Dotting the i's in three-component Biginelli-like condensations using 3-amino-1,2,4-triazole as a 1,3-binucleophile†
Yuriy V.
Sedash
a,
Nikolay Yu.
Gorobets
*a,
Valentin A.
Chebanov
a,
Irina S.
Konovalova
a,
Oleg V.
Shishkin
ab and
Sergey M.
Desenko
ab
aSSI “Institute for Single Crystals” of National Academy of Sciences of Ukraine, Lenin Ave. 60, Kharkiv 61001, Ukraine. E-mail: gorobets@isc.kharkov.com; Fax: +38 057 3410273; Tel: +38 0573410112
bKarazin Kharkiv National University, Svobody sq., 4, 61077 Kharkiv, Ukraine
First published on 3rd April 2012
Abstract
Alternative reaction pathways for Biginelli-like cyclocondensation reactions using 3-amino-1,2,4-triazole as a 1,3-binucleophile under different reaction conditions lead to several types of products. The literary data is generalized and discussed. Additional results going beyond the generalization are also revealed.
Introduction
The beginning of the 21st century was accompanied by an increase of interest in multicomponent reactions (MCRs)1,2 as an easier way to achieve diversity-orientated synthesis of complex organic molecules, especially, bioactive heterocycles. Among such reactions, the Biginelli multicomponent cyclocondensation is one of the oldest and at the same time a topical subject for research.3–6 The classical Biginelli reaction3 applying an aldehyde, urea or thiourea and an open chain β-dicarbonyl compound for the construction of 3,4-dihydropyrimidine-2(1H)ones (DHPMs) has been explored by a great number of researchers.4–10 This approach has received a second lease of life in the development of different types of Biginelli-like MCRs,11–14 where one or more starting reagents have certain differences from the classical starting compounds, but possess similar reactive sites. A rapidly developing approach applying aminoazoles as 1,3-binucleophiles in such reactions provides diverse DHPM derivatives and other heterocycles fused with azole rings.15,16 The stepwise character of the MCRs itself and the application of such polyfunctional 1,3-binucleophiles as 3-amino-1,2,4-triazoles having nonequivalent reaction centers can tentatively lead to at least eight possible products A–H from one set of the starting reagents (Scheme 1). Moreover, the use of salicylic aldehydes results in even more complex products 1, 2 (Fig. 1).17,18 Also one should take into account that Biginelli cyclocondensations, in some cases, may proceed by a formal hetero Diels–Alder mechanism to give reaction products with an alternative orientation of the dicarbonyl fragment 3 (Fig. 1).19 As a consequence of this diversity of the possible reaction products their structural elucidation often becomes problematic.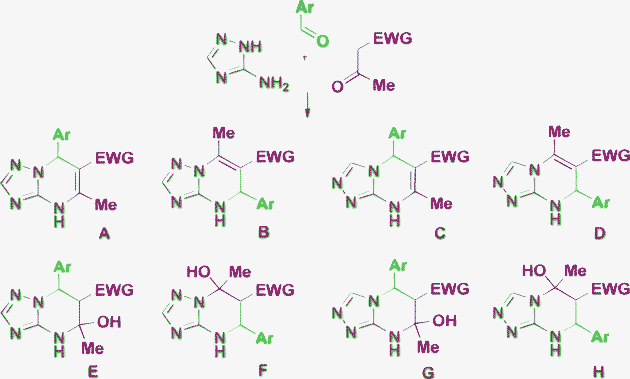 | ||
| Scheme 1 Possible products of Biginelli-like condensation using 3-amino-1,2,4-triazole as the urea component. | ||
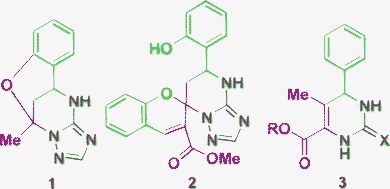 | ||
| Fig. 1 Unexpected heterocycles from Biginell-like condensations. | ||
In practice the reaction pathway and selectivity of such MCRs are frequently dependent on both the structure of the starting materials and the applied reaction conditions. Thus one set of the reagents may result in the formation of several different final heterocycles. Unfortunately, the existing literary data about the structure of such reaction products is not always comprehensive. This makes it difficult to work out the full picture of 3-amino-1,2,4-triazole reactivity in Biginelli-like condensations under different reaction conditions.
Here we aim to collect and generalize the published data concerning application of 3-amino-1,2,4-triazole in Biginelli-like MCRs and attempt to fulfil the conception of such reactions. Since some published data requires a re-examination of the obtained results this work combines a literature survey with experimental studies.
Results and discussion
Literature survey
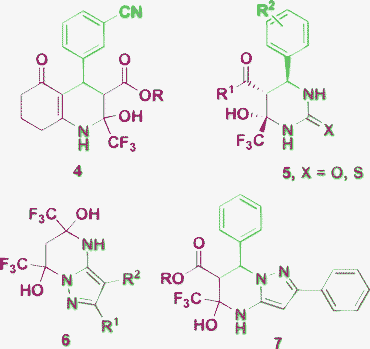
Most of the literature sources concerning Biginelli-like MCRs with 3-amino-1,2,4-triazoles describe structure A for the products that usually obtained under quite harsh conditions (Table 1, Entries 1–12, 14, 16, 17 and 19). Structure C is reported only as a side product accompanying the formation of the main product A (Table 1, Entries 7 and 17). Tetrahydro derivatives F have recently been described to be formed under mild conditions (Table 1, Entries 13–15). Interestingly, the application of 3-trifluoromethyl-3-oxoesters20 lead to the formation of E-type products that can be converted further into A-type compounds (Table 1, Entry 4). Additionally, Desenko et al.21 described an analogous two-component approach starting from benzylidene trifluoroacetone (Table 1, Entry 5). Thus, tetrahydrotriazolopyrimidines of type E can be considered as intermediates22 in the reaction pathway leading to thermodynamic products A and such intermediates could be isolated when the tetrahydropyrimidine ring is stabilized by a neighboring CF3 group. The same stabilization is also observed in other heterocycles, e.g.4–7, obtained by Biginelli and Hantzsch modified approaches using trifluoromethyl derivatives of the dicarbonyl component (Fig. 2).|
|
|||||
|---|---|---|---|---|---|
| Entry | R | Active methylene compound | Conditions | Product type | Reference |
| a Two-component reaction.b It seems there is a misprint in Scheme 1, page 771 of the original article,45 where the product is depicted as type C, but the given names for the same compounds correspond to A-type. These compounds are also described previously by Lipson et al.34 This typo was also replicated by SciFinder database. Similarly, in the review11 (page 3950, Scheme 19) the product of A-type is incorrectly referred as C-isomer (see the original article49 or Entry 14).c R, R1, R2, R3 are different substituents for a combinatorial library generation, see the original source51 for details. | |||||
| 1 | H |

