A new hypothesis of micro-region acid sites regarding the surface acidity of binary oxides
Yuesong
Shen
*
College of Materials Science and Engineering, State Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing University of Technology, Nanjing 210009, P. R. China. E-mail: sys-njut@163.com
First published on 10th May 2012
Abstract
According to Tanabe's hypothesis, a binary oxide exhibits only one acidity type or no acidity. Actually most binary oxides simultaneously exhibit Brønsted and Lewis acidities; Tanabe's hypothesis cannot explain this phenomenon. Taking into account the effects of material structure and the micro-concept of acid sites, on the basis of the Tanabe hypothesis, the author proposes a new hypothesis of micro-region acid sites regarding the surface acidity of binary oxides.
In 1974, Tanabe and coworkers proposed a hypothesis regarding the surface acidity of binary oxides.1 So far it has become a classic acid principle. The hypothesis includes two rules to determine acidity: (I) acidity generation is formed by an excess of negative or positive charge in a binary oxide; (II) Lewis acidity is assumed to appear in the presence of an excess of positive charge, Brønsted acidity is assumed to appear in the presence of an excess of negative charge. The determination rules are proposed on the basis of the following two postulates: (i) the coordination number of a positive element of a metal oxide, C1, and that of a second metal oxide, C2, are maintained even when mixed. (ii) The coordination number of a negative element (oxygen) of a major component oxide is retained for all the oxygens in a binary oxide.
According to the Tanabe hypothesis, a binary oxide always shows only one type of acidity (Brønsted or Lewis type) or no acidity (when there is no excess of charge). Actually most binary oxides simultaneously exhibit Brønsted and Lewis acidities. So we are confused by this bottleneck problem, because the Tanabe hypothesis is not able to explain this phenomenon. As seen in the model structures pictured according to postulates (i) and (ii), the Tanabe hypothesis emphasizes the single oxide content and the coordination number, but ignores the significant effect of material structure of the whole binary oxide. Even the material structures of two binary oxides that possess the same chemical composition may be completely different to each other. In materials science, besides the intrinsic properties determined by chemical composition, all other properties are mainly dependent on material structure.
To the best of our knowledge, an acid site is a micro-region concept, and can even be viewed as a point or center concept. Therefore the micro-region concept of an acid site is not applicable to large ranges such as the whole surface acidity of a binary oxide. Furthermore, the surface acidity of a binary oxide should not be simply regarded as the acidity sum of every acid site, because two acid sites may possess different acid types. If we have to put the micro-region acid site concept into a wide range application, such as the whole surface acidity of a binary oxide, the misplaced conception will induce an incorrect conclusion.
As stated above, the Tanabe hypothesis is still not universally applicable for all the real chemically-mixed binary oxides. Of course, if every binary oxide possessed a uniform composition and ideal homogeneous structure just as the model structures pictured according to postulates (i) and (ii), there is no doubt that the Tanabe hypothesis would be universally applicable for every binary oxide. But actually such an ideal binary oxide is very rare, even almost non-existent. The solid-phase structure of a real binary oxide is complex, usually a coexistence of homogeneous and heterogeneous phases. During calcination of binary oxides, single component oxide crystallizations usually exist in binary oxides. In this case, the original minor component oxide can also form a new binary oxide in a micro-region, where the minor original component oxide has become a major component oxide.
As we know, the major structural difference between crystal and amorphous is that a crystal has a long-range order structure, but an amorphous solid possesses a short-range order and long-range disorder structure. Thereby, for a real binary oxide system, it can be regarded that a binary oxide is made of large numbers of micro-regions, and each micro-region possesses a perfect homogeneous structure and uniform composition, just like a short-range order structure. In this case, the micro-region agrees well with the model structures pictured according to postulates (i) and (ii), and an acid site is formed by an excess of negative or positive charge in a micro-region.
In view of the limitations of Tanabe's hypothesis, taking into account the significant effect of material structure and the micro-concept of acid sites, on the basis of the Tanabe hypothesis, the current author proposes a new hypothesis of micro-region acid sites regarding the surface acidity of binary oxides, such as follows:
(I) The acid site generation of a binary oxide is formed by an excess of negative or positive charge in a micro-region with perfect homogeneous structure and uniform composition.
(II) Lewis acidity is assumed to appear in the presence of an excess of positive charge, Brønsted acidity is assumed to appear in the presence of an excess of negative charge.
(III) For a real binary oxide AOx–BOy (where AOx is a major component oxide) as depicted in Fig. 1, besides the major micro-regions of AOx–BOy (where AOx is a major component oxide), there may also exist minor micro-regions of BOy–AOx (where BOy is a major component oxide).
(IV) The major acidity of a binary oxide is dependent on the total acid amount of the major micro-regions.
The above determination rules are also proposed on the following two postulates:
(i) The coordination number of a positive element of a single oxide, C1, and that of a second metal oxide, C2, are maintained even when mixed.
(ii) The coordination number of a negative element (oxygen) of a major component oxide is retained for all the oxygens in a micro-region of a binary oxide.
For example, the structures of TiO2–SiO2 binary oxide, where TiO2 is the major component oxide, are shown in Fig. 2(a) and (b).
