Room-temperature synthesis of air-stable cobalt nanoparticles and their highly efficient adsorption ability for Congo red†
Xiaoming
Liang
and
Lijun
Zhao
*
Key Laboratory of Automobile Materials (Jilin University), Ministry of Education and School of Materials Science and Engineering, Jilin University, Changchun, 130022, China. E-mail: lijunzhao@jlu.edu.cn
First published on 17th May 2012
Abstract
A Co2+ ion was reduced by NaBH4 to form cobalt nanoparticles of 10–20 nm in size. The as-obtained Co nanoparticles were stable in air. Congo red was used to evaluate the Co nanoparticles' wastewater treatment capability. It was found that 150 ppm of Congo red could be removed from aqueous solution within 2 min with the Co nanoparticles, and the adsorption maximum is 800 mg g−1.
1. Introduction
In recent years, several wet-chemical methods have been developed to synthesize Co crystals with different morphologies, such as pyrolysis,1,2 solvothermal3 and hydrothermal decomposition,4 microfluidic synthesis,5 modified polyol processes6 and template-based methods.7 Moreover, some efforts have been focused on exploring the relations between their shapes and properties, for example, the catalytic activities of hierarchical nanostructured Co.8 It is likely that there is lack of a very simple and quick method to prepare Co nanoparticles. In addition, it will be significant to develop new application fields besides their magnetic and catalytic properties.Considerable attention has been paid to the environmental problems involving water treatment in recent years. Nowadays, the pollution of water resources by dyes from the textiles and mining industries has become a serious environmental problem. Nanosized magnetic particles are considered to be potential adsorbents for aqueous pollutants due to their high surface areas and the unique advantage of easy separation under external magnetic fields. Several reports have been published on the use of various types of magnetic nanoparticles for removal, separation and determination of dyes.9,10 The above-mentioned magnetic particles are almost all iron-based oxides. Although they show better adsorption capacities, a relatively long adsorption time is usually needed, because the most common form of adsorption by iron-based oxides is physical adsorption. Chemical adsorption will be an effective way to highly efficient adsorption, but there are few reports about chemical adsorption by magnetic materials. Herein, we used Co nanoparticles prepared at room temperature as an adsorbent to highly efficiently adsorb the dye in aqueous solution. For 150 ppm of Congo red (CR) solution, the dye can be completely removed within 2 min by the Co nanoparticles.
In this work, a room-temperature NaBH4 reduction method was used to quickly prepare air-stable Co nanoparticles on a large scale. Because of the magnetism of Co nanoparticles, they can be recovered by a magnet after adsorption. Hence, we attempt to use the as-prepared Co nanoparticles in the field of wastewater treatment, which may play important roles in the future of industrial effluents.
2. Experimental
All chemicals used were analytical grade without further purification. The reagents used for the synthesis were obtained from Beijing Chemicals Co. (Beijing, China). Nanosized cobalt particles were prepared by a very facile reduction method at ambient temperature. 0.2 g of CoCl2·6H2O and 0.1 g of NaBH4 were added to 10 mL of deionized water at the same time. When the hydrogen release stopped, we collected the gray–black powders with a magnet and washed them with deionized water and ethanol several times. The final powders were dried at room temperature in the air for 24 h.3. Results and discussion
The formation of cobalt nanoparticles during the reaction process was noticeable by the dramatic color change of the system after the introduction of the reducing agent. The overall reaction proposed for this process is| 2Co2+ + BH4−(s) + 2H2O→ 2Co(s) + 2H2(g) + 4H+ + BO2− |
To guarantee the synthesis of air-stable Co nanoparticles, controlling the dosage of H2O is an important factor. Because the release of hydrogen gas (H2) can protect the Co particles from being oxidized during the reaction process, less water can bring less oxygen, ensuring that the generating hydrogen can totally exclude the dissolved oxygen from the H2O. The whole reaction process is accompanied by the slowly generating hydrogen until the reaction is over. De-aired water by inert gases can also protect the Co particles from oxidation during the synthetic process, but it will complicate the preparation method and be against the adsorption charges on the surface of the Co nanoparticle. A simple preparation method and surface charges may favor those Co nanoparticles with a better adsorption ability. Hence, the optimum dosage of H2O is small, which can only ensure the formation of a transparent solution.