|
DMF, reflux, 3 h |

|
Desenko, 199330 |
| 2 | H |
|
DMF, reflux, 3 h |

|
Desenko, 199330 |
| 3 | H |

|
EtOH, HCl, reflux, 5–7 h |

|
Fedorova, 200343 |
| 4 | H |

|
a) EtOH, HCl, reflux, 12 h or DMF, 70 °C, 12 h |

|
Pryadeina, 200420 |
| b) TSA, benzene, reflux | |||||
| 5a | H |
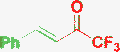
|
a) MeOH, rt, 2 days |

|
Desenko, 200421 |
| b) TSA, MeOH, reflux, 2 h | |||||
| NH2 |

|
DMF, 10 min, reflux |

|
Lipson, 200544 | |
| 7 | H |

|
AcOH, reflux, 4 h or DMF, reflux, 1.5 h |

|
Chebanov, 200533 |
| 8 | S-Alkyl |
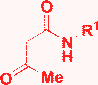
|
EtOH, MW, 120 °C, 5 min |

|
Chebanov, 200637 |
| 9b | H |

|
Solvent-free, 80 °C, 4 h |

|
Shaabani, 200645 |
| 10 | NH2 |

|
DMF, reflux, 30 min |

|
Chernyshev, 200746 |
| 11 | H |

|
DMF, 135 °C, 30 min |

|
Gladkov, 200747 |
| 12 | NH2 |

|
125–150 °C, fusion |
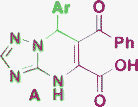
|
Gein, 200848 |
| 13 | H |

|
AcOH, rt, US, 30 min or AcOH, Δ 2 min or MW, 120 °C, 2 min |

|
Sakhno, 200840 |
| 14 | S-Alkyl |
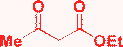
|
H2O, TSA, 80 °C, 10 h or EtOH, reflux, 18 h or MW, 150 °C, 30 min |
A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F ratio depends on the substituents F ratio depends on the substituents 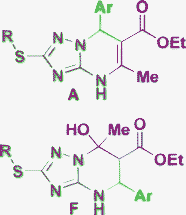 |
Chen, 200949 |
| 15 | H |
|
MeOH, HCl, 40 °C, 16 h |

|
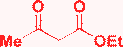
Gorobets, 201017 |
| 16 | NH2 |

|
Piperidine, THF, 70 °C |
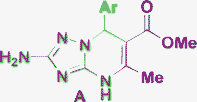
|
Brigance, 201150 |
| 17 | H |
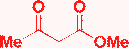
|
EtOH, HCl, reflux, 20 h |
A + C (admixture)  |
Světlík, 201118 |
| 18 | S-R |

|
a) EtOH, US, 25 °C, 1.5 h |
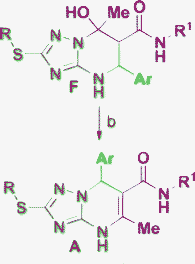
|
Muravyova, 201141 |
| b) DMF, reflux, 10 min | |||||
| 19c | R |

