.65 g (68%) of 3 as a yellow solid. MS (MALDI-TOF) m/z 644.19 (M+), calcd. 644.28. Melting point 105∼106 °C. 1H NMR (600 MHz, acetone-d6): δ (ppm) = 10.01 (s, 1H), 8.11 (s, 1H), 7.99 (s, 2H), 7.58 (d, 4H), 7.43 (d, 2H), 7.32–7.35 (m, 8H), 7.28 (d, 2H), 7.08–7.11 (m, 12H), 7.03 (d, 4H), 13C NMR (125 MHz, acetone-d6): δ (ppm) = 192.1, 147,8, 147.5, 139.2, 137.7, 131.2, 129.9, 129.4, 127.8, 125.6, 125.5, 124.6, 124.5, 123.4, 123.0. Anal. calcd. for C47H36N2O: C = 87.55; H = 5.63; N = 4.34. Found: C = 87.37; H = 5.59; N = 4.22.
10) to produce 3.65 g (68%) of 3 as a yellow solid. MS (MALDI-TOF) m/z 644.19 (M+), calcd. 644.28. Melting point 105∼106 °C. 1H NMR (600 MHz, acetone-d6): δ (ppm) = 10.01 (s, 1H), 8.11 (s, 1H), 7.99 (s, 2H), 7.58 (d, 4H), 7.43 (d, 2H), 7.32–7.35 (m, 8H), 7.28 (d, 2H), 7.08–7.11 (m, 12H), 7.03 (d, 4H), 13C NMR (125 MHz, acetone-d6): δ (ppm) = 192.1, 147,8, 147.5, 139.2, 137.7, 131.2, 129.9, 129.4, 127.8, 125.6, 125.5, 124.6, 124.5, 123.4, 123.0. Anal. calcd. for C47H36N2O: C = 87.55; H = 5.63; N = 4.34. Found: C = 87.37; H = 5.59; N = 4.22.
3-(3,5-Bis((E)-4-(diphenylamino)styryl)phenyl)-2-cyanoacrylic acid (RC-11).
2 (0.18 g, 0.28 mmol) and cyanoacetic acid (0.04 g, 0.47 mmol) were mixed with 5 ml acetonitrile and 5 ml chloroform. The mixture was refluxed for 5 h in the presence of piperidine (0.02 g, 0.23 mmol). After removal of the solvent, the crude product was purified by column chromatography (chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 1
methanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to produce 0.13 g (65%) RC-11 as a deep yellow solid. MS (MALDI-TOF) m/z 711.21 (M+), calcd. 711.29. Melting point 207∼208 °C. 1H NMR (600 MHz, DMSO-d6): δ (ppm) = 8.01 (s, 1H), 7.94 (s, 2H), 7.91 (s, 1H), 7.56 (d, 4H), 7.36–7.39 (m, 8H), 7.34 (d, 2H), 7.17 (d, 2H), 7.01–7.14 (12H), 7.01 (d, 4H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) = 163.5, 148.1, 147.5, 147.4, 138.8, 134.6, 131.3, 130.1, 129.6, 128.2, 126.3, 124.7, 123.8, 123.2, 119.5, 105.0. Anal. calcd. for C50H37N3O2: C 84.36; H = 5.24; N = 5.90. Found: C = 84.25; H = 5.17; N = 5.75.
1) to produce 0.13 g (65%) RC-11 as a deep yellow solid. MS (MALDI-TOF) m/z 711.21 (M+), calcd. 711.29. Melting point 207∼208 °C. 1H NMR (600 MHz, DMSO-d6): δ (ppm) = 8.01 (s, 1H), 7.94 (s, 2H), 7.91 (s, 1H), 7.56 (d, 4H), 7.36–7.39 (m, 8H), 7.34 (d, 2H), 7.17 (d, 2H), 7.01–7.14 (12H), 7.01 (d, 4H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) = 163.5, 148.1, 147.5, 147.4, 138.8, 134.6, 131.3, 130.1, 129.6, 128.2, 126.3, 124.7, 123.8, 123.2, 119.5, 105.0. Anal. calcd. for C50H37N3O2: C 84.36; H = 5.24; N = 5.90. Found: C = 84.25; H = 5.17; N = 5.75.
N-(4-(3-(4-(Diphenylamino)styryl)-5-vinylstyryl)phenyl)-N-phenylbenzenamine (3).
Potassium t-butoxide (0.65 g, 5.80 mmol) was added to methyltriphenylphosphonium iodide (2.63 g, 5.82 mmol) solution in 15 ml dry THF. After stirring for 15 min at room temperature, 2 (2.50 g, 3.88 mmol) in 10 ml dry THF was added dropwise. The solution was further stirred at room temperature for 6 h. Finally, the solid by-product was removed by filtration and the remaining liquid was concentrated and purified by column chromatography (hexane) to produce 1.99 g (80%) of 4 as a light yellow solid. MS (MALDI-TOF) m/z 642.22 (M+), calcd. 642.30. Melting point 75∼76 °C. 1H NMR (600 MHz, acetone-d6): δ (ppm) = 7.73 (s, 1H), 7.59 (s, 2H), 7.54 (d, 4H), 7.31–7.34 (m, 8H), 7.20 (d, 4H), 7.07–7.10 (m, 12H), 7.03 (d, 4H), 6.82 (q, 1H), 5.93 (d, 1H), 5.30 (d, 1H), 13C NMR (125 MHz, acetone-d6): δ (ppm) = 147.6, 147.5, 138.4, 138.3, 136.9, 131.7, 129.4, 128.6, 127.6, 126.7, 124.5, 124.4, 123.8, 123.3, 123.2, 123.1, 113.8. Anal. calcd. for C48H38N2: C 89.68; H = 5.96; N = 4.36. Found: C = 89.74; H = 5.82; N = 4.22.
5-(3,5-Bis(4-(diphenylamino)styryl)styryl)thiophene-2-carbaldehyde (4).
3 (0.30 g, 0.47 mmol), 5-bromothiophene-2-carbaldehyde (0.10 g, 0.52 mmol), palladium acetate (0.01 g, 0.04 mmol), potassium carbonate (0.15 g, 1.09 mmol), and tetra-n-butylammoniumbromide (0.17 g, 0.53 mmol) were mixed with 7 ml dry DMF. After degassing for 30 min with Ar, the mixture was heated under Ar at 90 °C for 2 days. The resultant black mixture was diluted with chloroform and washed with 1 M HCl and brine. After drying over MgSO4, the remaining liquid was concentrated and purified by column chromatography (chloroform) to produce 0.11 g (31%) of 5 as a deep yellow solid. MS (MALDI-TOF) m/z 752.19 (M+), calcd. 752.29. Melting point 118∼120 °C. 1H NMR (600 MHz, acetone-d6): δ (ppm) = 9.93 (s, 1H), 7.91 (s, 1H), 7.77 (s, 2H), 7.62 (d, 1H), 7.55 (d, 4H), 7.40 (d, 1H), 7.38 (d, 1H), 7.32–7.35 (m, 9H), 7.20 (d, 4H), 7.01–7.11 (m, 12H), 7.03 (d, 4H), 13C NMR (125 MHz, acetone-d6): δ (ppm) = 183.7, 148.5, 142.9, 139.7, 138.8, 137.9, 133.3, 132.5, 130.3, 132.5, 130.3, 129.8, 128.5, 128.2, 127.3, 125.6, 125.4, 124.6, 124.2, 124.1, 122.5. Anal. calcd. for C53H40N2OS: C 84.54; H = 5.35; N = 3.72. Found: C = 84.35; H = 5.20; N = 3.74.