The phase purity of the products was examined on a Rigaku-D/max 2500 V X-ray diffractometer (XRD) with Cu-Kα radiation (λ = 1.5418 Å). From the XRD pattern of Co nanoparticles (Fig. 1a), an obvious main peak of Co can be observed, but it is difficult to judge the Co phase. The absences of the characteristic diffraction peaks for Co nanoparticles show that the they are poorly crystallized during the process of room-temperature synthesis.
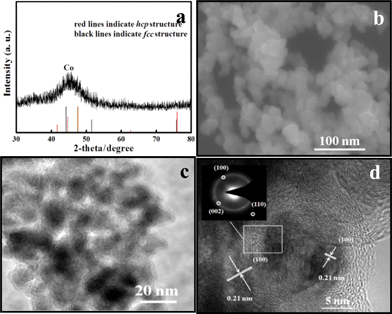 | ||
| Fig. 1 Room-temperature prepared Co nanoparticles (a) XRD figure; (b) SEM image; (c) TEM micrograph and (d) HRTEM photo. The inset in (d) shows a representative SAED pattern. | ||
The morphology and size of the as-synthesized products on the Au-coated silicon substrate were characterized by field-emission scanning electron microscopy (FESEM, JEOL JSM-6700F) and transmission electron microscopy (TEM, Philips Tecnai 20, 200 kV). Fig. 1b and 1c shows that there are regular Co nanoparticles with particle sizes in the range of 10–20 nm. Further insight into the structure of the Co nanoparticles was gained by using high-resolution transmission electron microscopy (HRTEM, JEOL-3010, 300 kV). In Fig. 1d, the lattice interplanar spacing is measured to be 0.21 nm, corresponding to the (100) plane of hexagonal-close-packed (hcp) Co. The corresponding Selected Area Electron Diffraction (SAED) pattern (inset in Fig. 1d) reveals the polycrystalline nature of the Co nanoparticles. By actual measurement of the distance between diffraction circles in the SAED pattern, the (100), (002) and (110) planes could be determined, respectively. It is such an easy method to prepare the Co nanoparticles as only CoCl2·6H2O, H2O and NaBH4 are used during the preparing process. Herein, the NaBH4 serves two important purposes. One is to reduce Co2+ ions to Co0, and the other is to control the shapes of the Co nanoparticles. The different morphologies of Co nanoparticles synthesized with different molar ratios of CoCl2·6H2O and NaBH4 are shown in Fig. S1†.
In the following adsorption experiments, we chose 0.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as the optimum molar ratio of CoCl2·6H2O and NaBH4 to synthesize the Co nanoparticles. In this ratio, the prepared Co nanoparticles possess the best adsorption capacity and efficiency (800 mg g−1), as shown in Fig. 2a. For the other four samples, with the increasing amount of NaBH4, the adsorption capacities are 599, 693, 733 and 396 mg g−1, respectively (Fig. 2a).
1 as the optimum molar ratio of CoCl2·6H2O and NaBH4 to synthesize the Co nanoparticles. In this ratio, the prepared Co nanoparticles possess the best adsorption capacity and efficiency (800 mg g−1), as shown in Fig. 2a. For the other four samples, with the increasing amount of NaBH4, the adsorption capacities are 599, 693, 733 and 396 mg g−1, respectively (Fig. 2a).
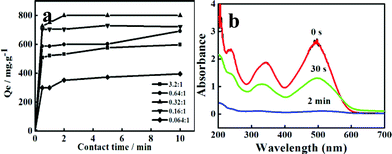 | ||
Fig. 2 (a) Adsorption capacities of Co nanoparticles prepared using different molar ratios of CoCl2·6H2O and NaBH4; (b) Successive UV-visible spectra of CR adsorption in the presence of the sample prepared with a molar ratio of CoCl2·6H2O and NaBH4 0.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 after different time intervals. 1 after different time intervals. | ||
With increasing industrial activities, wastewater from many industries such as printing, dyeing, tanning, chemical manufacturing, mining, battery manufacturing industries, etc. contains dyes. Most of them are harmful; they are not biodegradable and tend to accumulate in living organisms, causing various diseases and disorders. As an example of potential applications, the as-obtained cobalt nanoparticles were used as adsorbent in dye-containing aqueous solution, and the typical pollutant CR was used.
