Dilute magnetic semiconductor Cu2MnSnS4 nanocrystals with a novel zincblende and wurtzite structure†
Xiaolu
Liang
ab,
Peng
Guo
a,
Gang
Wang
a,
Ruiping
Deng
a,
Daocheng
Pan
*a and
Xianhua
Wei
*b
aState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 5625 Renmin Street, Changchun, 130022, P. R. China
bState Key Laboratory Cultivation Base for Nonmetal Composites and Functional Materials, Southwest University of Science and Technology, Mianyang, 621010, P. R. China. E-mail: pan@ciac.jl.cn; weisansao@yahoo.com.cn
First published on 15th March 2012
Abstract
Dilute magnetic semiconductor Cu2MnSnS4 nanocrystals with a novel zincblende and wurtzite structure have been successfully synthesized and reported for the first time. Cu+, Mn2+, and Sn4+ occupy the same position and have a random distribution in the zincblende and wurtzite unit cells. The nanocrystals exhibit a weak ferromagnetic behavior at low temperature.
During the past two decades, much attention has been focused on dilute magnetic semiconductor (DMS) nanocrystals by doping Mn ions in group II–VI and III–V semiconductors.1 In this context, binary GaN, ZnO, ZnS, ZnSe, CdS, and CdSe are the most commonly used host semiconductors.1 Nonetheless, these Mn2+ doped DMS nanocrystals usually have a relatively low concentration of dopants, generally less than 2 mol%.1 Fries et al. reported that a high Mn2+ concentration is detrimental to the magnetic properties for the binary semiconductors due to the strong anti-ferromagnetic (AFM) interactions between nearest-neighbor Mn ions, and the quaternary DMSs with a high Mn2+ concentration are being considered as material choices for avoiding large AFM exchange interactions.2 Among those quaternary Cu2MSnS4 (M = Fe, Ni, Mn, Co) materials, Cu2MnSnS4 with a stannite structure (space group I-42m, No. 121) has been extensively studied.2,3 Compared with the stannite structure, both the zincblende and wurtzite structures have been proved as novel methods to change the cationic distribution and Mn–Mn distance, which may result in peculiar magnetic properties.4 Note that Cu+, Mn2+, and Sn4+ occupy the same position and have a random distribution in the zincblende and wurtzite unit cells. Furthermore, unlike the Mn doped binary DMSs, there are more than two metal ions to occupy the cation positions in a random arrangement in quaternary Cu2MnSnS4, thus, to some extent, weakening the anti-ferromagnetic interaction between nearest-neighbor Mn ions. Therefore, it is critically important to synthesize Cu2MnSnS4 nanocrystals with a new zincblende and wurtzite structure in order to investigate the relations between the crystal structure and magnetic properties. However, there are no reports on the synthesis of nanocrystalline Cu2MnSnS4 with a zincblende and wurtzite structure in the literature.
It is well known that MnS has a metastable zincblende and wurtzite structure.6 Recently, Cu2SnS3 nanocrystals with a metastable zincblende and wurtzite structure have also been reported by our group.5 Therefore, it is quite possible to obtain the same zincblende and wurtzite structures for Cu2MnSnS4 nanocrystals by changing the synthetic conditions, since the nanocrystalline phase is strongly influenced by the capping agents, reaction temperatures, and the reactivity of precursors.5
Herein, we report on the synthesis of zincblende and wurtzite Cu2MnSnS4 nanocrystals by a hot-injection approach. The crystal structures of the nanocrystals can be controlled by changing the reactivity of sulfur precursors. The sulfur powder and thiourea dissolved in oleylamine are injected in a hot oleylamine solution containing copper, manganese, and tin precursors. The less reactive thiourea yields more metastable wurtzite Cu2MnSnS4 nanocrystals. Detailed synthesis procedures are provided in the ESI†. To the best of our knowledge, this is the first report of the synthesis of zincblende and wurtzite Cu2MnSnS4 nanocrystals.
As can be seen in Fig. 1 (bottom), the zincblende and wurtzite structures of Cu2MnSnS4 can be considered as being derived from those of ZnS, where 1/2, 1/4, and 1/4 Zn2+ ions are substituted by Cu+, Mn2+, and Sn4+, respectively. Note that Cu+, Mn2+, and Sn4+ ions share the same position and have a random distribution rather than a fixed position in the unit cells of zincblende and wurtzite.
