Rhodopseudomonas palustris purple bacteria fed Arthrospira maxima cyanobacteria: demonstration of application in microbial fuel cells
Alister E.
Inglesby
,
David A.
Beatty
and
Adrian C.
Fisher
*
Department of Chemical Engineering and Biotechnology, University of Cambridge, Pembroke Street, Cambridge, CB2 3RA, UK. E-mail: acf42@cam.ac.uk
First published on 9th March 2012
Abstract
Micro MFC devices support parallel, low cost and reproducible analysis for high-throughput screening and sensitivity analyses of biological and electrochemical performance parameters respectively. In this study a micro MFC (μMFC) device was developed, fabricated and operated to enable screening of the purple non-sulphur bacterium Rhodopseudomonas palustris (R. palustris, NCIMB1174 culture collection) using acetate and two renewable substrates, Arthrospira maxima (A. maxima) and glycerol. The use of A. maxima as a carbon source for the metabolism and growth of R. palustris as well as a substrate to generate power within an MFC provided positive results. R. palustris, with its wide range of metabolic modes, directly consumed solid substrate A. maxima with a biomass concentration increase of 0.041 ± 0.004 g DW/L over the growth cycle. Along with the batch phase flat plate μMFC (μMFC) a fed batch flat plate MFC (FBFP-MFC) and a continuous flat plate MFC (CFP-MFC) were used to evaluate the exoelectrogenic activity of R. palustris during this investigation. The μMFC system showed that power generation was independent of R. palustris growth phase and concentration. Further studies using flat plate-MFC systems concluded that current and power generation were not limited by substrate concentration or anolyte conductivity. Moreover, the use of A. maxima as a MFC feedstock resulted in the highest volumetric power among the three substrates investigated (10.4 mW m−3). The potential applications for cultivating R. palustris purple bacteria using A. maxima cyanobacteria as the primary carbon source are of great interest and offer a new approach to future energy systems.
Introduction
MFCs are bioelectrochemical systems (BES) that use bacteria and their associated bio-catalytic reactions for electric power generation.1,2 MFC technology has received much attention within the bioenergy field due to its potential for powering diverse technologies from wastewater treatment to autonomous sensors for long-term operations in low accessibility regions.3Micro engineered MFCs offer a significant number of advantages over macro-scale MFC's when power production is not the primary focus. These advantages have been highlighted via several research groups.4 Crittenden et al. (2006), although acquiring only very low current densities, demonstrated the capability of μMFCs to rapidly analyse the electrochemical performance of different electrodes. In this study the device began to generate electricity within minutes of the inoculation instead of hours or days as experienced in macro-scale MFC investigations. Moreover, in addition to the advantage of a rapid response, μMFC devices are also useful to for high throughput screening.3 Tiny sample sizes used and less material required for MFC construction enables researchers to generate results cheaply and in parallel, resulting in higher data outputs relative to similar investigations done with macro-scale devices.5 There are however, several challenges which researchers face when using μMFCs. Results during experimentation may be distorted due to the evaporation of tiny quantities of electrolytes.6 Moreover, relatively low volumetric power densities and Coulombic efficiencies often still limit μMFCs. It has been suggested that this arises from the high internal resistance of the systems.7
R. palustris is a purple non-sulphur bacterium that has the ability to change between four different modes of metabolism: photoautotrophic, photoheterotrophic, chemoautotrophic, chemoheterotrophic. It is normally a photoheterotroph, and thus is able to use light for energy but doesn't use carbon dioxide as the sole carbon source.8R. palustris's metabolic versatility has raised interest among researchers but little investigation has been made into its application and performance in MFC technology. Thus far only two investigations, by Xing et al. (2008) and Morishima et al. (2007), have been done to assess the exoelectrogenic activity of R. palustris.8,9 Xing et al. (2008) investigated electricity generation with the use of R. palustris DX-1 and wild type ATCC17001 (equivalent to NCIMB11774). Xing and colleagues stated that electricity generation was due to direct electron transfer. Morishima et al. (2007) investigated three strains of R. palustris; one wild type and two isolates, nifHD1d0941 and hupSL1d1518, which were derived to suppress and encourage the production of hydrogen respectively. The results from these investigations are presented in Table 1. Both studies loaded the anode chamber with a 20 mM carbon source, without external mediators and used a flouro-based membrane to separate the anode from an air cathode. It is noted that the DX-1 isolate produced by Xing and colleagues generated more than ten fold the power density than that of Morishima et al. (2007).
Exoelectrogenic bacteria are able to draw electric current from a wide range of soluble or dissolved complex organic wastes and renewable biomasses. This substrate flexibility makes MFC technology an attractive form of bioenergy. A number of different substrates have been explored as a feed for MFCs. Substrates, which have received major attention, include various types of artificial and real wastewaters and lignocellulosic biomass. Substrates are important as they provide the microbial community with an energy, nutrient or carbon source for metabolic reactions.10 Within an axenic culture the microbial communities affinity to consume a certain substrate will directly influence MFC electrochemical performance.11 In this study three substrates were investigated and are described below.
