A novel strategy to fabricate inorganic nanofibrous membranes for water treatment: use of functionalized graphene oxide as a cross linker†
Tong
Zhang
,
Jincheng
Liu
* and
Darren Delai
Sun
*
School of Civil and Environmental Engineering, Nanyang Technological University, Nanyang Avenue, Singapore 639798. E-mail: JCLiu@ntu.edu.sg; DDSun@ntu.edu.sg; Fax: +65-6791-0676; +65-6791-0676; Tel: +65-6790-9073; +65-6790-6273
First published on 13th April 2012
Abstract
A novel type of nanofibrous membrane was assembled using hierarchical K-OMS-2/GO-SO3H heterojunctions, and the membrane exhibited excellent permeability and selectivity in a dead-end microfiltration process.
Inorganic nanofibrous membranes have recently attracted increasing attention in the fields of catalysis, adsorption, fuel cells, sensors and filtration.1–6 In view of their mechanical resistance and excellent performance in the removal of pollutants, inorganic nanofibrous membranes with different structures and properties have been successfully applied for water purification.5–7 Successful application of inorganic membranes in water purification will depend upon the ability to prepare membranes with the desired pore size,8 which would affect the selectivity and permeability of the membrane. Previous studies have shown that various techniques such as self-assembly, electrospinning and selective etching of templates can be used to synthesize inorganic nanofibrous membranes.6,7,9,10 Unfortunately, these techniques for membrane synthesis suffer from poor selectivity and permeability, complicated technical requirements and high operational costs, thus severely restricting water purification applications.6,11 As the final step during the preparation of a traditional inorganic membrane, calcination is very important for controlling the pore size and pore structure of the membrane.12 Although it can downsize membrane pores and thus enhance membrane selectivity, this process often leads to the formation of pinholes and cracks within the membrane as well as an energy cost.13 Therefore, there is a growing need to develop a facile and economical method to fabricate inorganic nanofibrous membranes with good selectivity and permeability.
Graphene and its derivatives are promising candidates for potential applications in nanoelectronics, nanomedicine, supercapacitors and nanosensors due to their exceptional electronic, thermal, mechanical and optical properties.14,15 Current progress shows that free-standing graphene oxide (GO) paper can be formed by flow-directed assembly of individual graphene oxide sheets, since a stable suspension of GO can be obtained with the assistance of ultrasonic treatment.16,17 Although the GO paper exhibits excellent flexibility and high mechanical strength, it is not suitable for water filtration due to its poor permeability to fluids.16 One possible way of harnessing the advantages of GO for water filtration is to graft GO sheets onto some suitable inorganic nanofibers, which can be further fabricated into a nanofibrous membrane and applied for water purification. Since the linkage between GO and inorganic nanomaterials can be easily destroyed in a basic environment,18 it is essential to use sulfonic acid functionalized GO with strong nucleophilic capabilities to replace GO.