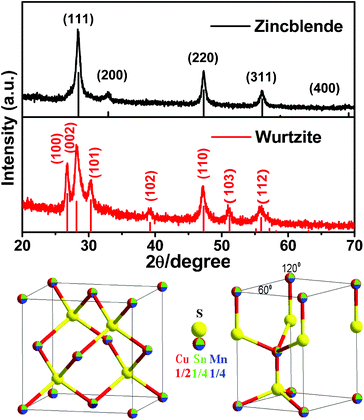 | ||
| Fig. 1 Top: the simulated and experimental XRD patterns of Cu2MnSnS4 nanocrystals with a zincblende (black line) and wurtzite (red line) structure. Bottom: the unit cells and structures of zincblende (left) and wurtzite (right) Cu2MnSnS4. | ||
Powder X-ray diffraction (XRD) was employed to check the crystal structure and calculate the lattice parameters. Fig. 1 (top) shows the simulated and experimental XRD patterns for the zincblende and wurtzite Cu2MnSnS4 nanocrystals. Our XRD patterns do not match that of the reported stannite structure (JCPDS, No. 51-0757).3 The comparisons were made between the simulated and experimental diffraction peak positions for zincblende and wurtzite structures (see Tables S1 and S2 in ESI†). The simulated and experimental XRD patterns match very well, confirming that these nanocrystals possess a zincblende and a wurtzite structure. When sulfur powder was used as precursor, the Cu2MnSnS4 nanocrystals crystallized in a zincblende structure with space group F-43m (No. 216) and unit cell parameter a = 5.4323 Å. The characteristic XRD patterns of the zincblende Cu2MnSnS4 nanocrystals exhibit three prominent peaks, which are indexed to the scattering from the (111), (220) and (311) planes, respectively. If the more stable thiourea is adopted as sulfur precursor, the wurtzite Cu2MnSnS4 nanocrystals with space group P63mc (No. 186) and unit cell parameters a = 3.8428 Å and c = 6.3313 Å will be obtained. The detailed crystal data and atomic coordinates of zincblende and wurtzite Cu2MnSnS4 nanocrystals are listed in the ESI†.
In addition, the zincblende and wurtzite structures of Cu2MnSnS4 nanocrystals can be further confirmed by selected area electron diffraction (SAED) images shown in Fig. S2 in the ESI†. Note that the lattice parameters of zincblende and wurtzite Cu2MnSnS4 nanocrystals measured from their SAED images agree with those calculated from the XRD patterns for zincblende and wurtzite Cu2MnSnS4 nanocrystals.
The morphologies and the sizes of zincblende and wurtzite Cu2MnSnS4 nanocrystals were characterized by means of transmission electron microscopy (TEM). Fig. 2a and b show low-resolution TEM images for zincblende and wurtzite Cu2MnSnS4 nanocrystals. The zincblende Cu2MnSnS4 nanocrystals have average diameters of 9.3 nm and exhibit a decent size distribution, and they are triangular in shape. Wurtzite Cu2MnSnS4 nanocrystals were composed of hexagonal plates and have a size of 23.1 nm. The continuous lattice fringes of zincblende and wurtzite Cu2MnSnS4 nanocrystals can be clearly observed in the high-resolution TEM images displayed in Fig. 2c and d, clearly indicating that they are single crystalline. The calculated d spacings corresponding to the (111) and (100) planes for zincblende and wurtzite Cu2MnSnS4 nanocrystals are 3.139 Å and 3.329 Å, which are in good agreement with those values determined by XRD patterns (3.137 Å and 3.327 Å for zincblende and wurtzite nanocrystals).
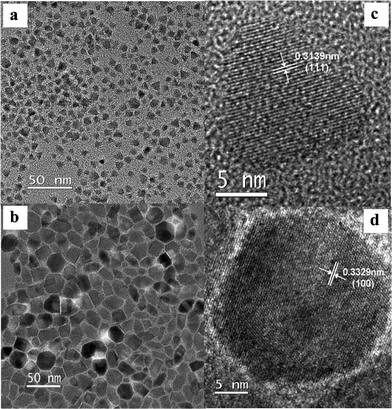 | ||
| Fig. 2 LR- (left) and HR-TEM (right) images of zincblende (top) and wurtzite (bottom) Cu2MnSnS4 nanocrystals. | ||
The chemical compositions of zincblende and wurtzite Cu2MnSnS4 nanocrystals were determined by energy disperse X-ray spectroscopy (EDS) (Fig. S3, ESI†). The composition ratios of Cu/Mn/Sn/S for the zincblende and wurtzite nanocrystals are very close to those of the co-precursors used, as well as to the stoichiometric composition of Cu2MnSnS4.