Acetate, C2H3O2
Acetate is a simple carbon source and is has been used in a number of studies to stimulate exoelectrogenic bacteria.12 Acetate is also the end product of metabolic pathways for several complex carbon sources and therefore is naturally present in degrading heavy carbon sources (wastewater) in significant quantities.13 Acetate has been used as a benchmark substrate for power densities in most MFC studies to date.10 It has therefore been used in this study as a basis for comparing the performance of two renewable substrates.Glycerol, C3H5(OH)3
Glycerol is a by-product of biodiesel production and an increasingly important renewable and clean fuel source. Demand for biodiesel is growing rapidly as it is considered to be the best substitution for diesel and can be used in any modern compression ignition engine.14 Glycerol is therefore produced in significant quantities as a result of increasing biodiesel production. For every 3 moles of acylesters (biodiesel) produced, 1 mole of glycerol is produced. Several researchers have investigated using pure and industrially produced glycerol in MFC technology.15,16 Clauwaert et al. (2008) demonstrated that electricity could be generated from pure and crude glycerol using a mixed consortia and achieved a competitive power output of 23 W m−3 at a current of 111 A m−3. Therefore this high-energy waste (19 MJ kg−1) presents an attractive substrate for MFC technology due to its abundance and power output potential.10Arthrospira maxima, C3.5H6.7O1.8N0.6S0.01
A. maxima is a free-floating filamentous cyanobacteria characterized by cylindrical, multicellular trichomes. These cyanobacteria are often referred to as algae because they are aquatic organisms capable of photosynthesis. They are in fact not related to the various types eukaryotic algae and, unlike eukaryotic algae, have a high-protein digestible cell wall.17 The biomass is mainly composed of carbohydrates (15–50%), protein (72–27%) and lipids (50–13%).18A. maxima presents an attractive substrate for MFC technology due to its high productivity and ease of harvesting. Moreover its nitrogen containing composition and soft digestible cell walls present great potential for its use as a substrate for exoelectrogenic organisms. No investigations have been done to test the exoelectrogenic activity or growth of R. palustris using A. maxima as the primary carbon source.In this paper there were five major objectives: (1) the performance of R. palustris in terms of growth using three different carbon sources acetate, glycerol and A. maxima was evaluated; (2) the development of a cost effective and reliable micro MFC device for screening of biological and electrochemical parameters influencing power production was compiled and presented; (3) the effect of the growth phase and culture concentration of R. palustris on current and power generation was determined; (4) electrochemical and biological performance parameters, which may have hindered power generation within the MFC systems were analysed and finally (5) the perspectives and opportunities for growth of R. palustris with A. maxima cyanobacteria as the primary carbon source was discussed.
Experimental
Cultures
| Chemical components | Concentration (g L−1) |
|---|---|
| a Recipe adapted from literature.19–21 | |
| Yeast extract | 0.200 |
| KH2PO4 | 1.700 |
| K2HPO4 | 1.700 |
| Na2S2O3 | 0.158 |
| PABA | 0.002 |
| MgSO4·7H20 | 0.200 |
| CaCl2·2H2O | 0.050 |
| NaCl | 0.400 |
| Iron citrate | 0.005 |
| Vitamins | Addition (1 L L−1 of each) |
| B1 | 0.12 g/100 ml |
| B12 | 0.001 g/100 ml |
| Trace element solution | Concentration (mg L−1) |
| ZnCl2 | 70.000 |
| MnCl2·4H20 | 100.000 |
| H3BO3 | 60.000 |
| CoCl2·6H20 | 200.000 |
| CuCl2·2H20 | 20.000 |
| NiCl2·6H20 | 20.000 |
| NaMoO4·2H20 | 40.000 |
Gosse et al. (2007), Chen et al. (2007) and Chen et al. (2008) all include acetate as the carbon source. Therefore the quantity of carbon within a mole of acetate was calculated and used together with the recommended concentration (20 mmol L−1, acetate) to determine the quantity of glycerol and A. maxima to be added to each respective media (Table 3).
| Carbon source | Empirical formula | Concentration (mmol L−1) | Concentration (g L−1) |
|---|---|---|---|
| Sodium acetate | C2H3NaO2 | 20.00 | 1.64 |
| Glycerol | C3H5(OH)3 | 13.33 | 1.23 |
| A. maxima | C3.5H6.7O1.8N0.6S0.01 | 11.43 | 0.99 |
Every day 800 μL samples were withdrawn from the growth bottles. Each sample was withdrawn in a Nuaire Biological Safety Cabinet to protect the integrity of the axenic culture. Samples underwent a number of analytical tests to recover all necessary growth data.