|
DMF, TMSCl 20 °C, 23–47 h |

|
Ryabukhin, 201151 |
The products D are not obtained by Biginelli-like MCRs, however, they were synthesized using other approaches.27 Finally, the products of structure B are observed when two molecules of cyclohexanone28 or acetophenone29 react with 3-amino-1,2,4-triazole derivatives. An example of the B-product from Biginelli-like condensations accompanying the main isomer A30 is given in Table 1, Entry 2. Moreover, compounds of type B are obtained by multistep approaches31,32 or via rearrangement of D.27
The analysis of 1H NMR spectral data collected to date for the isomeric structures of types A and C allow us to use the singlet of the CH proton of the triazole fragment as an easy spectral index to distinguish these isomers in the cases where this position of the triazole ring remains unsubstituted. These singlets for all the analyzed compounds of type A (for solutions in DMSO-d6, here and below) appear at 7.58–7.79 ppm,18,33–35 whereas for the C-type compounds these proton signals are usually deshielded, probably by the magnetic anisotropy of the neighboring aromatic ring,36 and are found at 7.94–8.34 ppm.18,33,35 The presence of NOE between the pyrimidine methyne group or aryl moiety and the triazole CH can also be an indicator of structure C formation.33
A distinct dependence of the 1H NMR chemical shift of the pyrimidine amino group (NH) on the structure of the azoloazine skeleton has also been described in several publications.28,30,37 It was established, that the A-like structures exhibited a signal for the azine NH proton at 9.5–10.5 ppm, whereas in the case of structures similar to B this signal was always strong-field shifted by 2–3 ppm. As an additional confirmation of these structures the absence (for A) or presence (for B) of NOE and COSY correlations between the CH and the NH groups in the pyrimidine ring were often used as well.37–42
On the other hand, to correctly distinguish the isomers E and F and determine the relative configuration of their chiral centers, application of correlation NMR methods or X-ray crystallography is generally required.17,20,21,41,49 In the report51 using 5-CF3-3-amino-1,2,4-triazole the authors assigned the E-type structure to tetrahydroderivative 8 that was obtained as an exception, whereas all the other library members were representatives of the A-type structure. The E structure of the product 8 was formulated based on previously described results20 (where the E was stabilized by the neighboring CF3-group, see Table 1, Entry 4) and the NOE experiments. However, the same NOE results can be expected for the alternative F-type positional isomer (9).
In ref. 49, the authors reported the formation of two products of type A and F obtained as a mixture or individually in an aqueous medium. The structure F was confirmed unambiguously by the X-ray diffraction study of a representative compound, 10a, containing a CH3 group in the dicarbonyl fragment (Fig. 3). However, the derivatives 10b–d, which contain a CF3 group, were not additionally analyzed and the structure F was assigned to these products just by analogy. Taking into account the discussed above effect of the CF3-group stabilization observed in the structures E, these result of structural elucidation also look ambiguous.
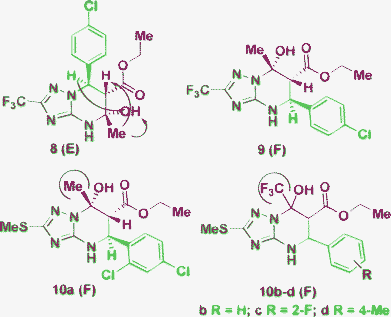 | ||
| Fig. 3 Difficulties in the structural elucidation in the case of F or E-isomer formation. NOEs are indicated by arrows. | ||
It can be assumed from the data collected above that Biginelli-like condensations using 3-amino-1,2,4-triazole lead to two main types of products: the A and F structures depending on the reaction conditions applied. It is worth noting that these products differ not only in the saturation of the pyrimidine core and uneliminated water molecule of F, but also by the aldehyde component reacted with the exocyclic amino group of the 3-amino-1,2,4-triazole to form the structure F instead of the endocyclic nitrogen of the triazole ring to form product A. At the same time structure E is reported for the CF3-derived dicarbonyl component. Finally, the formation of B and C as byproducts was described in the literature. Products of structure G and H are not known. Analysis of the literature has also revealed some other controversial data that require experimental re-investigation that is disclosed below.