Here we present a facile method to fabricate a novel type of inorganic nanofibrous membrane without calcination. GO sheets were firstly synthesized by Hummers’ method.19 Subsequently, sulfonated GO sheets were prepared using sodium 2-chloroethanesulfonate hydrate under ultrasonic conditions, and SO3H groups were formed on the GO sheet in this process. Then, the sulfonated GO sheets were grafted onto K-OMS-2 nanofibers to form a hierarchical structure (Fig. S1†). The GO-SO3H sheets have a strong affinity for the K-OMS-2 nanowires due to the coordination action between the sulfonic acid group and carbonic acid groups of GO-SO3H and the Mn center of the K-OMS-2 nanowire. Finally, the GO grafted K-OMS-2 nanowires were fabricated into a nanofibrous membrane through flow-directed assembly by filtration. Importantly, the presence of functionalized GO can act as a cross linker to assist the interweaving of inorganic nanofibers, resulting in the reduction of membrane pore sizes and the enhancement of the membrane rejection rate. It is also worth noting that the superhydrophilic properties of the K-OMS-2 nanowires and GO-SO3H would enhance the permeability of the synthesized nanofibrous membrane.6
The morphology of the synthesized materials was characterized with TEM, and some representative images are summarized in Fig. 1. As shown in Fig. 1A, the synthesized GO sheet is a transparent thin film with diameters of a few micrometers. The wrinkles and folds can be clearly observed, showing the two dimensional structure of the GO sheet. The sulfonated GO sheet is shown in Fig. 1B, which reveals that the GO sheet kept its original morphology after the sulfonation reaction. The GO sheets and GO-SO3H sheets are confirmed as single layer sheets by AFM patterns (Fig. S2 and S3†). In addition, the GO-SO3H sheet is smaller than the GO sheet due to the physical effect of ultrasound, indicating that GO-SO3H sheets have a relatively large contact area with K-OMS-2 nanowires as compared to GO sheets. Thus, the sulfonic acid group has more chance of forming coordinate bonds with the Mn centre of K-OMS-2 nanowires, which would facilitate the grafting of GO-SO3H onto K-OMS-2 nanowires. K-OMS-2 nanowires were prepared via a hydrothermal method.6 In a typical process, Mn2+ was oxidized by S2O82− under constant pressure and temperature for 4 days. As shown in Fig. 1C, the K-OMS-2 nanowire is about 100 nm in diameter. HRTEM (inset of Fig. 1C) reveals that the d-spacing of 0.48 nm corresponds to {002} planes of monoclinic K2-xMn8O16, which further confirms that the prepared nanowire is K-OMS-2. Fig. 1D shows that the GO-SO3H sheets were successfully grafted onto the K-OMS-2 nanowire, constructing a hierarchical heterojunction. Owing to the affinity of the sulfonate acid group toward the K-OMS-2 nanowire, GO-SO3H can be used as a cross linker to combine the K-OMS-2 nanowires more tightly, which facilitates the fabrication of a nanofibrous membrane with good selectivity. In our previous work,18 inorganic nanomaterials could be detached from GO sheets by the addition of NaOH solution. As shown in Fig. S4A,† the GO sheets cannot be grafted onto K-OMS-2 nanowires at a pH of less than 11. However, the strong nucleation capability of the sulfonic acid group can ensure the stability of the K-OMS-2/GO-SO3H composite in basic conditions (Fig. S4B†), which can extend its applications in water purification.
 | ||
| Fig. 1 TEM images of the synthesized (A) GO, (B) GO-SO3H, (C) K-OMS-2 nanowire, and (D) the hierarchical K-OMS-2/GO-SO3H heterojunction. | ||
XRD analysis of the synthesized materials is shown in Fig. 2A. The diffraction patterns of GO and GO-SO3H show the {001} peak of graphite oxide centered at 2θ = 11.9° and 10.5°, corresponding to interlayer spacings of 7.43 Å and 7.58 Å, respectively.20 It can be seen that the interlayer spacing of GO-SO3H is slightly larger than that of the original GO, due to the introduction of the ethane sulfonic acid group. Moreover, the XRD pattern of GO-SO3H shows a weak broader peak from the graphitic {002} diffraction plane centered at 2θ = 21.95°, which results from the disordered stacking of functionalized graphene sheets.20 This is caused by the decrease of oxygen containing groups under basic conditions and the transformation from epoxy group to ether group during the functionalization process.21,22 The XRD pattern of K-OMS-2/GO-SO3H shows clear diffraction peaks from the K-OMS-2 crystalline phase (JCPDS 44-1386), which are very similar to the K-OMS-2 nanowires. No marked reflections from the {001} diffraction plane of GO-SO3H are observed because the regular stacking of GO-SO3H is destroyed by the intercalation of K-OMS-2 nanowires.18,20