(E)-3-(5-(3,5-Bis((E)-4-(diphenylamino)styryl)styryl)thiophene-2-yl)-2-cyanoacrylic acid (RC-12).
4 (0.09 g, 0.12 mmol) and cyanoacetic acid (0.03 g, 0.35 mmol) were mixed with 3 ml acetonitrile and 3 ml chloroform. The mixture was refluxed for 5 h in the presence of piperidine (0.01 g, 0.12 mmol). After removal of the solvent, the crude product was purified by column chromatography (chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 1
methanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to produce 0.07 g (72%) RC-12 as a deep yellow solid. MS (MALDI-TOF) m/z 819.20 (M+), calcd. 819.29. Melting point 278∼280 °C. 1H NMR (600 MHz, DMSO-d6): δ (ppm) 8.01 (s, 1H), 7.78 (s, 2H), 7.72 (s, 1H), 7.66 (d, 1H), 7.64 (d, 1H), 7.54 (d, 4H), 7.32–7.38 (m, 10H), 7.05–7.16 (m, 16H), 6.99 (d, 4H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) = 163.7, 154.1, 147.7, 147.4, 147.3, 140.7, 138.6, 137.4, 136.4, 131.6, 130.8, 130.1, 129.1, 128.1, 127.7, 126.7, 125.0, 124.7, 123.8, 123.3, 122.4, 119.7, 109.9. Anal. calcd. for C56H41N3O2S: C 82.02; H = 5.04; N = 5.12. Found: C = 81.89; H = 4.99; N = 5.16.
1) to produce 0.07 g (72%) RC-12 as a deep yellow solid. MS (MALDI-TOF) m/z 819.20 (M+), calcd. 819.29. Melting point 278∼280 °C. 1H NMR (600 MHz, DMSO-d6): δ (ppm) 8.01 (s, 1H), 7.78 (s, 2H), 7.72 (s, 1H), 7.66 (d, 1H), 7.64 (d, 1H), 7.54 (d, 4H), 7.32–7.38 (m, 10H), 7.05–7.16 (m, 16H), 6.99 (d, 4H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) = 163.7, 154.1, 147.7, 147.4, 147.3, 140.7, 138.6, 137.4, 136.4, 131.6, 130.8, 130.1, 129.1, 128.1, 127.7, 126.7, 125.0, 124.7, 123.8, 123.3, 122.4, 119.7, 109.9. Anal. calcd. for C56H41N3O2S: C 82.02; H = 5.04; N = 5.12. Found: C = 81.89; H = 4.99; N = 5.16.
5-(5-(3,5-Bis(4-(diphenylamino)styryl)styryl)thiophene-2-yl)thiophene carbaldehyde (5).
3 (0.50 g, 0.78 mmol), 5-bromo-2,2′-bithiophene-5′-carbaldehyde (0.21 g, 0.77 mmol), palladium acetate (0.01 g, 0.04 mmol), potassium carbonate (0.22 g, 1.59 mmol), and tetra-n-butylammoniumbromide (0.25 g, 0.78 mmol) were mixed with 7 ml dry DMF. After degassing 30 min with Ar, the mixture was heated under Ar at 90 °C for 2 days. The resultant black mixture was diluted with chloroform and washed with 1 M HCl and brine. After drying over MgSO4, the remaining liquid was concentrated and purified by column chromatography (chloroform) to produce 0.27 g (42%) of 6 as an orange solid. MS (MALDI-TOF) m/z 834.17 (M+), calcd. 834.27. Melting point 109∼110 °C. 1H NMR (600 MHz, acetone-d6): δ (ppm) = 9.93 (s, 1H), 7.93 (s, 1H), 7.72 (s, 2H), 7.74–7.57 (m, 5H), 7.57 (q, 2H), 7.37 (s, 1H), 7.32–7.34 (m, 8H), 7.24 (d, 1H), 7.19 (d, 4H), 7.14 (d, 1H), 7.01–7.11 (m, 12H), 7.03 (d, 4H), 13C NMR (125 MHz, CDCl3): δ (ppm) = 182.4, 147.6, 147.5, 147.1, 144.7, 141.6, 138.5, 137.4, 137.1, 134.5, 131.2, 129.8, 129.3, 128.9, 127.5, 127.4, 126.8, 126.4, 124.7, 124.6, 124.1, 123.4, 123.3, 123.1, 121.5. Anal. calcd. for C57H42N2OS2: C 81.98; H = 5.07; N = 3.35. Found: C = 81.82; H = 5.01; N = 3.29.
(E)-3-(5-(5-(3,5-Bis((E)-4-(diphenylamino)styryl)styryl)thiophene-2-yl)thiophene-2-yl)-2-cyanoacrylic acid (RC-13).
5 (0.10 g, 0.12 mmol) and cyanoacetic acid (0.03 g, 0.35 mmol) were mixed with 3 ml acetonitrile and 3 ml chloroform. The mixture was refluxed for 5 h in the presence of piperidine (0.01 g, 0.12 mmol). After removal of solvent, the crude product was purified by column chromatography (chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 1
methanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to produce 0.07 g (69%) RC-13 as a red solid. MS (MALDI-TOF) m/z 901.21 (M+), calcd. 901.28. Melting point 198∼199 °C. 1H NMR (600 MHz, DMSO-d6): δ (ppm) = 8.18 (s, 1H), 7.76 (s, 2H), 7.73 (s, 1H), 7.65 (d, 1H), 7.60 (d, 4H), 7.52–7.54 (m, 2H), 7.38–7.42 (m, 10H), 7.32 (d, 1H), 7.19 (d, 2H), 7.11–7.15 (m, 14H), 7.04 (d, 4H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) = 163.5, 147.4, 147.3, 143.8, 138.6, 137.6, 137.3, 134.7, 131.6, 130.1, 129.4, 129.0, 128.9, 128.1, 127.0, 126.8, 125.1, 124.7, 124.5, 123.8, 123.7, 123.4, 122.4, 119.4, 110.4. Anal. calcd. for C60H43N3O2S2: C 79.88; H = 4.80; N = 4.66. Found: C = 79.65; H = 4.87; N = 4.75.