Experimental re-investigations
The three-component condensation of benzaldehyde, 1,3-indanedione and 3-amino-1,2,4-triazole was reported52 to result in product 11 of the B-type (Scheme 2). The products of this reaction, however, were insufficiently characterized (m.p., mass spectrometry, IR and elemental analysis are given in the article) and, in view of the results of our literature survey presented above, the formation of the compound 11 (type B) as a major product seems to be doubtful. Reproduction of the experimental procedure described in ref. 52 (reflux of an equimolar mixture of benzaldehyde, 1,3-indanedione and 3-amino-1,2,4-triazole in EtOH for 8 h) revealed the formation of only an ‘intermediate’ binary product 12. Our results are also in an agreement with publications by Heravi et al.53,54 who reported that the benzylidene derivative 12 was isolated from the same reaction mixture under different reaction conditions. This may be due to the unfavorability of the cyclocondensation of the S-cis-configuration of the enone fragment in ‘intermediate’ 12 along with the high rigidity of its skeleton. A low reactivity of the five-membered cyclic α,β-unsaturated ketones in such heterocyclizations with binuclephiles were also described previously.55 However, in a recent report51 product 13 of type A was obtained among the library members using TMSCl as a reaction promoter (Scheme 2).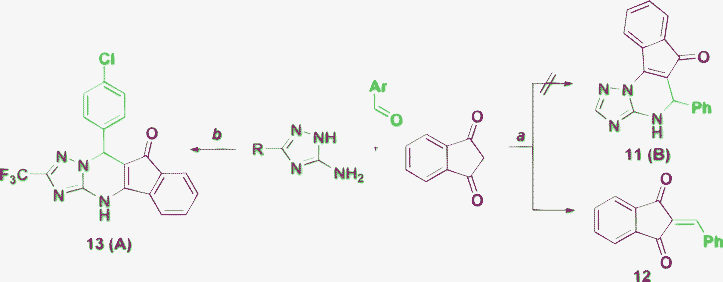 | ||
| Scheme 2 Reaction of indanedione under reaction conditions: a) EtOH, reflux, 8 h52; b) Me3SiCl, DMF, 20 °C, 23–47 h.51 | ||
The structure of type E suggested for the products obtained by Shaabani et al.56 in water at the room temperature without a catalyst has caused some doubts, since such compounds were isolated without the stabilizing effect of a neighboring fluorinated alkyl group. Moreover, such mild reaction conditions are favorable for the formation of E-type products (see Table 1, Entries 14, 15 and 18). Since the reaction conditions and structures of the starting reactants are different in all above mentioned publications, we revised this reaction using the conditions described in ref. 56 (Method A) and ref. 17 (Method B). In both cases we have obtained the products 14a,b instead of 15a,b (Scheme 3) which have the same spectral data as in the original article. The structure 14a was additionally confirmed by an X-ray diffraction study (Fig. 4). It should be pointed that ref. 56 was the first work where the products F were obtained and the reports containing the correct structural elucidation for such a compound type appeared later in the literature (see Table 1).
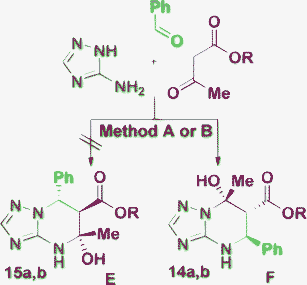 | ||
| Scheme 3 Problem in the structure determination for the product E/F obtained using the reaction conditions: water, rt, 4 h (Method A); MeOH, HCl, 40 °C, 16 h (Method B); a: R = Me, b: R = Et. | ||
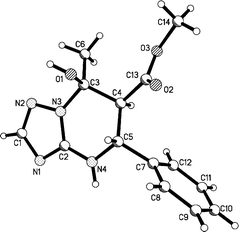 | ||
| Fig. 4 Molecular structure of the compound 14a according to X-ray data. | ||
The obtained product 14b under harsh conditions (as in Table 1, Entries 8, 14, 18) was transformed into the previously described derivative 16 (Scheme 4).43
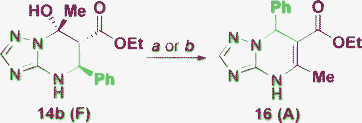 | ||
| Scheme 4 Reaction conditions: a) EtOH, MW, 120 °C, 20 min; b) DMF, reflux, 10 min. | ||
An analogous rearrangement followed by dehydratation (Table 1, Entry 18) has been recently reported.41
Experimental
General