Fig. 3 displays UV-vis-NIR absorption spectra of Cu2MnSnS4 nanocrystals with a zincblende and wurtzite structure. The optical band gap (Eg) was obtained by extrapolating the linear portion of the absorption spectrum to the hν axis, and the calculated optical band gaps for these two nanocrystals are almost the same and around 1.1 eV. Note that this band gap value is the most commonly used for highly efficient CIGSe and CZTSeS solar cells,7 suggesting that these dispersible Cu2MnSnS4 nanocrystals have a high potential application in thin film solar cells. It is also noteworthy that the absorption in the range of 1200 to 2500 nm cannot be observed, indicating the absence of CuxS nanocrystals in the final Cu2MnSnS4 nanocrystals.8
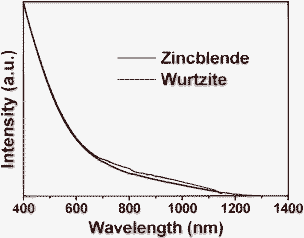 | ||
| Fig. 3 UV-vis-NIR absorption spectra of zincblende (black line) and wurtzite (red line) Cu2MnSnS4 nanocrystals. | ||
Magnetic properties of the samples were characterized by a SQUID magnetometer. The temperature-dependent magnetization of wurtzite Cu2MnSnS4 nanocrystals was studied between 2 and 300 K using zero-field-cooling (ZFC) and field-cooling (FC) procedures under a magnetic field of 100 Oe (Fig. 4a). The SQUID measurements revealed a magnetic transition at 4.0 K. A clear splitting between the ZFC and the FC curves indicates the existence of a magnetic ordering phenomenon below 4 K. The plot of χ−1–T (blue line in Fig. 4a) indicates classical Curie–Weiss behavior of this sample in the high temperature region. The magnetic susceptibility, χ(T), at the high-temperature limit can be represented by the Curie–Weiss law, χ(T) = C/(T – Θ), where C is the Curie constant, T is the temperature in Kelvin, and Θ is the Curie temperature. Moreover, the isothermal magnetization was investigated at 2 K and 300 K in magnetic fields up to ±50 kOe. Fig. 4b shows magnetization curves of wurtzite Cu2MnSnS4 nanocrystals at 2 K and 300 K. The hysteresis loop at 2 K is very weak, as shown in the inset of Fig. 4b. The as-synthesized wurtzite Cu2MnSnS4 nanocrystals are weakly ferromagnetic with a coercive force of 36 Oe at 2 K. However, the hysteresis loop obtained at 300 K does not show hysteresis behavior, and is weakly field dependent and linear, indicating that the nanocrystals become a paramagnetic material at 300 K. This result could be ascribed to the strong anti-ferromagnetic interaction at high temperature arising from the short-range exchange coupling between Mn(II) ions.4 Different from those Mn-doped nanocrystals, Mn concentrations relative to the cations are above 20 mol% in zincblende and wurtzite Cu2MnSnS4 nanocrystals (Table S3, ESI†).1 In addition, zincblende Cu2MnSnS4 nanocrystals exhibit magnetic behaviors similar to those of wurtzite Cu2MnSnS4 nanocrystals (see Fig. S4, ESI†).