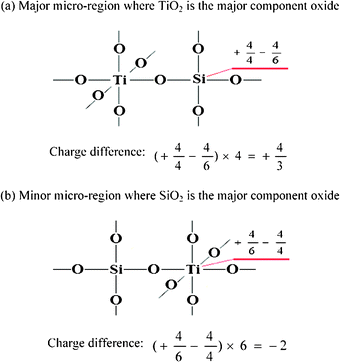 | ||
| Fig. 2 Model structures of TiO2–SiO2 (where TiO2 is the major component oxide) pictured according to my postulates (i) and (ii). | ||
According to the current new hypothesis, for the major micro-region where TiO2 is the major component oxide, the charge difference for one bond is +4/4 − 4/6 = +1/3, and for all the bonds the valence unit of +1/3 × 4 = +4/3 is the excess. In this case, Lewis acidity is assumed to appear upon the presence of an excess of negative charge. For the minor micro-region where SiO2 is the major component oxide, the charge difference for one bond is +4/6 − 4/4 = −1/3, and for all the bonds the valence unit of −1/3 × 6 = −2 is the excess. In this case, Brønsted acidity is assumed to appear upon the presence of an excess of positive charge.
As stated above, there are Lewis acid sites and Brønsted acid sites that simultaneously exist in the TiO2–SiO2 binary oxide. Due to TiO2 being the major component oxide, the proportion of the micro-regions of TiO2–SiO2 is bigger than that of the micro-regions of SiO2–TiO2. So the total acid amount of the micro-regions where TiO2 is the major component oxide is larger than that of the micro-regions where SiO2 is the major component oxide; this further illustrates that the major acidity of TiO2–SiO2 binary oxide (where TiO2 is the major component oxide) exhibits Lewis acidity, but TiO2–SiO2 binary oxide also simultaneously possesses certain Brønsted acidity. Doolin et al.2 have demonstrated that both Lewis and Brønsted acid sites simultaneously exist on the investigated TiO2–SiO2 binary oxide by using DRIFTS of adsorbed pyridine, and the Lewis acidity is the major acidity.
Let us examine another example of a SiO2–ZrO2 binary oxide. The structures of SiO2–ZrO2, where SiO2 is the major component oxide, are shown in Fig. 3(a) and (b).
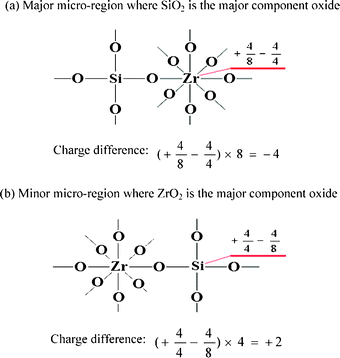 | ||
| Fig. 3 Model structures of SiO2–ZrO2 (where SiO2 is the major component oxide) pictured according to my postulates (i) and (ii). | ||
According to the current new hypothesis, for the major micro-regions where SiO2 is the major component oxide, the charge difference for one bond is +4/8 − 4/4 = −1/2, and for all the bonds the valence unit of −1/2 × 8 = −4 is the excess. In this case, Brønsted acidity is assumed to appear in the presence of an excess of negative charge. For the minor micro-regions where ZrO2 is the major component oxide, the charge difference for one bond is +4/4 − 4/8 = +1/2, and for all the bonds the valence unit of +1/2 × 4 = +2 is the excess. In this case, Lewis acidity is assumed to appear in the presence of an excess of positive charge.
As stated above, there are Brønsted acid sites and Lewis acid sites that simultaneously exist in the SiO2–ZrO2 binary oxide. Since SiO2 is the major component oxide, the proportion of the micro-regions of SiO2–ZrO2 is bigger than that of the micro-regions of ZrO2–SiO2. So the total acid amount of the micro-regions where SiO2 is the major component oxide is larger than that of the micro-regions where ZrO2 is the major component oxide, further illustrating that the major acidity of SiO2–ZrO2 binary oxide (where TiO2 is the major component oxide) exhibits Brønsted acidity, but SiO2–ZrO2 binary oxide also simultaneously possesses certain Lewis acidity. Anderson et al.3 have already confirmed that SiO2–ZrO2 binary oxide simultaneously possesses both Brønsted and Lewis acid sites by using IR spectra of adsorbed pyridine, and the Brønsted acid site density shows a maximum for the sample with 20 mol% ZrO2.