Starting materials and solvents were used as obtained from commercial suppliers. 1H NMR spectra (DMSO-d6 solutions) were recorded on a Varian Mercury VX-200 spectrometer, MS was performed on a Varian 1200 L GC-MS instrument with the use of direct exposure probe (EI, 70 eV). Microwave irradiation in sealed vials was carried out using an Emrys™ Creator EXP from Biotage (Uppsala) possessing a single-mode microwave cavity producing irradiation at 2.45 GHz.X-Ray diffraction study
The colourless crystals of 14a C14H16N4O3 are orthorhombic. At 293 K a = 5.6370(4), b = 10.2308(7), c = 24.765(2) Å, V = 1428.3(4) Å3, Mr = 288.31, Z = 4, space group P212121, dc = 1.341 g cm−3, μ(Mo-Kα) = 0.097 mm−1, F(000) = 608. Intensities of 9369 reflections (1498 independent, Rint = 0.054) were measured on the «Xcalibur-3» diffractometer (graphite monochromated Mo-Kα radiation, CCD detector, ω-scaning, 2Θmax = 50°). The structure was solved by direct method using the SHELXTL package.57 Positions of the hydrogen atoms were located from electron density difference maps and refined by “riding” model with Uiso = nUeq (n = 1.5 for methyl and hydroxyl groups and n = 1.2 for other hydrogen atoms) of the carrier atom. Full-matrix least-squares refinement against F2 in anisotropic approximation for non-hydrogen atoms using 1480 reflections was converged to wR2 = 0.096 (R1 = 0.044 for 1190 reflections with F > 4σ(F), S = 1.156). The final atomic coordinates, and crystallographic data for molecule 14a have been deposited to with the Cambridge Crystallographic Data Centre (CCDC 857098).Methyl 7-hydroxy-7-methyl-5-phenyl-4,5,6,7-tetrahydro[1,2,4]riazolo[1,5-a]pyrimidine-6-carboxylate 14a
Method A.56 A mixture of methylacetoacetate (0.116 g, 1 mmol), benzaldehyde (0.106 g, 1 mmol), and 3-amino-1,2,4-triazole (0.084 g, 1 mmol) was successively added to a vial containing a magnetic stirring bar and water (5 mL) and the reaction mixture was stirred at room temperature for 4 h. Thereafter the reaction mixture was filtrated and the crude product was recrystallized from ethanol (30 mL) to yield 0.140 g of 14a as a white powder (48%). The colorless crystals for X-ray diffraction study were obtained by a slow evaporation of saturated solution of this product in EtOH at room temperature.Method B. 17 To a mixture of benzaldehyde (1 mmol), 3-amino-1,2,4-triazole (1 mmol), and acetoacetic ester (1 mmol) in MeOH (1 mL) in a sealed round-bottomed flask, HCl (0.1 mmol, 0.05 mL, 4 N solution in EtOH) was added. The mixture was stirred at 40 °C for 16 h then cooled to room temperature, and the precipitate was filtered off, washed with MeOH (1 mL) and ether (3 × 1 mL), and dried. The compound was obtained in 55% yield. 1H NMR spectra for products obtained by Methods A and B are identical to described earlier as 15a.45 M.p. 158 °C (163–165 °C45); (DMSO-d6, 200 MHz): δ (ppm) 7.60 (s, 1H), 7.42 (br. s, 1H), 7.24–7.41 (m, 5H), 6.80 (s, 1H), 4.84 (d, J = 11.7 Hz, 1H), 3.38 (s, 3H), 3.24 (d, J = 5.5 Hz, 1H), 1.76 (s, 3H); MS m/z: 131 (51%) 171 (100%) 211 (19%) 245 (10%) 288 (M+, 35%).
It should be noted that the 1H NMR spectra of the tetrahydro derivatives 14a,b were measured in fresh solutions due to epimerisation in DMSO-d6. This has been described earlier for analogous compounds.17
Ethyl 7-hydroxy-7-methyl-5-phenyl-4,5,6,7-tetrahydro[1,2,4]striazolo[1,5-a]pyrimidine-6-carboxylate 14b
The product was obtained using Method A as a white powder. Yield 51%. M.p. 168–170 °C (176–178 °C45); 1H NMR (200 MHz, DMSO-d6) δ (ppm) 0.83–0.95 (m, 3 H) 1.78 (s, 3 H) 3.16–3.27 (m, 1 H) 3.85 (dd, J = 6.96, 4.76 Hz, 2 H) 4.83 (d, J = 11.72 Hz, 1 H) 6.76 (s, 1 H) 7.25–7.41 (m, 5 H) 7.42 (s, 1 H) 7.56 (s, 1 H). The 1H NMR data is identical to that described in the literature.45 MS m/z: 83 (36%) 117 (12%) 171 (100%) 211 (41%) 259 (11%) 302 (M+, 8%).Ethyl 4,7-dihydro-5-methyl-7-phenyl-[1,2,4]triazolo[1,5-a]pyrimidine-6-carboxylate 16