R. palustris micro MFC
 | ||
| Fig. 1 Schematic diagram of μMFC (not to scale). | ||
R. palustris flat plate microbial fuel cell (FP-MFC)
The μMFC was scaled up to perform tests that could not otherwise be done due to sample size limitations. Monitoring of the COD, VFAs and pH provided this study with further insight as to the metabolism adopted by the versatile R. palustris bacteria under the conditions maintained during investigation. Moreover, the requirement or non-requirement of R. palustris to acclimatise within an electrolytic environment was tested. A non-acclimatised culture was inoculated, grown and maintained in the FP-MFCs during the period of each run. | ||
| Fig. 2 Schematic diagram FP-MFC (not to scale). | ||
Fed batch mode operation (FBFP-MFC). 20 mL of inoculum and potassium ferricyanide (50 mM) was loaded into the anode and cathode chambers of the FP-MFC respectively. Samples were loaded with the use of a 1 mm hypodermic needle, 20 mL syringe and silicone tubing. Each FP-MFC was placed above an 8 W fluorescent light. The anode and cathode were connected to an external resistance. A Picolog AD24 (16 channel) data logger was connected in parallel to the external resistor. A PC and Picolog AD24 software allowed the voltage to be recorded for a given resistance. The anode was connected such that it acted as the working electrode whilst the cathode acted as the counter electrode.
Preliminary runs were conducted using two FBFP-MFCs to quantify the statistical error of the equipment in the results obtained. The anodic chamber of each FBFP-MFC was loaded with R. palustris in an acetate medium. Thereafter three FBFP-MFCs were used during the investigation with each anode chamber being inoculated with one of the given carbon sources. During each run each FBFP-MFC was fed at least twice concluding a minimum of three substrate consumption cycles. Three cycles provide enough data to evaluate the reproducibility of the working system.
Continuous mode operation (CFP-MFC). The FP-MFC was continuously fed with substrate at 1.4 × 10−2 mL min−1 (20 mL day−1) using an LKB Microperpex® peristaltic pump, which resulted in a hydraulic retention time (HRT) of 24 h. Flow was directed into and out of the anodic chamber using 1 mm (ID) silicone tubing. The feed and effluent port was sealed using a silicone sealant. The feed and effluent streams were introduced and extracted from the bottom and top of the anodic chamber respectively to maximise substrate mixing and consumption. The anode and cathode were connected directly to a potentiostat, which maintained peak power operation of the units.
Analytical techniques
All chemical oxygen demand (COD: a measure of the total quantity of oxygen required to oxidise all organic material into carbon dioxide and water) measurements were carried out using the AQUANAL reagent test protocol and a Thermo Scientific 8000 (Thermo Scientific, Colorado, USA) spectrophotometer. Volatile fatty acid (VFA: short chain fatty acids created through metabolism of R. palustris) concentration was determined using HPLC on a Hewlett Packard 1045 system equipped with a Hamilton PRP-X300 Organic Acid and Alcohol column (7 μm 4.1 × 250 mm) and a UV (210 nm wavelength) detector. The system was run isocratically using a mobile phase of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 10 mM potassium monobasic: tert-butanol at a flow rate of 1 mL min−1. The pressure in the column did not exceed 2000 psi. Sample injection volumes of 100 μL were used. The dry weight biomass concentration of R. palustris grown in acetate and glycerol media was determined by testing the optical density (OD) at 660 nm with a Thermo Scientific spectrophotometer. The determined OD was converted to a dry mass concentration (g DW/L) using a calibration curve. All samples were diluted, when necessary, to ensure that they did not exceed an optical density of 1. Final concentrations were then re-adjusted using the original dilution factor. Conductivity and pH were measured using the standard methods.
20 10 mM potassium monobasic: tert-butanol at a flow rate of 1 mL min−1. The pressure in the column did not exceed 2000 psi. Sample injection volumes of 100 μL were used. The dry weight biomass concentration of R. palustris grown in acetate and glycerol media was determined by testing the optical density (OD) at 660 nm with a Thermo Scientific spectrophotometer. The determined OD was converted to a dry mass concentration (g DW/L) using a calibration curve. All samples were diluted, when necessary, to ensure that they did not exceed an optical density of 1. Final concentrations were then re-adjusted using the original dilution factor. Conductivity and pH were measured using the standard methods.
Electrochemical methods
Fed batch mode operation. Polarisation curves were produced by variation of the external resistance within a range from the maximum of 1 MΩ to a minimum of 100 kΩ. The voltage output was recorded using a Picolog AD24 (16 channel) data logger. A stable output voltage was concluded prior to an external load step change. Polarisation curves enabled the optimum external resistance needed to acquire the maximum power output to be determined. FP-MFCs were subsequently operated at this optimum external load (1 MΩ) to analyse peak power output as a function of substrate consumption.