An Agilent Cary 50 UV–vis spectrophotometer was used for the determination of the CR concentration in the solutions. The adsorption of the CR from aqueous solution using the as-prepared cobalt powders was investigated. After the addition of cobalt powders to the CR-containing solution, the color of the solution changed from an initial deep red to colorless within 2 min. Fig. 2a shows the variation of adsorption amount with adsorption time, which proves that 100% removal of CR can be reached after 2 min. From the successive UV-visible spectra of CR adsorption (Fig. 2b), we can directly observe the quick decrease of the CR concentration. When 10 mg of Co particles was added into 50 mL of CR solution (150 mg L−1), over 50% of the CR can be removed within 30 s at room temperature, and 100% of CR can be absorbed after 2 min. The as-prepared cobalt powders exhibited much more favorable adsorptive capacity and a faster adsorption rate than most of materials reported in the literature (Table S2†), and thus can be potentially used to treat large amounts of aqueous solution containing CR within a short time and easily separated from aqueous solution.
The magnetic properties of the obtained products have been measured using a vsm-7300 vibrating sample magnetometer (VSM). The dye-loaded cobalt nanoparticles can be easily separated by using a conventional laboratory magnet bar, as shown in the inset of Fig. 3a. The black particles could be quickly attracted to the wall of the glass bottle and the dispersion became clear, suggesting the as-prepared Co nanoparticles are magnetic and can be potentially used as an easily recoverable adsorbent to remove dyes and other molecules in liquid-phase processes. The magnetic properties of Co nanoparticles and Co nanaparticles loaded with CR were evaluated, as shown in Fig. 3a. For the as-prepared Co nanoparticles, the saturation magnetization (Ms) is 80 emu g−1 and the coercivity (Hc) is 220 Oe. The decreasing Ms and increasing Hc compared to the bulk Co material (Ms = 163.1 emu g−1 and Hc = 10 Oe) may be due to the incomplete crystallization through room-temperature preparation. After adsorption of CR, the Ms is 49 emu g−1 and the Hc is 160 Oe for Co nanoaprticles. Hence, the Co nanoparticles loaded with CR are also easily attracted to the magnetic bar.
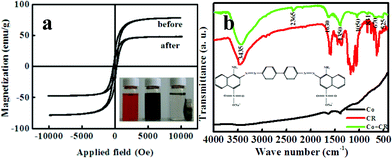 | ||
| Fig. 3 (a) Room-temperature magnetic hysteresis loop of the as-prepared cobalt nanoparticles before and after CR adsorption; (b) FTIR spectra. Inset “a” is the photo of sewage treatment process (CR solution, CR solution with Co nanoparticles, Co loaded with CR seperation by a magnet); Inset “b” is the molecular structure of CR. | ||
We also carried out FT-IR (NEXUS, 670) studies to investigate the adsorption mechanism of the Co nanoparticles before and after CR adsorption, as shown in Fig. 3b. By contrastive analysis of the three FTIR spectra, we are certain that the surfaces of Co powders have been loaded with CR. For the FT-IR spectrum of pure Co (black line), the bond at 1630 cm−1 was undoubtedly due to the O–H bend vibration of the absorption water, but the bond at 1350 cm−1 derives from the existence of the O–H bond of constituent water.11 There are no metal oxide bonds, indicating that the obtained cobalt nanoparticles were not oxidized in the synthesis process and were air-stable. As seen from the Co + CR spectrum (green line), the FTIR spectra of the magnetic powders display weak bonds at 525 to 831 cm−1, which is believed to be associated with the stretching vibrations of Co2+–O2–. Hence, oxidation and reduction reactions are happening during the adsorption process. Furthermore, the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in CR is still observed from the Co + CR spectrum, but it shifts to a lower wave number of 1050 cm−1.12,13 Compared with the FT-IR spectroscopy of CR (red line), the obvious decrease in the aromatic ring absorption peak of the S
O stretching vibration in CR is still observed from the Co + CR spectrum, but it shifts to a lower wave number of 1050 cm−1.12,13 Compared with the FT-IR spectroscopy of CR (red line), the obvious decrease in the aromatic ring absorption peak of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond after treatment indicated the destruction of the CR structure by the Co nanoparticles. Therefore, chemical adsorption is the main adsorption mode between the Co nanoparticles and CR.
O bond after treatment indicated the destruction of the CR structure by the Co nanoparticles. Therefore, chemical adsorption is the main adsorption mode between the Co nanoparticles and CR.