Method A. 37 Crude product 14b (604 mg) was placed into a MW process vial (for 2.5 mL reaction mixture volume), 1.5 mL of EtOH was added and the reaction mixture was irradiated for 5 min at 120 °C. After cooling, the vial was left to stand at room temperature for a day to complete precipitation. The precipitate was filtered off, washed with EtOH and dried. A white powder, yield 39%, was obtained.Method B. 41 Crude product 14b (604 mg) in 0.4 mL of DMF was heated to reflux for 10 min. After cooling, acetone (10 mL) was added and the precipitate formed was filtered off, washed with acetone and dried to give a white powder 16. Yield 51%. 1H NMR spectra for products obtained by Methods A and B are identical to those described earlier.43 M.p. 195 °C (190-192 °C43); 1H NMR (DMSO-d6 ,200 MHz): δ (ppm) 10.78 (s, 1H), 7.63 (s, 1H), 7.10–7.40 (m, 5H), 6.25 (s, 1H), 3.94 (d, J = 7.0 Hz, 2H), 2.40 (s, 3H), 1.01 (t, J = 7.1 Hz, 3H). MS m/z: 77 (15%) 161 (25%) 179 (19%) 207(45%) 255 (100%) 284 (M+, 37%).
Conclusions
In this work we have collected and critically analyzed the literature devoted to the reactivity of 3-amino-1,2,4-triazole in Biginelli-like MCRs taking into account that these condensations may formally result in the formation of at least eight types of product A–H (Fig. 1). Such an approach has the advantage of a broader view of the problem that led us to certain conclusions on the way to the moment when all the i's will finally be dotted in this subject. The general results are represented in Scheme 5.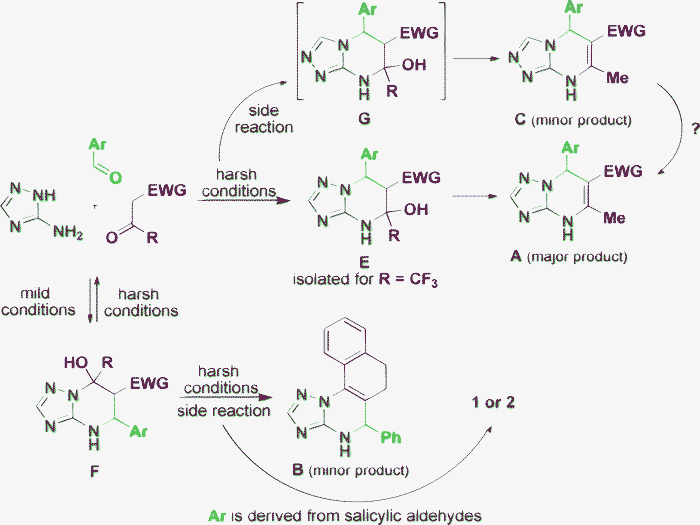 | ||
| Scheme 5 Graphical conclusions. | ||
The system of three starting components has two main ways for the realization of their reactive potential: via the tetrahydro derivatives F or E. We believe that application of mild reaction conditions results in the formation of the first kinetic product containing a newly formed pyrimidine ring F. Further development under harsher conditions depends on the substituent nature in the CH-acid and aldehyde components. The product B derived from the corresponding intermediate F is formed only as a minor component of the reaction mixture using tetralone,30 but the intermediate F was not isolated in this work. The competitive A/B-formation is typical behavior for the cases where both the CH-acid and ‘aldehyde’ components are represented by ketones.29 Also, the application of salicylic aldehydes under harsh conditions results in a fixation of the core F by an oxygen bridge (compounds 1 and 2, Fig. 1). All the other unambiguous published results indicate that product F, under harsh conditions, undergoes complete or partial retro-cyclization into its acyclic precursors that results in the formation of the main thermodynamic product Avia its intermediate E which is generally too unstable to be isolated. However, such intermediates E can be isolated when stabilized by the CF3-substituent.
The formation of product C in only small amounts can be caused by at least two reasons. The first is the competition between the two endocyclic nitrogen atoms in the triazole ring which are able to participate in heterocyclizations. The hydrazine nitrogen is more reactive and thus A is obtained in fair amounts while C is only a minor product. Another possibility is a Dimroth-type rearrangement (C → A) known for similar azolopyrimidines.58 Thus, it can be suggested that an equilibrium between the thermodynamic products C and A exists and is significantly shifted to the side A. The same two reasons can work in the case of D/B-competition, which is indirectly confirmed by the results of Said et al.27 Correspondingly, the elusive products G and H are intermediates of the minor product C and the “double minor” product D, which have never been isolated from Biginelli-like condensations.