Continuous mode operation. The peak power was determined with the use of an algorithm-controlled potentiostat. The algorithm employed a ‘perturb and observe’ (PO) model to control the applied voltage. The average power density output at an applied voltage, between the OCP of the device and 0 volts, was measured and compared to the power density output obtained at a previous applied voltage. These consecutive readings were evaluated and used to predict a new voltage to apply that would yield a higher power density value. The potentiostat applied the new voltage and the average power density output obtained was used to predict another voltage to apply. The algorithm compensated for the possibility of an underlying, “long term” first order change in power output. The algorithm continued iterating in this fashion and hence an approximation of the peak power density over time was generated.
Results and discussion
R. palustris growth on the proposed renewable substrates
The growth of R. palustris using acetate and glycerol, loaded in each case with a carbon concentration of 40 mM, was recorded (Fig. 3a). Three runs were conducted to determine the experimental error and reproducibility of the results obtained. The doubling time of R. palustris with a starting concentration of 0.006 g DW/L was 1.2 ± 0.2 and 0.9 ± 0.1 days for acetate and glycerol respectively. Each achieved a maximum growth rate of 9.8 ± 4.2 and 6.9 ± 0.1 mg DW/day (Fig. 3a). These generation (doubling) times are comparable with Oh et al. (2002) who anaerobically cultivated R. palustris using a 50 mM acetate medium at 30 °C under white light at 3.6 W m−2 (555 nm).22 Oh and colleagues only achieved a doubling time of approximately 2 days (maximum growth rate: 4.5 mg DW/day). Harwood and Gibson (1987) tested the anaerobic growth of R. palustris in several aromatic carbon sources and reported a maximum doubling time of 10 h using 10 mM cinnamate at 30 °C in natural light.23 Acetate exhibited significant variability at the point and rate at which growth shifted from the linear growth phase into the stationery phase. Although the glycerol substrate induced a more rapid doubling time of the axenic culture, during the three runs R. palustris consuming acetate achieved a higher final dry weight concentration (0.018 ± 0.001 g DW/L) than R. palustris in glycerol (0.017 ± 0.001 g DW/L). This indicates that R. palustris was able to metabolise acetate more effectively for growth than glycerol (Fig. 3a). | ||
| Fig. 3 (a) R. palustris growth curve in acetate and glycerol media (left). The initial substrate concentration of glycerol and acetate was 1.23 and 1.64 g L−1 respectively. (b) R. palustris growth in A. maxima media (right). Growth estimated using solid and soluble COD. Initial substrate concentration of A. maxima was 0.99 g L−1. All growth bottles were operated at 30 °C with an irradiance 0.4 W cm−2. | ||
The growth of R. palustris on A. maxima could not be recorded using optical density methods. A. maxima contains green chlorophyll and blue phycocyanin and moreover was introduced as a substrate in solid phase making OD measurements infeasible. Solid and soluble COD and VFA analyses enabled an estimate of R. palustris's growth in an A. maxima media to be made (Fig. 3b). The solid COD composed of both R. palustris and A. maxima, whilst soluble COD indicated the quantity of substrate that had solubilised. During the first day a large proportion of the A. maxima substrate was solubilised, increasing and decreasing the soluble and solid COD respectively (Fig. 3b). Therefore, growth during this phase could not be determined. Subsequently the solid COD increased between day 2 and 4 from 460 ± 30 to 610 ± 70 mg COD/L. Although R. palustris continued to consume the solid substrate, lowering solid COD, biomass growth added to the solid COD resulting in a net increase between day 2 and 4. R. palustris thus had a minimum increase in concentration of 140 ± 80 mg COD/L.
The soluble COD and VFAs declined to below detectable levels (< 50 mg L−1) within the 4-day period. This suggested that R. palustris did not hydrolyse the solid substrate but rather consumed it directly, which was consistent with literature that states R. palustris produces a cell surface adhesion, which allows it to stick to solid substrates.24 Sasikala and Ramana (1998) demonstrated R. palustris's ability to biodegrade solid substrates (e.g. starch) but did not contrast growth rates as a function of substrate phase.25 Although the solid COD remained constant after day 4, colour change and OD660 nm measurements confirmed complete consumption of the residual solid substrate and consequent growth of R. palustris. The initial dry weight concentration of the R. palustris inoculum before the addition of A. maxima was 0.006 ± 0.000 g DW/L (OD660 nm: 0.160 ± 0.01). Subsequent to substrate consumption, after all green-blue colour had disappeared (8 days), the dry weight concentration was 0.047 ± 0.004 g DW/L (OD660 nm: 1.199 ± 0.1), which confirmed bacterial growth. No previous studies have investigated R. palustris's affinity for the consumption of a cyanobacteria substrate.