1) to produce 0.07 g (69%) RC-13 as a red solid. MS (MALDI-TOF) m/z 901.21 (M+), calcd. 901.28. Melting point 198∼199 °C. 1H NMR (600 MHz, DMSO-d6): δ (ppm) = 8.18 (s, 1H), 7.76 (s, 2H), 7.73 (s, 1H), 7.65 (d, 1H), 7.60 (d, 4H), 7.52–7.54 (m, 2H), 7.38–7.42 (m, 10H), 7.32 (d, 1H), 7.19 (d, 2H), 7.11–7.15 (m, 14H), 7.04 (d, 4H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) = 163.5, 147.4, 147.3, 143.8, 138.6, 137.6, 137.3, 134.7, 131.6, 130.1, 129.4, 129.0, 128.9, 128.1, 127.0, 126.8, 125.1, 124.7, 124.5, 123.8, 123.7, 123.4, 122.4, 119.4, 110.4. Anal. calcd. for C60H43N3O2S2: C 79.88; H = 4.80; N = 4.66. Found: C = 79.65; H = 4.87; N = 4.75.
2.2. Fabrication and analyses of dye-sensitized solar cells (DSSCs)
The photo-anodes composed of nanocrystalline TiO2 were prepared using the procedure reported previously.8 FTO glass plates (Nippon Sheet Glass, Solar 4 mm thickness) were used as transparent conducting electrodes. After TiCl4 treatment, a paste composed of 20 nm anatase TiO2 particles for the transparent nanocrystalline layer was coated onto the FTO glass plates by screen printing. This coating–drying procedure was repeated to increase the thickness to about 9 μm. After drying the nanocrystalline TiO2 layer, a paste for the scattering layer containing 400 nm anatase particles (CCIC, HPW-400) was deposited by two screen printings. The resulting layer was composed of a 9 μm transparent layer and a 5 μm scattering layer, the thicknesses being measured using an Alpha-step 200 surface profilometer (Tencor Instruments, San Jose, CA). The TiO2 electrodes were gradually heated under an air flow, and then the TiO2 electrodes were treated by TiCl4 and sintered at 500 °C for 30 min. After cooling to about 80 °C, the TiO2 electrodes were immersed in the RC-11, RC-12 and RC-13 (in ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF = 1
THF = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions (0.3 mM dye and 5 mM 3a,7a-dihydroxy-5b-cholic acid, Cheno) and kept at room temperature overnight. Counter electrodes were prepared by coating with a drop of H2PtCl6 solution (2 mg Pt in 1 mL ethanol) on a FTO plate (TEC 15/2.2 mm thickness, Libbey-Owens-Ford Industries) and heating at 400 °C for 15 min. The dye-stained TiO2 electrode and Pt counter electrode were assembled into a sealed sandwich-type cell by hot-pressing with a 25 μm thick transparent hot-melt film (Surlyn 1702, DuPont). An electrolyte solution (Z960: 1.0 M 1,3-dimethylimidazolium iodide, 0.030 M iodine, 0.050 M LiI, 0.10 M guanidinium thiocyanate, and 0.50 M tert-butylpyridine in a 15/85 (v/v) mixture of valeronitrile and acetonitrile) was prepared and injected into the inter-electrode space from the counter electrode side through a predrilled hole, which was then sealed with a Bynel sheet and a thin glass cover by heating. In order to measure the conversion efficiencies accurately, by avoiding the scattered light from the edge of the glass electrodes of the dyed-TiO2 layer, the light-shading metal mask with an aperture was used on the DSSCs, so that the active area of DSCs was fixed at 0.16 cm2, following the area of aperture.9
1) solutions (0.3 mM dye and 5 mM 3a,7a-dihydroxy-5b-cholic acid, Cheno) and kept at room temperature overnight. Counter electrodes were prepared by coating with a drop of H2PtCl6 solution (2 mg Pt in 1 mL ethanol) on a FTO plate (TEC 15/2.2 mm thickness, Libbey-Owens-Ford Industries) and heating at 400 °C for 15 min. The dye-stained TiO2 electrode and Pt counter electrode were assembled into a sealed sandwich-type cell by hot-pressing with a 25 μm thick transparent hot-melt film (Surlyn 1702, DuPont). An electrolyte solution (Z960: 1.0 M 1,3-dimethylimidazolium iodide, 0.030 M iodine, 0.050 M LiI, 0.10 M guanidinium thiocyanate, and 0.50 M tert-butylpyridine in a 15/85 (v/v) mixture of valeronitrile and acetonitrile) was prepared and injected into the inter-electrode space from the counter electrode side through a predrilled hole, which was then sealed with a Bynel sheet and a thin glass cover by heating. In order to measure the conversion efficiencies accurately, by avoiding the scattered light from the edge of the glass electrodes of the dyed-TiO2 layer, the light-shading metal mask with an aperture was used on the DSSCs, so that the active area of DSCs was fixed at 0.16 cm2, following the area of aperture.9
For photovoltaic measurements of the DSSCs, the irradiation source was a 450 W xenon light source (Osram XBO 450, USA) using a Tempax 113 solar filter. The output power of the AM 1.5 solar simulator was calibrated by using a reference Si photodiode equipped with a colored matched IR-cutoff filter (KG-3, Schott) in order to reduce the mismatch in the region of 350–750 nm between the simulated light and AM 1.5 to less than 2%. The measurement of IPCE was plotted as a function of excitation wavelength using the incident light from a 300 W xenon lamp (ILC Technology, USA), which was focused through a Gemini-180 double monochromator (Jobin Yvon Ltd., U.K.).