The measured maximum adsorption capacity (qmax) for the adsorption of CR on cobalt nanoparticles synthesized at different temperatures and the qmax values calculated from the Langmuir isotherm model of other adsorbents for CR adsorption are listed in Table S2†. Except for Fe3O4@mesoC nanocapsules (its Ms value is only 5.5 emu g−1 which will be against the separation rate), the Co nanoparticles (Ms = 80 emu g−1) used in this study have the highest qmax value. Compared with other adsorbents, Co nanoparticles have the best adsorption and separation rates. Furthermore, the simplicity of the preparation method, acceptable magnetic properties after adsorption and highly efficient adsorption capacity may make Co nanoparticles a better adsorbent than the others for CR adsorption.
4. Conclusions
In summary, we synthesized air-stable Co nanoparticles (10–20 nm) by a facile NaBH4 reduction method at room temperature. The dosage of NaBH4 has an important influence on the morphologies of the Co nanoparticles. We ascertained the optimum molar ratio of CoCl2·6H2O and NaBH4 for fabricating Co nanoparticles to be 0.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; in this ratio, the Co nanoparticles possess the best adsorption ability. Furthermore, the Ms value of the Co nanoparticles after adsorption for CR is 49 emu g−1, which facilitates the Co with CR to be recovered quickly. For 150 ppm of CR solution, the adsorption can be complete within 2 min and the measured qmax is 800 mg g−1. By contrastive analysis of the FT-IR spectra, chemical adsorption is identified as the main adsorption mechanism.
1; in this ratio, the Co nanoparticles possess the best adsorption ability. Furthermore, the Ms value of the Co nanoparticles after adsorption for CR is 49 emu g−1, which facilitates the Co with CR to be recovered quickly. For 150 ppm of CR solution, the adsorption can be complete within 2 min and the measured qmax is 800 mg g−1. By contrastive analysis of the FT-IR spectra, chemical adsorption is identified as the main adsorption mechanism.
Acknowledgements
The financial supports from the Natural Science Foundation of Jilin Province (20101542) of China and the Youth Research Foundation of Jilin Province (201101060).References
- L. Guo, F. Liang, X. G. Wen, S. H. Yang, L. He, W. Z. Zheng, C. P. Chen and Q. P. Zhong, Adv. Funct. Mater., 2007, 17, 425–430 CrossRef CAS.
- S. H. Liu, H. T. Gao, E. Y. Ye, M. Low, S. Lim, S. Y. Zhang, X. H. Lieu, S. Tripathy, W. Tremel and M. Y. Han, Chem. Commun., 2010, 46, 4749–4751 RSC.
- X. Wang, F. L.Yuan, P. Hu, L. J. Yu and L. Y. Bai, J. Phys. Chem. C, 2008, 112, 8773–8778 CAS.
- F. Cao, R. P. Deng, J. K. Tang, S. Y. Song, Y. Q. Lei and H. J. Zhang, CrystEngComm, 2011, 13, 223–229 RSC.
- Y. J. Song, H. Modrow, L. L. Henry, C. K. Saw, E. E. Doomes, V. Palshin, J. Hormes and C. S. S. R. Kumar, Chem. Mater., 2006, 18, 2817–2827 CrossRef CAS.
- A. Dakhlaoui, L. S. Smiri, G. Babadjian, F. Schoenstein, P. Molinié and N. Jouini, J. Phys. Chem. C, 2008, 112, 14348–14354 CAS.
- D. D. Li, R. S. Thompson, G. Bergmann and J. G. Lu, Adv. Mater., 2008, 20, A1–4 CrossRef.
- Y. B. Cao, X. Zhang, Jun. M. Fan, P. Hu, L. Y. Bai, H. B. Zhang, F. L. Yuan and Y. F. Chen, Cryst. Growth Des., 2011, 11, 472–479 CAS.
- M. Iram, C. Guo, Y. Guan, A. Ishfaq and H. Liu, J. Hazard. Mater., 2010, 181, 1039–1050 CrossRef CAS.
- X. Y. Hou, J. Feng, X. H. Liu, Y. M. Ren, Z. J. Fan and M. L. Zhang, J. Colloid Interface Sci., 2011, 353, 524–529 CrossRef CAS.
- B. Smith, Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, 1999; p p. 98 Search PubMed.
- F. H. Li, Y. Bao, J. Chai, Q. X. Zhang, D. X. Han and L. Niu, Langmuir, 2010, 26, 12314–12320 CrossRef CAS.
- S. D. Deng, X. H. Li and H. Fu, Corros. Sci., 2011, 53, 760–768 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20240a/ |
| This journal is © The Royal Society of Chemistry 2012 |