Development of a cost effective and reproducible micro-scale microbial fuel cell for screening R. palustris and renewable substrates
The objective of this study was not to optimise the reactor design and materials of construction for maximum power output of the given system. The μMFC system was developed for high throughput screening of R. palustris's affinity to consume previously untested substrate media and its ability to produce current. Troubleshooting was done in order to construct a system that provided reliable and reproducible results.Single compartment air cathode versus two-chambered ferricyanide cathode system
Initially an air cathode system, using an indium tin oxide (iTO) (Visiontek Systems Ltd) anode and a carbon platinum cathode flat plate micro-device was proposed for high-throughput screening of R. palustris on different media. The consistency of the system was tested, loading three identical μMFCs with R. palustris in fresh acetate. The reproducibility of results was questionable as the relative error of the peak power between cells was ±31% (standard error presented in Fig. 4a). System error was a result of the inconsistencies among carbon platinum cathodes and iTO anodes. | ||
| Fig. 4 (a) μMFC air cathode system and (b) μMFC potassium ferricyanide cathode system error analysis. μMFC systems were operated under ambient conditions with natural light. R. palustris (0.026 ± 0.000 g DW/L) used acetate (1.64 g L−1) as a carbon source. | ||
To improve the consistency of results, the μMFC system was redesigned to include a second chamber for a potassium ferricyanide cathode system. Moreover, a more robust stainless steel electrode replaced the iTO electrode. These modifications also made for a more cost efficient high throughput screening micro device. The relative error of the peak power for the new system, testing three μMFC, loaded with R. palustris in acetate media was reduced to ±15% (standard error presented in Fig. 4b). Since the two-chamber ferricyanide system produced the most reproducible results and eliminated the inconsistent iTO and carbon platinum electrodes, it was deemed the best arrangement to use for further tests in both the μMFC and FP-MFC systems.
Electricity generation from R. palustris in the μMFC
The typical polarisation curve for R. palustris fed with acetate (Fig. 5) indicated that activation losses were insignificant. This result was due to the provision of an adequate electrode material, surface area and the direct electron transport mechanism exploited by R. palustris.9 After peak power was reached the current declined and in many occasions an overshoot-phenomenon was experienced. An overshoot results from electrons being drained from the anolyte due to the system's greater demand for electrons at lower resistances.26
 | ||
| Fig. 5 Typical polarisation and power curve for R. palustris re-suspended in an acetate (1.64 g L−1) media. | ||
The system overload in all cases was only temporary as the bacteria adapted to the dynamic environment, demonstrating their robustness.26 Insignificant concentration polarisation was experienced in the micro MFCs.
The electrogenic activity of R. palustris in a glycerol media, as with acetate, typically only exhibited ohmic polarisation and was therefore a linear function of current. Typically no overshoot was experienced (Fig. 6). Linear polarisation curves are most often encountered using MFCs.1 Ohmic resistance therefore played the dominant role in determining peak power. The gradient of the linear function could therefore be used to approximate the internal resistance of the system, which was 15 ± 4 MΩ.
 | ||
| Fig. 6 Typical polarisation and power curve for R. palustris re-suspended in a glycerol (1.23 g L−1) media. | ||
R. palustris fed with A. maxima substrate similarly experienced the overshoot phenomenon (Fig. 7). Peak power values were obtained at a lower external resistance (7.6–15 MΩ) relative to the soluble carbon sources (15–33 MΩ). The external resistance at peak power can be used to approximate the internal resistance of the system, when polarisation curves are non-linear.1 The peak power experienced at a lower external resistance was a result of both a greater OCP (A. maxima, 390.2 ± 20.4 mV > glycerol, 354.6 ± 23.4 mV; acetate, 331.9 ± 23.0 mV), and sustained current. OCP is representative of the cell electromotive force (emf), omitting internal losses, which are implicated by ohmic polarisation and other current dependent overpotentials. Therefore a higher OCP is indicative of each systems affinity to pump electrons from a point of low potential (anode) to high potential (cathode).27 The sustained current, during consumption of the A. maxima media at lower external resistances, indicated the axenic cultures affinity to donate electrons to the anode at a lower redox potential, relative to the soluble substrate mediums.
 | ||
| Fig. 7 Polarisation and power curve for R. palustris re-suspended in an A. maximum (0.99 g L−1) media. | ||
Xing et al. (2008) generated power using R. palustris (strain DX-1) in 20 mM acetate media, without external mediators and with a flouro-based membrane to separate the anode from an air cathode. Power curves produced were semi-circular, which is indicative of a linear polarisation curve that is dominated by ohmic losses. Although an overshoot phenomenon was experienced in this study, all polarisation curves were otherwise linear, dominated by ohmic losses and in alignment with the work done by Xing and colleagues.