 | ||
| Fig. 2 (A) XRD spectra and (B) FTIR spectra of the synthesized (a) GO, (b) GO-SO3H, (c) K-OMS-2, (d) K-OMS-2/GO-SO3H. Inset: FTIR spectra of GO-SO3H ranging from 1500 cm−1 to 1000 cm−1. | ||
Fig. 2B shows the FTIR spectra of GO, GO-SO3H, K-OMS-2 and K-OMS-2/GO-SO3H. The FTIR spectrum of GO indicates that the broad band ranging from 3600 cm−1 to 3000 cm−1 and the band near 1631 cm−1 can be assigned to the H–O–H stretching vibrations of adsorbed water molecules.23 The bands at 1730 cm−1 and 1039 cm−1 are related to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O stretching vibrations of COOH groups,24 indicating the graphite was oxidized into hydrophilic GO with hydroxyl and carboxyl groups. For the spectrum of GO-SO3H, the weak band centered at 1265 cm−1 and a weak shoulder band centered at 1162 cm−1 (inset of Fig. 2B) are respectively attributed to the C–O–C bond stretching vibration and the S
O and C–O stretching vibrations of COOH groups,24 indicating the graphite was oxidized into hydrophilic GO with hydroxyl and carboxyl groups. For the spectrum of GO-SO3H, the weak band centered at 1265 cm−1 and a weak shoulder band centered at 1162 cm−1 (inset of Fig. 2B) are respectively attributed to the C–O–C bond stretching vibration and the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of sulfonic acid,25,26 which reveals that the SO3H group was successfully grafted onto the GO sheet by etherification. For the spectrum of K-OMS-2, the bands centered at 716 cm−1 and 531 cm−1 result from the characteristic vibrations of the O–Mn–O bonding.27 After combining with GO-SO3H, the FTIR spectrum of K-OMS-2/GO-SO3H shows a clear band centered at 1390 cm−1, which can be attributed to the coordination between Mn and the carboxylic group from GO-SO3H.28 However, no clear band connected to the coordination between the SO3H group of GO-SO3H and the Mn center can be distinguished, due to the small number of sulfonic groups in the sample.
O stretching vibrations of sulfonic acid,25,26 which reveals that the SO3H group was successfully grafted onto the GO sheet by etherification. For the spectrum of K-OMS-2, the bands centered at 716 cm−1 and 531 cm−1 result from the characteristic vibrations of the O–Mn–O bonding.27 After combining with GO-SO3H, the FTIR spectrum of K-OMS-2/GO-SO3H shows a clear band centered at 1390 cm−1, which can be attributed to the coordination between Mn and the carboxylic group from GO-SO3H.28 However, no clear band connected to the coordination between the SO3H group of GO-SO3H and the Mn center can be distinguished, due to the small number of sulfonic groups in the sample.
To further identify the SO3H group in the synthesized K-OMS-2/GO-SO3H sample, an XPS measurement was conducted. The survey spectrum of the K-OMS-2/GO-SO3H indicates that the sample contains Mn, C, O, K, and S, as shown in Fig. S5.†Fig. 3A–C show the high resolution XPS spectra of C 1s taken for the different samples, and the Gaussian curve fittings of C 1s were performed to describe the different carbon bonds in the materials. Although both the C–OH groups and the HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups appeared in all three samples, the C–OH groups are considerably decreased for GO-SO3H and K-OMS-2/GO-SO3H, indicating that the C–OH groups were partly replaced by C–O–SO3H groups during the sulfonation reaction. Furthermore, a single S 2p peak at 168 eV in the high resolution XPS spectrum in Fig. 3D confirms the existence of a SO3H group in the K-OMS-2/GO-SO3H composite.29,30Fig. 3E shows the high resolution of Mn 2p, and a Gaussian curve fitting of Mn 2p3/2 displays that the Mn 2p3/2 binding energies were centered at 641.7 eV and 643.0 eV, representing Mn3+ and Mn4+ respectively.31 The strong nucleophilic effect of the SO3H group in GO-SO3H toward Mn atoms can effectively contribute to the combination of K-OMS-2 and GO-SO3H together with coordination between the carboxylic acid groups and Mn atoms.