2.3. Computational investigation
Calculations on the structure and the simulated spectra were performed using Density Functional Theory, DFT, and its Time-Dependent formulation, TDDFT, as implemented in the Gaussian program suite.10 The ground state geometry for the protonated dyes were optimized in the gas phase within the B3LYP functional11 using a 6-31G* basis set.12 At the optimized ground state geometries, we performed TDDFT (MPW1K/6-31G*)13 excited state calculations in the gas phase as well as in ethanol, adopting the non-equilibrium Conductor-like Polarizable Continuum Model, CPCM.14 This computational set-up has been previously shown to adequately describe the electronic and optical properties of similar push–pull dyes.15,16
3. Results and discussions
3.1. Preparation of RC-dyes
The detailed procedures of the dye synthesis are shown in Scheme 1 in the experimental part; firstly, the key intermediate of compound 2 with a bistriphenylamine donor was synthesized by a Heck coupling reaction between vinyltriphenylamine and dibromobenzaldehyde in the presence of palladium acetate, tetrabutylammonium bromide, and potassium carbonate. Then, the thiophene-based linkers were introduced via the Heck reaction after modification of the aldehyde group to vinyl functionality, on the focal point of the donors. Thiophene derivatives have been widely used as a building block for efficient sensitizers, because of their well-known high polarizability as well as their tunable spectroscopic and electrochemical properties. For comparison, RC-11 was prepared to have no linker part while RC-12 and RC-13 have monothiophene and bithiophene linkers between the donor and the acceptor, respectively. Finally, a cyanoacrylic acid moiety has been attached to the sensitizers by the Knoevenagel condensation reaction as an acceptor and anchoring group. The bistriphenylamine derivatives, 2 and 3, can be quite useful synthetic intermediates resulting in an efficient electron-donating moiety, which can later be connected to many different types of linkers and/or acceptors through highly reactive aldehyde and vinyl functional groups on the focal points. By introducing a bulky bistriphenylamine as an electron donating component in RC-dyes, several advantages can be anticipated; stronger electron donating capability by two antennae of triphenylamine5 and more efficient retardation of charge recombination between photoelectrons injected into the CB of TiO2 and the oxidized form (I3−) of the redox couple, due to the larger steric hindrance induced by the bulky periphery of the RC-dyes.17
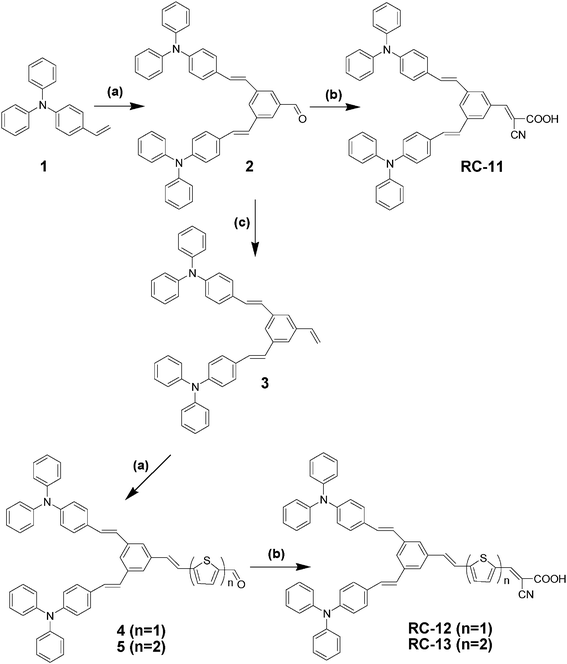 |
| | Scheme 1 Synthesis scheme of RC-11, RC-12 and RC-13. (a) Pd(OAc)2, Bu4NBr, K2CO3, DMF, 90 °C, 2 days; (b) acetic acid–chloroform, reflux, 5 h; (c) methyltriphenylphosphine iodide, potassium t-butoxide, THF, rt, 6 h. | |
3.2. Optical and electrochemical properties of RC-dyes
Fig. 2 shows the UV-vis absorption and emission spectra of RC-sensitizers dissolved in THF. All RC-dyes show relatively high molar extinction coefficients (ε > 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1), and the highest value was obtained for RC-13 (70
000 M−1 cm−1), and the highest value was obtained for RC-13 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1 at 386 nm). These optical properties of RC-sensitizers are summarized in Table 1. The optimized molecular geometries of the RC-dyes were shown to have quite a planar geometry, except for the terminal triphenylamine moiety (vide infra). Thus, the observed high molar extinction coefficients of RC-sensitizers probably originate from increased electron delocalization over the conjugated structures on the planar geometries and the efficient π–π* electron transitions.6a The electrochemical energy levels of the RC dyes were analyzed by differential pulse voltammetry (DPV) in DMF. Ag wire was used as a quasi-reference electrode with ferrocene as an internal standard. The ground-state oxidation potentials (Es+/s) of RC dyes, corresponding to the highest occupied molecular orbital (HOMO), were measured as being located at around 1.00 V vs. a normal hydrogen electrode (NHE). That was more positive than the redox couple (I−/I3−, ∼0.35 V), ensuring that there is a sufficient driving force for dye regeneration. The zeroth–zeroth energy values of the dyes (E0-0), which are related to the band gap energy (Eg), were estimated from the intercept of the normalized absorption and emission spectra. The lowest unoccupied molecular orbital (LUMO) level of the RC-dyes was obtained by subtracting E0-0 from the HOMO, and was also found to be more negative than the TiO2 conduction band (∼−0.5 V vs. NHE) for efficient electron injection. Therefore, these new organic dyes have proper electronic energy levels as a promising sensitizer in DSSCs.
900 M−1 cm−1 at 386 nm). These optical properties of RC-sensitizers are summarized in Table 1. The optimized molecular geometries of the RC-dyes were shown to have quite a planar geometry, except for the terminal triphenylamine moiety (vide infra). Thus, the observed high molar extinction coefficients of RC-sensitizers probably originate from increased electron delocalization over the conjugated structures on the planar geometries and the efficient π–π* electron transitions.6a The electrochemical energy levels of the RC dyes were analyzed by differential pulse voltammetry (DPV) in DMF. Ag wire was used as a quasi-reference electrode with ferrocene as an internal standard. The ground-state oxidation potentials (Es+/s) of RC dyes, corresponding to the highest occupied molecular orbital (HOMO), were measured as being located at around 1.00 V vs. a normal hydrogen electrode (NHE). That was more positive than the redox couple (I−/I3−, ∼0.35 V), ensuring that there is a sufficient driving force for dye regeneration. The zeroth–zeroth energy values of the dyes (E0-0), which are related to the band gap energy (Eg), were estimated from the intercept of the normalized absorption and emission spectra. The lowest unoccupied molecular orbital (LUMO) level of the RC-dyes was obtained by subtracting E0-0 from the HOMO, and was also found to be more negative than the TiO2 conduction band (∼−0.5 V vs. NHE) for efficient electron injection. Therefore, these new organic dyes have proper electronic energy levels as a promising sensitizer in DSSCs.
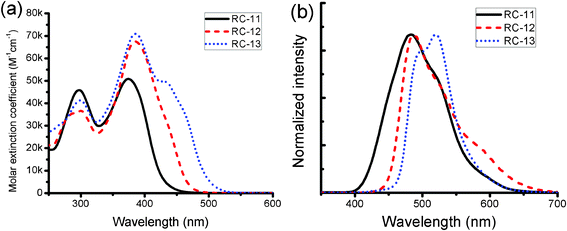 |
| | Fig. 2 (a) Absorption and (b) emission spectra of RC-11, RC-12 and RC-13 in THF. The excitation wavelength for the emission spectra was 300 nm. | |
Table 1 Absorption, emission, and electrochemical properties of RC dyes
| Dye |
Absorptiona |
Emissiona |
Energy level |
|
λ
max/nm |
ε/M−1 cm−1 |
λ
max/nm |
E
0-0
b/eV |
E
s+/s
c
|
E
s+/s*
d
|
|
Absorption and emission spectra were measured in THF (10−5 M).
E
0-0 was estimated from the intercept of normalized absorption and emission spectra.
The ground-state oxidation potential of dyes were measured in DMF by DPV. Potentials measured vs. Fc+/Fc were converted to those vs. the normal hydrogen electrode (NHE) by addition of + 0.63 V.
The excited-state oxidation potential, Es+/s*, was calculated from Es+/s − E0-0.
|
|
RC-11
|
376 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
483 |
2.93 |
0.96 |
−1.97 |
|
RC-12
|
384 |
67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
487 |
2.77 |
1.01 |
−1.76 |
|
RC-13
|
386 |
70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
520 |
2.58 |
1.03 |
−1.55 |
3.3. Theoretical investigation of RC-dyes
The optimized molecular structures of RC-11∼13 are characterized by a planar donor–acceptor arrangement, except for the terminal triphenylamine moiety, which is enforced by the conjugation occurring along the push–pull system. Thus, by extending the conjugation along the acceptor axis in the RC-11∼13 series, we expect a stabilization of the related unoccupied orbitals. A summary of the electronic structure for the considered systems is reported in Fig. 3 along with their relevant molecular orbitals.