Power generation as a function of R. palustris concentration and growth phase
Wang et al. (2010) showed that the growth curve phase with respect to culturing time of Escherichia coli influenced electricity performance.28 The study concluded that the best time to inoculate the MFC was in middle of the logarithmic phase and during the phase transition from logarithmic to stationery phase. The current drawn via electron transport from R. palustris to the stainless steel electrode, in this study, remained relatively constant irrespective of R. palustris concentration or growth phase indicating that these factors were not limiting current production (Fig. 8a). A. maxima drew the highest average current (24 ± 4.7 mA m−3 anode or 0.10 ± 0.02 mA m−2 anode), glycerol second (19 ± 5.5 mA m−3 anode or 0.08 ± 0.02 mA m−2 anode) and acetate the lowest (9.0 ± 1.8 mA m−3 anode or 0.04 ± 0.01 mA m−2 anode). R. palustris growing in A. maxima media, however, experienced an increase in volumetric current on day 1, which remained consistent thereafter. The initial low current experienced seemed to be a result of the time required by R. palustris to synthesise cellular machineries for solid substrate metabolism. The current drawn from the acetate and glycerol media remained constant during the 4-day growth period (Fig. 8a).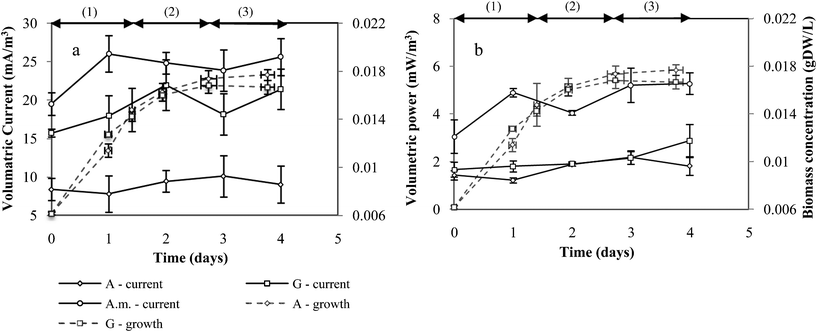 | ||
| Fig. 8 (a) Volumetric current at peak power (left) and (b) volumetric peak power (right) as a function of R. palustris growth and growth phase. μMFCs were run under ambient conditions with natural light over the period of a day. The current at peak power for R. palustris in A. maxima (A.m.), acetate (A) and glycerol (G) media was determined at an external resistance of 7.6–15, 15–33, 15–33 MΩ respectively. A 50 mM potassium ferricyanide catholyte was used. Growth phase: (1) linear growth phase; (2) linear-stationery phase transition; (3) stationery growth phase. | ||
Similarly, the power density produced by R. palustris in A. maxima media increased between day 1 and 2 (Fig. 8b). R. palustris in A. maxima outperformed glycerol and acetate in terms of power generation. The OCP for the A. maxima system was higher than glycerol and acetate, which concluded average OCPs within the same band of error (354.6 ± 23.4 and 331.9 ± 23.0 mV respectively). Moreover, A. maxima is a complex organic, offering R. palustris a multitude of pathways for metabolism, and consequently electron transfer to the anode.24 This was validated through a VFA analysis of the solubilised organics, which consisted predominantly of acetic acid, propionic acid and valeric acid. The results suggest that these conditions encouraged a higher power output.
The power outputs of R. palustris in acetate and glycerol were equivalent (Fig. 8b). Xing et al. (2008), however, tested R. palustris (strain DX-1) on different substrates in a 50 mM phosphate buffer solution using a 1 kΩ resistor and concluded that R. palustris on acetate media generated approximately 2 times more power (460 mW m−2) than when in glycerol media (220 mW m−2). Contradictory results may have stemmed from different strains of R. palustris analysed. Xing and colleagues did test a wild type R. palustris ATCC 1700T, equivalent to that investigated in this study, but reported that no current was generated.
R. palustris in A. maxima, glycerol and acetate within the μMFCs did not generate high maximum power and current outputs (Table 4). This was a result of the μMFCs high internal resistance (external resistance at peak power). High internal resistance resulted from a number of factors. The low ratio of PEM to electrode surface area, low conductivity of the anolyte and reactor design may have contributed to the resistance.
Electricity generation using the flat plate MFC in fed-batch mode (FBFP-MFC)
 | ||
| Fig. 9 Volumetric power and soluble COD profiles of acetate, glycerol and A. maxima FBFP-MFCs across three cycles. | ||
R. palustris fed A. maxima media again consistently generated the highest quantity of power, whilst R. palustris in acetate and glycerol produced equal power outputs, when considering experimental error. The reproducibility of the results produced by the FBFP-MFC system was good (Fig. 9). The system did not appear to be substrate limited after initial loadings as in each case the power output increased to a peak at which point each peak was sustained for at least 12 h before becoming substrate limited. Furthermore the initial loading concentration did not influence the maximum power output reached. Acetate and glycerol's soluble COD concentration increased between cycles, due to incomplete oxidation between runs, but the power output did not increase (Fig. 9).