O groups appeared in all three samples, the C–OH groups are considerably decreased for GO-SO3H and K-OMS-2/GO-SO3H, indicating that the C–OH groups were partly replaced by C–O–SO3H groups during the sulfonation reaction. Furthermore, a single S 2p peak at 168 eV in the high resolution XPS spectrum in Fig. 3D confirms the existence of a SO3H group in the K-OMS-2/GO-SO3H composite.29,30Fig. 3E shows the high resolution of Mn 2p, and a Gaussian curve fitting of Mn 2p3/2 displays that the Mn 2p3/2 binding energies were centered at 641.7 eV and 643.0 eV, representing Mn3+ and Mn4+ respectively.31 The strong nucleophilic effect of the SO3H group in GO-SO3H toward Mn atoms can effectively contribute to the combination of K-OMS-2 and GO-SO3H together with coordination between the carboxylic acid groups and Mn atoms.
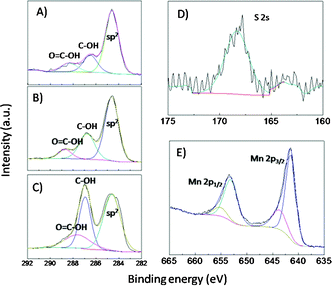 | ||
| Fig. 3 High-resolution C 1s XPS spectra of: (A) K-OMS-2/GO-SO3H (B) GO-SO3H (C) GO; high-resolution XPS spectra of the Mn 2p (D) and S 2p regions (E) taken on the K-OMS-2/GO-SO3H. | ||
An inorganic nanofibrous membrane was fabricated using the synthesized K-OMS-2/GO-SO3H heterojunctions via a filtration process. The digital photo of the synthesized free-standing nanofibrous membrane is shown in Fig. 4A. The 35 mm-diameter membrane was fabricated via a filtration process, and larger diameter membranes could also be produced. The inset of Fig. 4A indicates that the synthesized membrane possesses excellent flexibility, which can be freely shaped by tweezers. Fig. 4B shows the top view FESEM image of the membrane, which reveals a relatively flat topology with no observed cracks. From the high-resolution FESEM image (Fig. 4D), it can be observed that the open porous network was formed by overlapping and interweaving of the ultra long hierarchical K-OMS-2/GO-SO3H heterojunctions, and the membrane pore sizes range from 0.05 μm to 0.2 μm. Compared to the K-OMS-2 nanowire membrane without calcination (Fig. S6†), the membrane in Fig. 4D shows much more compact nanowire bundles, due to the presence of GO-SO3H as a cross linker, which downsizes the membrane pore and thus increases the rejection capacity of the membrane in the water purification process. GO-SO3H sheets can enable the K-OMS-2 nanowires to bind together and thus form a free-standing and flexible membrane, which was further confirmed by the AFM image in Fig. S7.† In addition, a cross-sectional SEM image (Fig. 4C) of the synthesized membrane reveals that the membrane is composed of many layers, and each layer is assembled by the bundles of hierarchical K-OMS-2/GO-SO3H heterojunctions. The tightly interwoven K-OMS-2/GO-SO3H heterojunctions can endow the membrane with a compact functional layer.