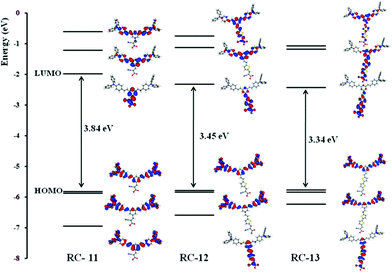 |
| | Fig. 3 Comparison between HOMO, HOMO − 1, HOMO − 2, LUMO, LUMO + 1 and LUMO + 2 molecular orbitals of the RC-11∼13 dyes in ethanol solution. | |
In line with the electronic structure of related push–pull dyes,15,16 the HOMO and HOMO − 1 of the RC dyes are in all cases localized on the donor moiety of the dye while the LUMO is delocalized over the acceptor moiety. Thus, it is expected that the efficient photo-excited electron transfer from the HOMO to the LUMO of the dyes, and then to the conduction band of TiO2, will occur through the close position of the LUMO to the anchoring group in the excited state. The HOMO and HOMO − 1 are a degenerate couple, with contributions extending through the double donor moieties. Since the donor moiety is the same in all dyes, the HOMO energies do not show substantial variations along the series, which was also confirmed by almost the same electrochemical oxidation potentials, ∼1 V (Table 1). On the other hand, the LUMOs of RC-11∼13 are in all cases delocalized over the acceptor moieties, with an increasing delocalization as the bridging moiety is extended from RC-11 to RC-13. The LUMO energy reflects this trend, decreasing from −1.98 to −2.33 to −2.43 eV along the RC-11∼13 series.
A comparison of the calculated and experimental absorption spectra for RC-11∼13 showed a good agreement between the calculated and experimental spectra along the series, allowing us to characterize the visible absorption of the investigated compounds as essentially originating from charge transfer excitations occurring along the push–pull system of the dyes, although this is mixed with an increasing π–π* character when moving from RC-11 to RC-13.
3.4. Photovoltaic properties of RC-dyes
The photovoltaic properties of RC dye-incorporated DSSCs are shown in Fig. 4 and summarized in Table 2. Under standard solar irradiation conditions of simulated AM 1.5, the RC-13 sensitized cell exhibited the best power conversion efficiency of 5.67% with a short circuit current (JSC) of 10.78 mA cm−2, a open circuit voltage of 0.71 V and a fill factor (ff) of 0.74, outperforming those from RC-11 (2.05%) and RC-12 (3.46%). According to the current results obtained from the typical DSSCs sensitized with a series of RC dyes, the presence and length of the thiophene linker in RC dyes appear to be critical in determining the overall power conversion efficiencies in the DSSCs thereof. Without any linkage between the donor and acceptor parts, the lowest efficiency of 2.05% was obtained for RC-11, while the efficiencies are gradually increased from RC-12 with a monothiophene linker to RC-13 with a dithiophene one. Similar results have been reported before about other organic sensitizers;18 it is believed that both broader and stronger light-absorption from sensitizers are the main reasons for this enhancement. Therefore, it is evident that a proper linker between the promising donor and acceptor unit is very critical for maximizing the capacity of the organic sensitizer composed of D–π–A. Another important factor governing the overall power conversion efficiency of RC dye-based DSSCs was observed to be that the solvent, which dissolves RC dyes, should self-assemble onto the TiO2 surface. The different dye baths for semiconductor sensitization have a crucial effect on the performance of the DSSCs due to some reasons such as the different adsorbed amounts and binding modes of dyes anchored to the TiO2 surface in various solvents as reported before.19 At the current research stage, the mixed solvent of EtOH and THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, v/v) was found to give the best result while in each pure solvent, exhibiting a much less efficient record with RC dyes. More detailed investigations are currently under way to elucidate the effect of solvent on the behavior of the self-assembly of these bulky organic dyes and their final photovoltaic performances. To check the wavelength-dependent conversion efficiencies, the incident photon to current spectra (IPCE) from the RC dye-sensitized cells were measured (Fig. 4(b)). The IPCE spectra become more efficient in a broader range from RC-11viaRC-12 to RC-13, which is in accordance with their absorption spectra (Fig. 1). In our best dye (RC-13), the high IPCE values (>80%) over the wide spectrum range from about 400 nm to 550 nm, with a maximum value 88% at 520 nm, were observed. This high IPCE value from the RC-13 dye over its absorption range certifies that the current molecular configuration (bistriphenylamine-dithiophene-cyanoacrylic acid) could be a promising one for the further improvement of metal-free organic sensitizers.
1 ratio, v/v) was found to give the best result while in each pure solvent, exhibiting a much less efficient record with RC dyes. More detailed investigations are currently under way to elucidate the effect of solvent on the behavior of the self-assembly of these bulky organic dyes and their final photovoltaic performances. To check the wavelength-dependent conversion efficiencies, the incident photon to current spectra (IPCE) from the RC dye-sensitized cells were measured (Fig. 4(b)). The IPCE spectra become more efficient in a broader range from RC-11viaRC-12 to RC-13, which is in accordance with their absorption spectra (Fig. 1). In our best dye (RC-13), the high IPCE values (>80%) over the wide spectrum range from about 400 nm to 550 nm, with a maximum value 88% at 520 nm, were observed. This high IPCE value from the RC-13 dye over its absorption range certifies that the current molecular configuration (bistriphenylamine-dithiophene-cyanoacrylic acid) could be a promising one for the further improvement of metal-free organic sensitizers.
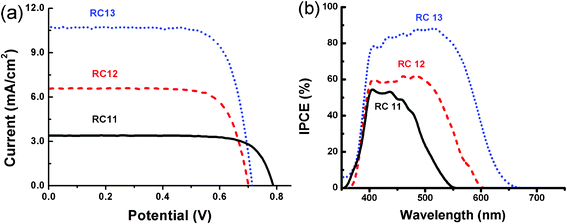 |
| | Fig. 4 (a) Photocurrent density–voltage curves of DSSCs with RC-11, RC-12 and RC-13 under AM 1.5 simulated sunlight (100 mW cm−2) and (b) the IPCE action spectra of DSSCs with RC-11, RC-12 and RC-13. | |
Table 2 Photovoltaic performances of DSSCs of dyesab
| Dye |
Jsc/mA cm−2 |
Voc/mV |
Fill factor |
η (%) |
Measured under simulated AM 1.5G conditions (100 mW cm−2).
THF–ethanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and t-BuOH/ACN were used as a dye loading solvent for RC dyes and N719, respectively. 1 and t-BuOH/ACN were used as a dye loading solvent for RC dyes and N719, respectively.
|
| RC-11 |
3.38 |
0.79 |
0.77 |
2.05 |
| RC-12 |
6.56 |
0.70 |
0.75 |
3.46 |
| RC-13 |
10.78 |
0.71 |
0.74 |
5.67 |
| N719 |
17.01 |
0.74 |
0.75 |
9.43 |
4. Conclusions
A series of bistriphenylamine donor-based sensitizers (coded as RC-dyes) with high molar extinction coefficients (5.1∼7.1 × 104 M−1 cm−1) have been synthesized based on a facile preparation route, which could easily modulate diverse structures in typical organic chromophores for applications in DSSCs. In addition to proper selection of a donor and acceptor unit, the bridging π-conjugation component was found to be crucial in designing efficient sensitizers toward an ideal molecular converter by strong absorption of incident light. Though the best power conversion efficiency from an RC 13-sensitized cell was 5.67% at the current stage, there is much room left for further enhancement by introducing some functional groups into the main structure of RC dyes and finding a more appropriate solvent to dissolve RC dyes in a favorable way for effective self-assembly onto the surface of TiO2.