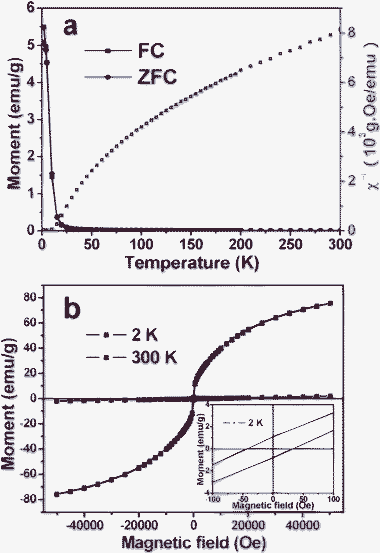 | ||
| Fig. 4 (a) Temperature dependence of the magnetization for Cu2MnSnS4 nanocrystals with a wurtzite structure, measured under conditions of zero-field-cooled and field-cooled in a magnetic field of 100 Oe. (b) The field dependent magnetization curves of Cu2MnSnS4 nanocrystals at 2 and 300 K; inset: the magnification of the hysteresis loop at 2 K. | ||
In conclusion, dilute magnetic semiconductor Cu2MnSnS4 nanocrystals with a novel zincblende and wurtzite structure have been successfully synthesized for the first time. The Cu+, Mn2+, and Sn4+ occupy the same position in the unit cells and have a random distribution, which may influence the magnetic properties of Cu2MnSnS4 nanocrystals compared with traditional stannite-type Cu2MnSnS4. These dispersible and low-cost Cu2MnSnS4 nanocrystals have an optimal band gap of 1.1 eV, which makes them have a high potential to reduce the manufacturing and material costs of thin film solar cells. In addition, this finding has enabled us to synthesize other dilute magnetic Cu2MSnS4 (M = Co, Fe, Ni) semiconductor nanocrystals with a zincblende and wurtzite structure. The synthesis and applications of these Cu2MSnS4 nanocrystals are currently underway in our laboratory.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No.21071142; 51172229), the Fund for Creative Research Groups (Grant No. 20921002), the Natural Science Foundation for Young Scientists of Jilin Province (20100105).References
- (a) M. L. Reed, M. K. Ritums, H. H. Stadelmaier, M. J. Reed, C. A. Parker, S. M. Bedair and N. A. El-Masry, Mater. Lett., 2001, 51, 500 CrossRef CAS; (b) Y. M. Kim, M. Yoon, I. W. Park, Y. J. Park and J. H. Lyou, Solid State Commun., 2004, 129, 175 CrossRef CAS; (c) Z. Deng, L. Tong, M. Flores, S. Lin, J. Cheng, H. Yan and Y. Liu, J. Am. Chem. Soc., 2011, 133, 5389 CrossRef CAS; (d) D. J. Norris, N. Yao, F. T. Charnock and T. A. Kennedy, Nano Lett., 2001, 1, 3 CrossRef CAS; (e) Y. Jun, J. Lee, J. Choi and J. Cheon, J. Phys. Chem. B, 2005, 109, 14795 CrossRef CAS; (f) K. M. Hanif, R. W. Meulenberg and G. F. Strouse, J. Am. Chem. Soc., 2002, 124, 11495 CrossRef CAS.
- T. Fries, Y. Shapira, F. Palacio, M. C. Morón, G. J. McIntyre, R. Kershaw, A. Wold and E. J. McNiff, Phys. Rev. B: Condens. Matter, 1997, 56, 5424 CrossRef CAS.
- (a) M. Wintenberger, Mater. Res. Bull., 1979, 14, 1195 CrossRef CAS; (b) J. Allemand and M. Wintenberger, Bull. Soc. Fr. Mineral. Cristallogr., 1970, 93, 14 CAS.
- (a) T. Fukushima, K. Yamauchi and S. Picozzi, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 014102–1 CrossRef; (b) G. Nénert and T. T. M. Palstra, J. Phys.: Condens. Matter, 2009, 21, 176002–1 CrossRef.
- (a) Q. Liu, Z. Zhao, Y. Lin, P. Guo, L. Li, D. Pan and X. Ji, Chem. Commun., 2011, 47, 964 RSC; (b) Z. Li, Y. Ji, R. Xie, S. Grisham and X. Peng, J. Am. Chem. Soc., 2011, 133, 17248 CrossRef CAS.
- C. Sombuthawee, S. B. Bonsall and F. A. Hummel, J. Solid State Chem., 1978, 25, 391 CrossRef CAS.
- (a) M. A. Green, K. Emery, D. L. King, S. Igari and W. Warta, Progr. Photovolt.: Res. Appl., 2005, 13, 49 CrossRef CAS; (b) K. Ramanathan, M. A. Contreras, C. L. Perkins, S. Asher, F. S. Hasoon, J. Keane, D. Young, M. Romero, W. Metzger, R. Noufi, J. Ward and A. Duda, Progr. Photovolt.: Res. Appl., 2003, 11, 225 CrossRef CAS; (c) Q. Guo, G. M. Ford, W. Yang, B. C. Walker, E. A. Stach, H. W. Hillhouse and R. Agrawal, J. Am. Chem. Soc., 2010, 132, 17384 CrossRef CAS; (d) T. K. Todorov, K. B. Reuter and D. B. Mitzi, Adv. Mater., 2010, 22, E156–E159 CrossRef CAS.
- (a) R. Xie, M. Rutherford and X. Peng, J. Am. Chem. Soc., 2009, 131, 5691 CrossRef CAS; (b) J. Podder, R. Kobayashi and M. Ichimura, Thin Solid Films, 2005, 472, 71 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: detailed synthesis of nanocrystals; crystal data, SAED, EDS spectra, and chemical compositions. See DOI: 10.1039/c2ra20198d/ |
| This journal is © The Royal Society of Chemistry 2012 |