The number of publications about different variations of the Biginelli approach is increasing like a snowball today. Also the application of non-conventional activation methods and reaction media indicates the urgency of this subject. In this article we have restricted the subject to the application of 3-amino-1.2.4-triazole derivatives and observed how diverse this ‘narrow’ area in fact is. In the light of the generalization of the current results on Biginelli-like condensations involving 3-amino-1.2.4-triazole presented here and taking into account the emerging literary data concerning other “non-classical” products from these reaction18 it can hardly be stated that all the i's are dotted for the moment. Moreover, we did not concern ourselves here with the many intriguing t's that have to be further crossed such as the tautomerism and reactivity of the reaction products as well as the mechanistic and stereochemical aspects of their formation that still require additional research. Nevertheless, we believe the given generalization of the facts concerning such multicomponent heterocyclization will become a reliable platform for the design of new synthetic approaches leading to diverse heterocycles based on 3-amino-1,2,4-triazoles and other aminoazoles.
References
- J. Zhu and H. Bienayme, ed., Multicomponent Reactions, WILEY-VCH Verlag GmbH & Co.KGaA, Weinheim, 2005 Search PubMed.
- C. Kalinski, M. Umkehrer, L. Weber, J. Kolb, C. Burdack and G. Ross, Mol. Diversity, 2010, 14, 513 CrossRef CAS.
- P. Biginelli, Gazz. Chim. Ital., 1893, 23, 360 Search PubMed.
- C. O. Kappe, Acc. Chem. Res., 2000, 33, 879 CrossRef CAS.
- J. G. Ma, J. M. Zhang, H. H. Jiang, W. Y. Ma and J. H. Zhou, Chin. Chem. Lett., 2008, 19, 375 CrossRef CAS.
- C. O. Kappe, Tetrahedron, 1993, 49, 6937 CrossRef CAS.
- C. O. Kappe, J. Org. Chem., 1997, 62, 7201 CrossRef CAS.
- M. A. Kolosov, V. D. Orlov, D. A. Beloborodov and V. V. Dotsenko, Mol. Diversity, 2008, 13, 5 CrossRef.
- S. V. Vdovina and V. A. Mamedov, Russ. Chem. Rev., 2008, 77, 1017 CrossRef CAS.
- M. d. M. Sanchez Duque, C. Allais, N. Isambert, T. Constantieux and J. Rodriguez, Top. Heterocycl. Chem., 2010, 23, 227 CrossRef.
- J.-P. Wan and Y. Liu, Synthesis, 2010, 3943 CrossRef CAS.
- A. Shaabani, A. Bazgir and H. R. Bijanzadeh, Mol. Diversity, 2004, 8, 141 CrossRef CAS.
- M. M. Abelman, S. C. Smith and D. R. James, Tetrahedron Lett., 2003, 44, 4559 CrossRef CAS.
- X. Jing, Z. Li, X. Pan, Q. Wang, C. Yan and H. Zhu, Synth. Commun., 2009, 39, 3796 CrossRef CAS.
- V. A. Chebanov, S. M. Desenko and T. W. Gurley, Azaheterocycles Based on α,β-Unsaturated Carbonyls, Springer, 2008 Search PubMed.
- V. A. Chebanov, K. A. Gura and S. M. Desenko, Top. Heterocycl. Chem., 2010, 23, 41 CrossRef CAS.
- N. Yu. Gorobets, Yu. V. Sedash, K. S. Ostras, O. V. Zaremba, S. V. Shishkina, V. N. Baumer, O. V. Shishkin, S. M. Kovalenko, S. M. Desenko and E. V. Van der Eycken, Tetrahedron Lett., 2010, 51, 2095 CrossRef CAS.
- J. Světlík and V. Kettmann, Tetrahedron Lett., 2011, 52, 1062 CrossRef.
- J. Zhu, M. Zhang, B. Liu and X. Li, Chem. Lett., 2009, 38, 56 CrossRef CAS.
- M. V. Pryadeina, Ya. V. Burgart, V. I. Saloutin, M. I. Kodess, E. N. Ulomskii and V. L. Rusinov, Russ. J. Org. Chem., 2004, 40, 902 CrossRef CAS.
- S. M. Desenko, E. S. Gladkov, V. G. Nenaidenko, O. V. Shishkin and S. V. Shishkina, Chem. Heterocycl. Compd., 2004, 40, 65 CrossRef CAS.
- C. O. Kappe and S. F. Falsone, Synlett, 1998, 718 CrossRef CAS.
- J. D. Moseley, Tetrahedron Lett., 2005, 46, 3179 CrossRef CAS.
- O. C. Agbaje, O. O. Fadeyi, S. A. Fadeyi, L. E. Myles and C. O. Okoro, Bioorg. Med. Chem. Lett., 2011, 21, 989 CrossRef CAS.
- E. E. Emelina, A. A. Petrov and A. V. Firsov, Russ. J. Org. Chem., 2003, 39, 277 CrossRef CAS.
- M. V. Pryadeina, Ya. V. Burgart, V. I. Saloutin, P. A. Slepukhin, E. V. Sadchikova and E. N. Ulomskii, Russ. J. Org. Chem., 2009, 45, 242 CrossRef CAS.
- S. A. Said, A. E.-G. E. Amr, N. M. Sabry and M. M. Abdalla, Eur. J. Med. Chem., 2009, 44, 4787 CrossRef CAS.
- S. M. Desenko, V. D. Orlov and K. Estrada, Chem. Heterocycl. Compd., 1990, 26, 839 CrossRef.
- K. Wermann and M. Hartmann, Synthesis, 1991, 189 CrossRef CAS.