Acknowledgements
Prof. Jong-Beom Baek and Dr Mohammad K. Nazeeruddin acknowledge the financial support from the World Class University (WCU) program supported by National Research Foundation and Ministry of Education, Science and Technology of Korea. The support by the “Human Resource Development (project name: Advanced track for Si-based solar cell materials and devices, project number: 20104010100660)” of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Knowledge Economy was acknowledged by H. J. Lee.
References
- B. O’Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
-
(a) A. Yella, H.-W Lee, H. N. Tsao, C. Yi, A. K. Chandiran, Md. K. Nazeeruddin, E. Wei-Guang Diau, C.-Yu Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629–634 CrossRef CAS;
(b) G. Zhang, H. Bala, Y. Cheng, D. Shi, X. Lv, Q. Yu and P. Wang, Chem. Commun., 2009, 2198–2200 RSC.
-
(a) M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Müller, P. Liska, N. Vlachopoulos and M. Grätzel, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS;
(b) M. K. Nazeeruddin, P. Pechy, T. Renouard, S. M. Zakeeruddin, R. Humphry-Baker, P. Comte, P. Liska, L. Cevey, E. Costa, V. Shklover, L. Spiccia, G. B. Deacon, C. A. Bignozzi and M. Grätzel, J. Am. Chem. Soc., 2001, 123, 1613–1624 CrossRef CAS.
-
(a) A. Mishra, M. K. R. Fischer and P. Bäuerle, Angew. Chem., Int. Ed., 2009, 28, 2474–2499 CrossRef;
(b) Y. Ooyama and Y. Harima, Eur. J. Org. Chem., 2009, 2903–2934 CrossRef CAS.
- Z. Ning and H. Tian, Chem. Commun., 2009, 5483–5495 RSC.
-
(a) H. Choi, I. Raabe, D. Kim, F. Teocoli, C. Kim, K. Song, J. H. Yum, J. J. Ko, M. K. Naeeruddin and M. Grätzel, Chem.–Eur. J., 2010, 16, 1193–1201 CrossRef CAS;
(b) H. Chen, H. Huang, X. Huang, J. N. Clifford, A. Forneli, E. Palomares, X. Zheng, L. Zheng, X. Wang, P. Shen, B. Zhao and S. Tan, J. Phys. Chem. C, 2010, 114, 3280–3286 CrossRef CAS.
- M. Behl, E. Hattemer, M. Brehmer and R. Zentel, Macromol. Chem. Phys., 2002, 203, 503–510 CrossRef CAS.
- D. P. Hagberg, J.-H. Yum, H. J. Lee, F. De Angelis, T. Marinado, K. M. Karlsson, R. Humphry-Baker, L. Sun, A. Hagfeldt, M. Grätzel and Md. K. Nazeeruddin, J. Am. Chem. Soc., 2008, 130, 6259–6266 CrossRef CAS.
- S. Ito, Md. K. Nazeeruddin, P. Liska, P. Comte, R. Charvet, P. Pechy, M. Jirousek, A. Kay, S. M. Zakeeruddin and M. Grätzel, Progr. Photovolt.: Res. Appl., 2006, 14, 589–601 CrossRef CAS.
- Gaussian 09, Revision A.1
M. J. Frisch
et al.
Gaussian, Inc.: Wallingford CT, 2009.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- V. A. Rassolov, J. A. Pople, M. A. Ratner and T. L. Windus, J. Chem. Phys., 1998, 109, 1223–1229 CrossRef CAS.
- B. J. Lynch, P. L. Fast, M. Harris and D. G. Truhlar, J. Phys. Chem. A, 2000, 104, 4811–4815 CrossRef CAS.
- M. Cossi, N. Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24, 669–681 CrossRef CAS.
- A. Abbotto, N. Manfredi, C. Marinzi, F. De Angelis, E. Mosconi, J.-H. Yum, Z. Xianxi, M. K. Nazeeruddin and M. Grätzel, Energy Environ. Sci., 2009, 2, 1094–1101 CAS.
- M. Pastore, E. Mosconi, F. De Angelis and M. Grätzel, J. Phys. Chem. C, 2010, 114, 7205–7212 CAS.
- S. A. Haque, S. Handa, K. Peter, E. Palomares, M. Thelakkat and J. R. Durrant, Angew. Chem., Int. Ed., 2005, 44, 5740–5744 CrossRef CAS.
-
(a) W. H. Liu, I. C. Wu, C. H. Lai, P. T. Chou, Y. T. Li, C. L. Chen, Y. Y. Hsu and Y. Chi, Chem. Commun., 2008, 5152 RSC;
(b) D. P. Hagberg, T. Marinado, K. M. Karlsson, K. Nonomura, P. Qin, G. Boschloo, T. Bricnk, A. Hägfelt and L. Sun, J. Org. Chem., 2007, 72, 9550 CrossRef CAS.
- H. Tian, X. Yang, R. Chen, R. Zhang, A. Hägfelt and L. Sun, J. Phys. Chem. C, 2008, 112, 11023–11033 CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000∼70
000∼70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) have been synthesized and then tested as a sensitizer in DSSCs. The chemical structures of sensitizers used in this study are shown in Fig. 1.
000) have been synthesized and then tested as a sensitizer in DSSCs. The chemical structures of sensitizers used in this study are shown in Fig. 1.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to produce 3.65 g (68%) of 3 as a yellow solid. MS (MALDI-TOF) m/z 644.19 (M+), calcd. 644.28. Melting point 105∼106 °C. 1H NMR (600 MHz, acetone-d6): δ (ppm) = 10.01 (s, 1H), 8.11 (s, 1H), 7.99 (s, 2H), 7.58 (d, 4H), 7.43 (d, 2H), 7.32–7.35 (m, 8H), 7.28 (d, 2H), 7.08–7.11 (m, 12H), 7.03 (d, 4H), 13C NMR (125 MHz, acetone-d6): δ (ppm) = 192.1, 147,8, 147.5, 139.2, 137.7, 131.2, 129.9, 129.4, 127.8, 125.6, 125.5, 124.6, 124.5, 123.4, 123.0. Anal. calcd. for C47H36N2O: C = 87.55; H = 5.63; N = 4.34. Found: C = 87.37; H = 5.59; N = 4.22.