During the three cycles of power generation, R. palustris in acetate, glycerol and A. maxima produced a maximum volumetric power output, of 5.3, 4.9 and 10.4 mW m−3 respectively (0.15, 0.14, 0.29 mW m−2). The FBFP-MCF system had substrate consumption cycle times of approximately 5 days. Xing et al. (2008) generated higher power outputs and experienced power generation cycle times of approximately 2.5 days.
Continuous phase operation of the flat plate MFC (CFP-MFC)
The CFP-MFC was run using an algorithm-controlled potentiostat to consistently obtain the system's peak power. The CFP-MFC was used to confirm that substrate concentration was not the limiting factor in power generation, as postulated through observations made in the FBFP-MFC system. R. palustris in A. maxima was proven in both the μMFC and FBFP-MFC to be a superior substrate for current and power generation and thus was only considered during this investigation. A. maxima media (0.99 g L−1) was fed continuously into CFP-MFC for 6 days until a steady state current (56 ± 4 mA m−3) and power output (6.4 ± 1.2 mW m−3) was reached. This steady state condition was monitored for 2 days before doubling the A. maxima feed concentration (1.98 g L−1). Doubling the concentration of nutrient in the feed had no effect on current (51 ± 3 mA m−3) and power generation (6.2 ± 0.7 mW m−3) thus confirming that substrate concentration did not limit the FP-MFC system analysed (Fig. 10, zones 1 and 2). However, each time the feed concentration was changed the CFP-MFC system entered a more volatile transition phase (Fig. 10, zone ‘T’) whereby the exoelectrogenic bacteria were acclimatising to the new environment as a function of feed concentration. | ||
| Fig. 10 Steady state peak power for CFP-MFC inoculated with R. palustris in A. maxima media continuously fed at 20 mL day−1. (1) Concentration of A. maxima in feed was 0.99 g L−1. (2) Concentration of A. maxima in feed was 1.98 g L−1. (3) Concentration of A. maxima in feed returned to original 0.99 g L−1. (T) Transition period for system to return to steady state after perturbation of concentration of A. maxima in feed. | ||
Perspectives and opportunities for growth of R. palustris purple bacteria on A. maxima cyanobacteria
In this study, it has been demonstrated that R. palustris bacteria can consume whole cell A. maxima biomass and sustain high levels of growth productivity. This characteristic opens up a wide variety of potential applications; given the number of uses the bacteria has as both a biomass source and product generator.Conclusions
Although wild type R. palustris (NCIMB1174 culture collection) achieved a higher average generation time in aqueous glycerol media (0.9 ± 0.1 days) than in aqueous acetate media (1.2 ± 0.2 days). At the end of the recorded growth period (4 days) the dry weight concentration of R. palustris in acetate media (0.018 ± 0.001 g DW/L) was higher than that in glycerol media (0.017 ± 0.001 g DW/L). Previous work had not yet been done on R. palustris's ability to consume A. maxima as a carbon source. This study confirmed that R. palustris directly metabolises solid substrate A. maxima. This result was verified through solid COD analysis, and through initial and final OD (660 nm) analyses, which confirmed an increased dry weight concentration of R. palustris in an A. maxima media. R. palustris in A. maxima solid substrate media, however required an initial period for acclimatisation for synthesis of cellular machineries for solid substrate metabolism.The μMFCs developed provided reliable results, having a relative error in the peak power of ±15%. The μMFC exhibited acceptable reproducibility for low-cost high-throughput comparative analyses of electrochemical and biological variables, which directly affect power generation within MFC systems. R. palustris produced a constant current and power output within all media types irrespective of the growth phase and culture concentration. This suggested that power production was neither limited by exoelectrogenic bacteria concentration nor different phases of growth. A comparative analysis, using a μMFC and a FP-MFC, of R. palustris's ability to generate power using acetate, glycerol and A. maxima showed that the complex organic, A. maxima, was the superior substrate with the greatest current (μMFC, 27.9 mA m−3 (max); FP-MFC, 23 ± 5 mA m−3) and power output (μMFC, 5.9 mW m−3 (max); FBFP-MFC, 10.4 ± 1.1 mW m−3) due to a higher OCP, diversity of metabolic pathways offered and possibly as a result of a higher nitrogen content.
The results from the fed batch FP-MFCs indicated that MFC performance, loaded with different substrates, had not been limited by substrate concentration. This was confirmed using an identical FP-MFC run in continuous mode. Varying the concentration of A. maxima from 0.99 g L−1 to 1.98 g L−1 did not have any effect on the volumetric power output (Fig. 10).
The ability of R. palustris to consume whole cell A.maxima opens up a wide variety of potential applications and indeed provides for an interesting advance in potential substrates used in bioprocesses.
References
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schröder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Environ. Sci. Technol., 2006, 40, 5181–92 CrossRef CAS.