 | ||
| Fig. 4 (A) Digital photo of the K-OMS-2/GO-SO3H membrane. (B) Top view FESEM image of the K-OMS-2/GO-SO3H membrane. (C) FESEM image of the cross-section. (D) High-resolution top view FESEM image of the K-OMS-2/GO-SO3H membrane. | ||
To investigate the permeability of the K-OMS-2/GO-SO3H membrane, membrane fluxes of deionized water under different transmembrane pressures (TMP) were studied in a lab-scale dead-end filtration apparatus. As shown in Fig. 5A, the permeate flux of the K-OMS-2/GO-SO3H membrane is highly correlated with TMP (R2 = 0.991) since the only resistance present in the experiments is the intrinsic membrane resistance (Rm). The K-OMS-2 nanowires were tightly combined because of the crosslinking effect of GO-SO3H, and the K-OMS-2/GO-SO3H membrane flux increased proportionately with increasing TMP. However, in the absence of GO-SO3H, the degree of binding of the nanowires varied with increasing TMP, resulting in the poorly fitted regression line (R2 = 0.937) in Fig. 5A. Although the K-OMS-2/GO-SO3H membrane has a lower permeability than the K-OMS-2 membrane due to the presence of the GO-SO3H sheets, the GO-SO3H sheets can enhance the separation efficiency of the nanofibrous membrane. Standard polystyrene (PS) microsphere solutions with different particle sizes were filtered by the synthesized membranes. As shown in Fig. 5B, the synthesized K-OMS-2/GO-SO3H membrane displays a much higher separation efficiency than the K-OMS-2 nanowire membrane, and the retention rates of PS microspheres increase with increasing particle size. Since the pore size of a membrane can be defined as the diameter of latex microspheres which are 90% retained by the membrane,32 the pore size of the K-OMS-2/GO-SO3H membrane can be characterized as being around 0.2 μm, classifying it under the microfiltration membrane category. Fig. 5C shows that the 0.2 μm PS microspheres can be retained and subsequently accumulated to form a cake layer on the surface of the membrane, and it is predictable that pollutants with larger particle sizes (larger than 0.2 μm) would be efficiently removed by the membrane. The synthesized K-OMS-2/GO-SO3H membrane is milking the profits from both the K-OMS-2 nanowires and the GO-SO3H sheets, and consequently possesses excellent permeability and selectivity.
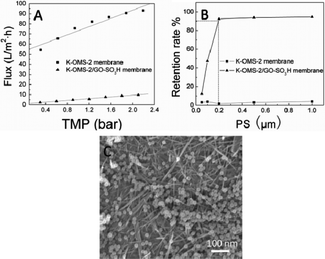 | ||
| Fig. 5 (A) Deionized water permeability of the synthesized membranes. (B) Retention rates of standard PS microspheres with different particle sizes by the synthesized membranes, TMP: 0.3 bar. (C) The upper-surface FESEM image of the K-OMS-2/GO-SO3H membrane after filtration of the 0.2 μm PS microsphere suspension. | ||
In conclusion, we have successfully synthesized a hierarchical K-OMS-2/GO-SO3H heterojunction, and a free-standing, flexible nanofibrous membrane was further fabricated using a filtration method. It is important to note that GO-SO3H can act as a cross linker to combine the K-OMS-2 nanowires, which enhances the rejection capacity of the membrane in the filtration process. Although the effect of GO-SO3H sheets on the membrane performance needs to be further investigated, there is no doubt that the synthesized membrane possesses good permeability and selectivity in the water purification process. As a microfiltration membrane, it exhibited excellent rejection capacity on pollutants with particle sizes larger than 0.2 μm. We believe that the synthesized membrane can have great potential in membrane applications. Moreover, this work provides a novel methodology towards the fabrication of nanofibrous membranes using other inorganic nanomaterials with one dimensional structures.
Acknowledgements
We are grateful for the financial support received from the Prime Minister's Office of Singapore via an initiative called The Enterprise Challenge under award number P00579/1273, Singapore Environment & Water Industry (EWI) Development Council under award number MEWR 621/06/166 and the Public Utilities Board of Singapore.References
- D. S. Sholl and J. K. Johnson, Science, 2006, 312, 1003–1004 CrossRef CAS.
- K. Tan and S. K. Obendorf, J. Membr. Sci., 2007, 305, 287–298 CrossRef CAS.
- D. Yang, X. Niu, Y. Liu, Y. Wang, X. Gu, L. Song, R. Zhao, L. Ma, Y. Shao and X. Jiang, Adv. Mater., 2008, 20, 4770–4775 CrossRef CAS.
- R. Takemori and H. Kawakami, J. Power Sources, 2010, 195, 5957–5961 CrossRef CAS.
- H. W. Liang, X. Cao, W. J. Zhang, H. T. Lin, F. Zhou, L. F. Chen and S. H. Yu, Adv. Funct. Mater., 2011, 21, 3851–3858 CrossRef CAS.