10) to produce 3.65 g (68%) of 3 as a yellow solid. MS (MALDI-TOF) m/z 644.19 (M+), calcd. 644.28. Melting point 105∼106 °C. 1H NMR (600 MHz, acetone-d6): δ (ppm) = 10.01 (s, 1H), 8.11 (s, 1H), 7.99 (s, 2H), 7.58 (d, 4H), 7.43 (d, 2H), 7.32–7.35 (m, 8H), 7.28 (d, 2H), 7.08–7.11 (m, 12H), 7.03 (d, 4H), 13C NMR (125 MHz, acetone-d6): δ (ppm) = 192.1, 147,8, 147.5, 139.2, 137.7, 131.2, 129.9, 129.4, 127.8, 125.6, 125.5, 124.6, 124.5, 123.4, 123.0. Anal. calcd. for C47H36N2O: C = 87.55; H = 5.63; N = 4.34. Found: C = 87.37; H = 5.59; N = 4.22.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 1
methanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to produce 0.13 g (65%) RC-11 as a deep yellow solid. MS (MALDI-TOF) m/z 711.21 (M+), calcd. 711.29. Melting point 207∼208 °C. 1H NMR (600 MHz, DMSO-d6): δ (ppm) = 8.01 (s, 1H), 7.94 (s, 2H), 7.91 (s, 1H), 7.56 (d, 4H), 7.36–7.39 (m, 8H), 7.34 (d, 2H), 7.17 (d, 2H), 7.01–7.14 (12H), 7.01 (d, 4H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) = 163.5, 148.1, 147.5, 147.4, 138.8, 134.6, 131.3, 130.1, 129.6, 128.2, 126.3, 124.7, 123.8, 123.2, 119.5, 105.0. Anal. calcd. for C50H37N3O2: C 84.36; H = 5.24; N = 5.90. Found: C = 84.25; H = 5.17; N = 5.75.
1) to produce 0.13 g (65%) RC-11 as a deep yellow solid. MS (MALDI-TOF) m/z 711.21 (M+), calcd. 711.29. Melting point 207∼208 °C. 1H NMR (600 MHz, DMSO-d6): δ (ppm) = 8.01 (s, 1H), 7.94 (s, 2H), 7.91 (s, 1H), 7.56 (d, 4H), 7.36–7.39 (m, 8H), 7.34 (d, 2H), 7.17 (d, 2H), 7.01–7.14 (12H), 7.01 (d, 4H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) = 163.5, 148.1, 147.5, 147.4, 138.8, 134.6, 131.3, 130.1, 129.6, 128.2, 126.3, 124.7, 123.8, 123.2, 119.5, 105.0. Anal. calcd. for C50H37N3O2: C 84.36; H = 5.24; N = 5.90. Found: C = 84.25; H = 5.17; N = 5.75.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 1
methanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to produce 0.07 g (72%) RC-12 as a deep yellow solid. MS (MALDI-TOF) m/z 819.20 (M+), calcd. 819.29. Melting point 278∼280 °C. 1H NMR (600 MHz, DMSO-d6): δ (ppm) 8.01 (s, 1H), 7.78 (s, 2H), 7.72 (s, 1H), 7.66 (d, 1H), 7.64 (d, 1H), 7.54 (d, 4H), 7.32–7.38 (m, 10H), 7.05–7.16 (m, 16H), 6.99 (d, 4H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) = 163.7, 154.1, 147.7, 147.4, 147.3, 140.7, 138.6, 137.4, 136.4, 131.6, 130.8, 130.1, 129.1, 128.1, 127.7, 126.7, 125.0, 124.7, 123.8, 123.3, 122.4, 119.7, 109.9. Anal. calcd. for C56H41N3O2S: C 82.02; H = 5.04; N = 5.12. Found: C = 81.89; H = 4.99; N = 5.16.
1) to produce 0.07 g (72%) RC-12 as a deep yellow solid. MS (MALDI-TOF) m/z 819.20 (M+), calcd. 819.29. Melting point 278∼280 °C. 1H NMR (600 MHz, DMSO-d6): δ (ppm) 8.01 (s, 1H), 7.78 (s, 2H), 7.72 (s, 1H), 7.66 (d, 1H), 7.64 (d, 1H), 7.54 (d, 4H), 7.32–7.38 (m, 10H), 7.05–7.16 (m, 16H), 6.99 (d, 4H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) = 163.7, 154.1, 147.7, 147.4, 147.3, 140.7, 138.6, 137.4, 136.4, 131.6, 130.8, 130.1, 129.1, 128.1, 127.7, 126.7, 125.0, 124.7, 123.8, 123.3, 122.4, 119.7, 109.9. Anal. calcd. for C56H41N3O2S: C 82.02; H = 5.04; N = 5.12. Found: C = 81.89; H = 4.99; N = 5.16.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 1
methanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to produce 0.07 g (69%) RC-13 as a red solid. MS (MALDI-TOF) m/z 901.21 (M+), calcd. 901.28. Melting point 198∼199 °C. 1H NMR (600 MHz, DMSO-d6): δ (ppm) = 8.18 (s, 1H), 7.76 (s, 2H), 7.73 (s, 1H), 7.65 (d, 1H), 7.60 (d, 4H), 7.52–7.54 (m, 2H), 7.38–7.42 (m, 10H), 7.32 (d, 1H), 7.19 (d, 2H), 7.11–7.15 (m, 14H), 7.04 (d, 4H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) = 163.5, 147.4, 147.3, 143.8, 138.6, 137.6, 137.3, 134.7, 131.6, 130.1, 129.4, 129.0, 128.9, 128.1, 127.0, 126.8, 125.1, 124.7, 124.5, 123.8, 123.7, 123.4, 122.4, 119.4, 110.4. Anal. calcd. for C60H43N3O2S2: C 79.88; H = 4.80; N = 4.66. Found: C = 79.65; H = 4.87; N = 4.75.