- M. Rosenbaum, Z. He and L. T. Angenent, Curr. Opin. Biotechnol., 2010, 21, 259–64 CrossRef CAS.
- H. Hou, L. Li, Y. Cho, P. de Figueiredo and A. Han, PLoS One, 2009, 4, e6570 Search PubMed.
- S. R. Crittenden, C. J. Sund and J. J. Sumner, Langmuir, 2006, 22, 9473–6 CrossRef CAS.
- D. F. Call and B. E. Logan, Biosens. Bioelectron., 2011, 26, 4526–31 CrossRef CAS.
- M. Chiao, K. B. Lam and L. Lin, J. Micromech. Microeng., 2006, 16, 2547–2553 CrossRef CAS.
- H.-Y. Wang, A. Bernarda, C.-Y. Huang, D.-J. Lee and J.-S. Chang, Bioresour. Technol., 2011, 102, 235–243 CrossRef CAS.
- K. Morishima, M. Yoshida, A. Furuya, T. Moriuchi, M. Ota and Y. Furukawa, J. Micromech. Microeng., 2007, 17, S274–S279 CrossRef CAS.
- D. Xing, Y. Zuo, S. Cheng, J. M. Regan and B. E. Logan, Environ. Sci. Technol., 2008, 42, 4146–51 CrossRef CAS.
- D. Pant, G. Van Bogaert, L. Diels and K. Vanbroekhoven, Bioresour. Technol., 2010, 101, 1533–43 CrossRef CAS.
- K.-J. Chae, M.-J. Choi, J.-W. Lee, K.-Y. Kim and I. S. Kim, Bioresour. Technol., 2009, 100, 3518–3525 CrossRef CAS.
- D. R. Bond and D. R. Lovley, Appl. Environ. Microbiol., 2003, 69, 1548–1555 CrossRef CAS.
- J. C. Biffinger, J. N. Byrd, B. L. Dudley and B. R. Ringeisen, Biosens. Bioelectron., 2008, 23, 820–826 CrossRef CAS.
- D. Y. C. Leung, X. Wu and M. K. H. Leung, Appl. Energy, 2010, 87, 1083–1095 CrossRef CAS.
- P. Clauwaert, D. Van Der Ha and W. Verstraete, Biotechnol. Lett., 2008, 30, 1947–1951 CrossRef CAS.
- V. R. Nimje, C.-Y. Chen, C.-C. Chen, H.-R. Chen, M.-J. Tseng, J.-S. Jean and Y.-F. Chang, Bioresour. Technol., 2011, 102, 2629–2634 CrossRef CAS.
- O. Ciferri and O. Tiboni, Annu. Rev. Microbiol., 1985, 39, 503–26 CrossRef CAS.
- B. Sialve, N. Bernet and O. Bernard, Biotechnol. Adv., 2009, 27, 409–16 CrossRef CAS.
- J. L. Gosse, B. J. Engel, F. E. Rey, C. S. Harwood, L. E. Scriven and M. C. Flickinger, Biotechnol. Prog., 2007, 23, 124–130 CrossRef CAS.
- C. Chen, W. Lu, J. Wu and J. Chang, Int. J. Hydrogen Energy, 2007, 32, 940–949 CrossRef CAS.
- C. Chen, G. Saratale, C. Lee, P. Chen and J. Chang, Int. J. Hydrogen Energy, 2008, 33, 6886–6895 CrossRef CAS.
- Y. Oh, E.-H. Seol, E. Yeol and S. Park, Int. J. Hydrogen Energy, 2002, 27, 1373–1379 CrossRef CAS.
- C. S. Harwood and J. Gibson, Appl. Environ. Microbiol, 1988, 54, 712–717 CAS.
- F. W. Larimer, L. Hauser, M. L. Land, D. A. Pelletier, P. Chain, J. Lamerdin, S. Malfatti, L. Do, J. T. Beatty, A. S. Lang, F. R. Tabita, J. L. Gibson, T. E. Hanson, C. Bobst, J. L. Torres, Y. Torres, C. Peres, F. H. Harrison, J. Gibson and C. S. Harwood, Nat. Biotechnol., 2004, 22, 55–61 CrossRef CAS.
- C. Sasikala and C. V. Ramana, Adv. Microb. Physiol., 1998, 39, 339–377 CrossRef CAS.
- I. Ieropoulos, J. Winfield and J. Greenman, Bioresour. Technol., 2010, 101, 3520–5 CrossRef CAS.
- M. Villano, F. Aulenta, C. Ciucci, T. Ferri, A. Giuliano and M. Majone, Bioresour. Technol., 2010, 101, 3085–90 CrossRef CAS.
- C.-T. Wang, W.-J. Chen and R.-Y. Huang, Int. J. Hydrogen Energy, 2010, 35, 7217–7223 CrossRef CAS.
- S. Cheng and B. Logan, Electrochem. Commun., 2007, 9, 492–496 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