- J. Yuan, X. Liu, O. Akbulut, J. Hu, S. L. Suib, J. Kong and F. Stellacci, Nat. Nanotechnol., 2008, 3, 332–336 CrossRef CAS.
- X. Zhang, T. Zhang, J. Ng and D. D. Sun, Adv. Funct. Mater., 2009, 19, 3731–3736 CrossRef CAS.
- Q. Xu and M. A. Anderson, J. Mater. Res., 1991, 6, 1073–1081 CrossRef CAS.
- T. Zhang, Y. Wang, J. Ng and D. D. Sun, RSC Adv., 2012, 2, 3638 RSC.
- W. Jia, Y. Wang, J. Basu, T. Strout, C. B. Carter, A. Gokirmak and Y. Lei, J. Phys. Chem. C, 2009, 113, 19525–19530 CAS.
- P. Kohli, C. C. Harrell, Z. Cao, R. Gasparac, W. Tan and C. R. Martin, Science, 2004, 305, 984–986 CrossRef CAS.
- R. Mallada and M. Menéndez, Inorganic membranes: synthesis, characterization and applications, Elsevier, Amsterdam, 2008 Search PubMed.
- X. B. Ke, H. Y. Zhu, X. P. Gao, J. W. Liu and Z. F. Zheng, Adv. Mater., 2007, 19, 785–790 CrossRef CAS.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS.
- C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2009, 48, 7752–7777 CrossRef CAS.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. B. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457–460 CrossRef CAS.
- C. Chen, Q. H. Yang, Y. Yang, W. Lv, Y. Wen, P. X. Hou, M. Wang and H. M. Cheng, Adv. Mater., 2009, 21, 3007–3011 CrossRef CAS.
- J. Liu, H. Bai, Y. Wang, Z. Liu, X. Zhang and D. D. Sun, Adv. Funct. Mater., 2010, 20, 4175–4181 CrossRef CAS.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- P. Liu, K. Gong, P. Xiao and M. Xiao, J. Mater. Chem., 2000, 10, 933–935 RSC.
- X. Fan, W. Peng, Y. Li, X. Li, S. Wang, G. Zhang and F. Zhang, Adv. Mater., 2008, 20, 4490–4493 CrossRef CAS.
- J. Liu, Y. Wang, S. Xu and D. D. Sun, Mater. Lett., 2010, 64, 2236–2239 CrossRef CAS.
- T. Szabóa, O. Berkesic and I. Dékánya, Carbon, 2005, 43, 3186–3189 CrossRef.
- G. I. Titelman, V. Gelman, S. Bron, R. L. Khalfin, Y. Cohen and H. Bianco-Peled, Carbon, 2005, 43, 641–649 CrossRef CAS.
- K. Krishnamoorthy, R. Mohan and S. J. Kim, Appl. Phys. Lett., 2011, 98, 244101 CrossRef.
- H. M. Li, J. C. Liu, F. M. Zhu and S. A. Lin, Polym. Int., 2001, 50, 421–428 CrossRef CAS.
- L. Shi, N. Li and C. Wang, J. Hazard. Mater., 2010, 178, 1137–1140 CrossRef CAS.
- S. J. Parikh and J. Chorover, Geomicrobiol. J., 2005, 22, 207–218 CrossRef CAS.
- M. Okamura, A. Takagaki, M. Toda, J. N. Kondo, K. Domen, T. Tatsumi, M. Hara and S. Hayashi, Chem. Mater., 2006, 18, 3039–3045 CrossRef CAS.
- Y. Wu, Z. Fu, D. Yin, Q. Xu, F. Liu, C. Lu and L. Mao, Green Chem., 2010, 12, 696–700 RSC.
- T. Zhang, X. Zhang, J. Ng, H. Yang, J. Liu and D. D. Sun, Chem. Commun., 2011, 47, 1890–1892 RSC.
- S. Nakao, J. Membr. Sci., 1994, 96, 131–165 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: details of experimental information available. See DOI: 10.1039/c2ra20300f/ |
| This journal is © The Royal Society of Chemistry 2012 |