1) to produce 0.07 g (69%) RC-13 as a red solid. MS (MALDI-TOF) m/z 901.21 (M+), calcd. 901.28. Melting point 198∼199 °C. 1H NMR (600 MHz, DMSO-d6): δ (ppm) = 8.18 (s, 1H), 7.76 (s, 2H), 7.73 (s, 1H), 7.65 (d, 1H), 7.60 (d, 4H), 7.52–7.54 (m, 2H), 7.38–7.42 (m, 10H), 7.32 (d, 1H), 7.19 (d, 2H), 7.11–7.15 (m, 14H), 7.04 (d, 4H), 13C NMR (125 MHz, DMSO-d6): δ (ppm) = 163.5, 147.4, 147.3, 143.8, 138.6, 137.6, 137.3, 134.7, 131.6, 130.1, 129.4, 129.0, 128.9, 128.1, 127.0, 126.8, 125.1, 124.7, 124.5, 123.8, 123.7, 123.4, 122.4, 119.4, 110.4. Anal. calcd. for C60H43N3O2S2: C 79.88; H = 4.80; N = 4.66. Found: C = 79.65; H = 4.87; N = 4.75.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF = 1
THF = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions (0.3 mM dye and 5 mM 3a,7a-dihydroxy-5b-cholic acid, Cheno) and kept at room temperature overnight. Counter electrodes were prepared by coating with a drop of H2PtCl6 solution (2 mg Pt in 1 mL ethanol) on a FTO plate (TEC 15/2.2 mm thickness, Libbey-Owens-Ford Industries) and heating at 400 °C for 15 min. The dye-stained TiO2 electrode and Pt counter electrode were assembled into a sealed sandwich-type cell by hot-pressing with a 25 μm thick transparent hot-melt film (Surlyn 1702, DuPont). An electrolyte solution (Z960: 1.0 M 1,3-dimethylimidazolium iodide, 0.030 M iodine, 0.050 M LiI, 0.10 M guanidinium thiocyanate, and 0.50 M tert-butylpyridine in a 15/85 (v/v) mixture of valeronitrile and acetonitrile) was prepared and injected into the inter-electrode space from the counter electrode side through a predrilled hole, which was then sealed with a Bynel sheet and a thin glass cover by heating. In order to measure the conversion efficiencies accurately, by avoiding the scattered light from the edge of the glass electrodes of the dyed-TiO2 layer, the light-shading metal mask with an aperture was used on the DSSCs, so that the active area of DSCs was fixed at 0.16 cm2, following the area of aperture.9
1) solutions (0.3 mM dye and 5 mM 3a,7a-dihydroxy-5b-cholic acid, Cheno) and kept at room temperature overnight. Counter electrodes were prepared by coating with a drop of H2PtCl6 solution (2 mg Pt in 1 mL ethanol) on a FTO plate (TEC 15/2.2 mm thickness, Libbey-Owens-Ford Industries) and heating at 400 °C for 15 min. The dye-stained TiO2 electrode and Pt counter electrode were assembled into a sealed sandwich-type cell by hot-pressing with a 25 μm thick transparent hot-melt film (Surlyn 1702, DuPont). An electrolyte solution (Z960: 1.0 M 1,3-dimethylimidazolium iodide, 0.030 M iodine, 0.050 M LiI, 0.10 M guanidinium thiocyanate, and 0.50 M tert-butylpyridine in a 15/85 (v/v) mixture of valeronitrile and acetonitrile) was prepared and injected into the inter-electrode space from the counter electrode side through a predrilled hole, which was then sealed with a Bynel sheet and a thin glass cover by heating. In order to measure the conversion efficiencies accurately, by avoiding the scattered light from the edge of the glass electrodes of the dyed-TiO2 layer, the light-shading metal mask with an aperture was used on the DSSCs, so that the active area of DSCs was fixed at 0.16 cm2, following the area of aperture.9

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1), and the highest value was obtained for RC-13 (70
000 M−1 cm−1), and the highest value was obtained for RC-13 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1 at 386 nm). These optical properties of RC-sensitizers are summarized in Table 1. The optimized molecular geometries of the RC-dyes were shown to have quite a planar geometry, except for the terminal triphenylamine moiety (vide infra). Thus, the observed high molar extinction coefficients of RC-sensitizers probably originate from increased electron delocalization over the conjugated structures on the planar geometries and the efficient π–π* electron transitions.6a The electrochemical energy levels of the RC dyes were analyzed by differential pulse voltammetry (DPV) in DMF. Ag wire was used as a quasi-reference electrode with ferrocene as an internal standard. The ground-state oxidation potentials (Es+/s) of RC dyes, corresponding to the highest occupied molecular orbital (HOMO), were measured as being located at around 1.00 V vs. a normal hydrogen electrode (NHE). That was more positive than the redox couple (I−/I3−, ∼0.35 V), ensuring that there is a sufficient driving force for dye regeneration. The zeroth–zeroth energy values of the dyes (E0-0), which are related to the band gap energy (Eg), were estimated from the intercept of the normalized absorption and emission spectra. The lowest unoccupied molecular orbital (LUMO) level of the RC-dyes was obtained by subtracting E0-0 from the HOMO, and was also found to be more negative than the TiO2 conduction band (∼−0.5 V vs. NHE) for efficient electron injection. Therefore, these new organic dyes have proper electronic energy levels as a promising sensitizer in DSSCs.
900 M−1 cm−1 at 386 nm). These optical properties of RC-sensitizers are summarized in Table 1. The optimized molecular geometries of the RC-dyes were shown to have quite a planar geometry, except for the terminal triphenylamine moiety (vide infra). Thus, the observed high molar extinction coefficients of RC-sensitizers probably originate from increased electron delocalization over the conjugated structures on the planar geometries and the efficient π–π* electron transitions.6a The electrochemical energy levels of the RC dyes were analyzed by differential pulse voltammetry (DPV) in DMF. Ag wire was used as a quasi-reference electrode with ferrocene as an internal standard. The ground-state oxidation potentials (Es+/s) of RC dyes, corresponding to the highest occupied molecular orbital (HOMO), were measured as being located at around 1.00 V vs. a normal hydrogen electrode (NHE). That was more positive than the redox couple (I−/I3−, ∼0.35 V), ensuring that there is a sufficient driving force for dye regeneration. The zeroth–zeroth energy values of the dyes (E0-0), which are related to the band gap energy (Eg), were estimated from the intercept of the normalized absorption and emission spectra. The lowest unoccupied molecular orbital (LUMO) level of the RC-dyes was obtained by subtracting E0-0 from the HOMO, and was also found to be more negative than the TiO2 conduction band (∼−0.5 V vs. NHE) for efficient electron injection. Therefore, these new organic dyes have proper electronic energy levels as a promising sensitizer in DSSCs.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, v/v) was found to give the best result while in each pure solvent, exhibiting a much less efficient record with RC dyes. More detailed investigations are currently under way to elucidate the effect of solvent on the behavior of the self-assembly of these bulky organic dyes and their final photovoltaic performances. To check the wavelength-dependent conversion efficiencies, the incident photon to current spectra (IPCE) from the RC dye-sensitized cells were measured (Fig. 4(b)). The IPCE spectra become more efficient in a broader range from RC-11viaRC-12 to RC-13, which is in accordance with their absorption spectra (Fig. 1). In our best dye (RC-13), the high IPCE values (>80%) over the wide spectrum range from about 400 nm to 550 nm, with a maximum value 88% at 520 nm, were observed. This high IPCE value from the RC-13 dye over its absorption range certifies that the current molecular configuration (bistriphenylamine-dithiophene-cyanoacrylic acid) could be a promising one for the further improvement of metal-free organic sensitizers.
1 ratio, v/v) was found to give the best result while in each pure solvent, exhibiting a much less efficient record with RC dyes. More detailed investigations are currently under way to elucidate the effect of solvent on the behavior of the self-assembly of these bulky organic dyes and their final photovoltaic performances. To check the wavelength-dependent conversion efficiencies, the incident photon to current spectra (IPCE) from the RC dye-sensitized cells were measured (Fig. 4(b)). The IPCE spectra become more efficient in a broader range from RC-11viaRC-12 to RC-13, which is in accordance with their absorption spectra (Fig. 1). In our best dye (RC-13), the high IPCE values (>80%) over the wide spectrum range from about 400 nm to 550 nm, with a maximum value 88% at 520 nm, were observed. This high IPCE value from the RC-13 dye over its absorption range certifies that the current molecular configuration (bistriphenylamine-dithiophene-cyanoacrylic acid) could be a promising one for the further improvement of metal-free organic sensitizers.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and t-BuOH/ACN were used as a dye loading solvent for RC dyes and N719, respectively.
1 and t-BuOH/ACN were used as a dye loading solvent for RC dyes and N719, respectively.