- S. M. Desenko, V. D. Orlov, N. V. Getmanskii, O. V. Shishkin, S. V. Lindeman and Yu. T. Struchkov, Chem. Heterocycl. Compd., 1993, 29, 406 CrossRef.
- S. A. Komykhov, K. S. Ostras, A. R. Kostanyan, S. M. Desenko, V. D. Orlov and H. Meier, J. Heterocycl. Chem., 2005, 42, 1111 CrossRef CAS.
- I. E. Varshalomidze, A. G. Golikov and A. P. Krivenko, Chem. Heterocycl. Compd., 2009, 45, 1014 CrossRef CAS.
- V. A. Chebanov, Ya. I. Sakhno, S. M. Desenko, S. V. Shishkina, V. I. Musatov, O. V. Shishkin and I. V. Knyazeva, Synthesis, 2005, 2597 CrossRef CAS.
- V. V. Lipson, S. M. Desenko, M. G. Shirobokova and V. V. Borodina, Chem. Heterocycl. Compd., 2003, 39, 1213 CrossRef CAS.
- R. V. Rudenko, S. A. Komykhov, V. I. Musatov, I. S. Konovalova, O. V. Shishkin and S. M. Desenko, J. Heterocycl. Chem., 2011, 48, 888 CrossRef CAS.
- S. M. Desenko, V. D. Orlov, V. V. Lipson, A. A. Kaganovskii and V. T. Zuong, Dokl. Akad. Nauk Ukr. SSR, Ser. B: Geol., Khim. Biol. Nauki, 1990, 45 CAS.
- V. A. Chebanov, E. A. Muravyova, S. M. Desenko, V. I. Musatov, I. V. Knyazeva, S. V. Shishkina, O. V. Shishkin and C. O. Kappe, J. Comb. Chem., 2006, 8, 427 CrossRef CAS.
- V. A. Chebanov, V. E. Saraev, S. M. Desenko, V. N. Chernenko, I. V. Knyazeva, U. Groth, T. N. Glasnov and C. O. Kappe, J. Org. Chem., 2008, 73, 5110 CrossRef CAS.
- V. A. Chebanov, Ya. I. Sakhno, S. M. Desenko, V. N. Chernenko, V. I. Musatov, S. V. Shishkina, O. V. Shishkin and C. O. Kappe, Tetrahedron, 2007, 63, 1229 CrossRef CAS.
- Ya. I. Sakhno, S. M. Desenko, S. V. Shishkina, O. V. Shishkin, D. O. Sysoyev, U. Groth, C. O. Kappe and V. A. Chebanov, Tetrahedron, 2008, 64, 11041 CrossRef CAS.
- E. A. Muravyova, S. M. Desenko, R. V. Rudenko, S. V. Shishkina, O. V. Shishkin, Yu. V. Sen'ko, E. V. Vashchenko and V. A. Chebanov, Tetrahedron, 2011, 67, 9389 CrossRef CAS.
- Ya. I. Sakhno, S. V. Shishkina, O. V. Shishkin, V. I. Musatov, E. V. Vashchenko, S. M. Desenko and V. A. Chebanov, Mol. Diversity, 2010, 14, 523 CrossRef CAS.
- O. V. Fedorova, M. S. Zhidovinova, G. L. Rusinov and I. G. Ovchinnikova, Russ. Chem. Bull., 2003, 52, 1768 CrossRef CAS.
- V. V. Lipson, S. M. Desenko, V. V. Borodina, M. G. Shirobokova and V. I. Musatov, Russ. J. Org. Chem., 2005, 41, 114 CrossRef CAS.
- A. Shaabani, E. Farhangi and A. Rahmati, Comb. Chem. High Throughput Screening, 2006, 9, 771 CrossRef CAS.
- V. M. Chernyshev, A. N. Sokolov and V. A. Taranushich, Russ. J. Appl. Chem., 2007, 80, 1691 CrossRef CAS.
- E. S. Gladkov, V. A. Chebanov, S. M. Desenko, O. V. Shishkin, S. V. Shishkina, D. Dallinger and C. O. Kappe, Heterocycles, 2007, 73, 469 CrossRef CAS.
- V. L. Gein, E. P. Tsyplyakova, G. A. Stashina and V. A. Bakulev, Russ. J. Org. Chem., 2011, 44, 478 CrossRef.
- Q. Chen, L.-L. Jiang, C.-N. Chen and G.-F. Yang, J. Heterocycl. Chem., 2009, 46, 139 CrossRef CAS.
- R. P. Brigance, W. Meng, A. Fura, T. Harrity, A. Wang, R. Zahler, M. S. Kirby and L. G. Hamann, Bioorg. Med. Chem. Lett., 2010, 20, 4395 CrossRef CAS.
- S. V. Ryabukhin, A. S. Plaskon, S. Yu. Boron, D. M. Volochnyuk and A. A. Tolmachev, Mol. Diversity, 2010, 15, 189 CrossRef.
- E. M. Afsah, H. A. Etman, W. S. Hamama and A. F. Sayed-Ahmed, Boll. Chim. Farm., 1998, 137, 244 CAS.
- M. M. Heravi, L. Ranjbar, F. Derikvand, B. Alimadadi, H. A. Oskooie and F. F. Bamoharram, Mol. Diversity, 2008, 12, 181 CrossRef CAS.
- M. M. Heravi, F. Derikvand and L. Ranjbar, Synth. Commun., 2010, 40, 677 CrossRef CAS.
- N. N. Kolos, V. A. Chebanov and V. D. Orlov, Chem. Heterocycl. Compd., 1999, 35, 1085 CrossRef CAS.
- A. Shaabani, A. Rahmati, A. H. Rezayan, M. Darvishi, Z. Badri and A. Sarvari, QSAR Comb. Sci., 2007, 26, 973 CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2007, 64, 112 CrossRef.
- C. F. H. Allen, H. R. Beilfuss, D. M. Burness, G. A. Reynolds, J. F. Tinker and J. A. VanAllan, J. Org. Chem., 1959, 24, 787 CrossRef CAS.
Footnote |
| † CCDC reference numbers 857098. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra20195j |
| This journal is © The Royal Society of Chemistry 2012 |