In order to further verify the validity of the current new hypothesis, we have examined the surface acidity of 20 types of binary oxides as shown in Table 1. The case where the Tanabe hypothesis predicts that a binary oxide should generate acidity (Brønsted acid sites or Lewis acid sites) is marked by a B or L in the fourth column of Table 1. The case where the current new hypothesis predicts that a binary oxide should generate acidity (Brønsted acid sites and/or Lewis acid sites) is marked by B and/or L in the fifth column of Table 1, and the major acid site is marked in bold font. The experimental results cited from the literature are presented in the sixth column. Cases where the result predicted by the hypothesis agrees with one of the experimental results are marked with 50%, and cases that completely agree with the experimental results are marked with 100%, and cases of disagreement are marked with 0. As seen in Table 1, an agreement of the prediction of Tanabe's hypothesis with the experimental results is found at 50%. Accordingly, the validity of Tanabe's hypothesis is 50%. But the agreement of the prediction of the current new hypothesis with the experimental results is found at 100%. Accordingly, the validity of the current new hypothesis is 100%. Therefore, it has been verified that the current new hypothesis is much better and more applicable for real binary oxides.
| Binary oxides | α = V/Ca | Acidity site type | Validity of hypotheses | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Major | Minor | α major | α minor | Hypotheses | Experimental results | Reference | Tanabe | This paper | |
| Tanabe | This paper | ||||||||
| a V: valence of positive element, C: coordination number of positive element. | |||||||||
| WO3–ZrO2 | 6/6 | 4/8 | B | B, L | B, L | 4 | 50% | 100% | |
| TiO2–ZrO2 | 4/6 | 4/8 | B | B, L | B, L | 5 | 50% | 100% | |
| TiO2–Al2O3 | 4/6 | 3/6 | B | B, L | B, L | 6 | 50% | 100% | |
| Al2O3–TiO2 | 3/6 | 4/6 | L | L, B | L, B | 7 | 50% | 100% | |
| MgO–Al2O3 | 2/6 | 3/6 | L | L, B | L, B | 8 | 50% | 100% | |
| MoO3–Sb2O4 | 6/6 | 4/6 | B | B, L | B, L | 9 | 50% | 100% | |
| Sb2O4–V2O5 | 4/6 | 5/6 | L | L, B | L, B | 10 | 50% | 100% | |
| SiO2–Sb2O3 | 4/4 | 3/6 | B | B, L | B, L | 11 | 50% | 100% | |
| SiO2–Ta2O5 | 4/4 | 5/6 | B | B, L | B, L | 12 | 50% | 100% | |
| SiO2–SnO2 | 4/4 | 4/6 | B | B, L | B, L | 13 | 50% | 100% | |
| SiO2–MgO | 4/4 | 2/6 | B | B, L | B, L | 14 | 50% | 100% | |
| SiO2–Al2O3 | 4/4 | 3/6 | B | B, L | B, L | 14 | 50% | 100% | |
| SiO2–ZrO2 | 4/4 | 4/8 | B | B, L | B, L | 15 | 50% | 100% | |
| SnO2–SiO2 | 4/6 | 4/4 | L | L, B | L, B | 16 | 50% | 100% | |
| TiO2–SiO2 | 4/6 | 4/4 | L | L, B | L, B | 2 | 50% | 100% | |
| TiO2–V2O5 | 4/6 | 5/6 | L | L, B | L, B | 17 | 50% | 100% | |
| SiO2–Ga2O3 | 4/4 | 3/6 | B | B, L | B, L | 18 | 50% | 100% | |
| SiO2–Sc2O3 | 4/4 | 3/6 | B | B, L | B, L | 18 | 50% | 100% | |
| Nb2O5–MoO3 | 5/6 | 6/6 | L | L, B | L, B | 19 | 50% | 100% | |
| Nb2O5–WO3 | 5/6 | 6/6 | L | L, B | L, B | 19 | 50% | 100% | |
Additionally, sometimes we meet a special case where there is no excess charge on the surface of a binary oxide according to Tanabe's hypothesis and the current new hypothesis. In this case, the binary oxide should have no surface acidity, but actually the binary oxide still exhibits certain acidity. For the example of Fe2O3–Cr2O3, the structures of Fe2O3–Cr2O3 binary oxide, where Fe2O3 is the major component oxide, are shown in Fig. 4(a) and (b).
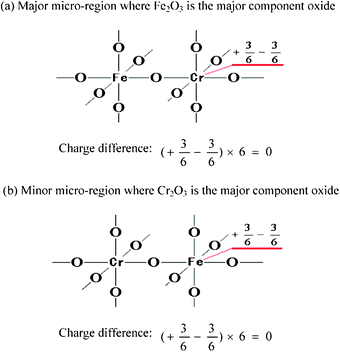 | ||
| Fig. 4 Model structures of Fe2O3–Cr2O3 (where Fe2O3 is the major component oxide) pictured according to postulates (i) and (ii). | ||
In Fe2O3–Cr2O3, there is no excess charge in any part of its composition according to the model structure written by postulates (i) and (ii), as illustrated in Fig. 4. Therefore, the binary oxide is not expected to show any acidic property. However, this prediction does not agree with the experimental result that both Brønsted and Lewis acid centers are detected on the surface of the investigated Fe2O3–Cr2O3 binary oxide.20 Why? The major reason is that there often exist some defects in a real binary oxide, including structural defects and non-stoichiometric defects. The structural defects (oxygen vacancies, lattice defects, impurity atoms and so on) and non-stoichiometric defects can both induce a coordination number change and cause excesses of charge at defect centers; accordingly acid sites are formed. Moreover, if the cation radii of two single oxides are very different to each other, the two single oxides cannot form a continuous solid solution in their binary oxide. However, the perfect Fe2O3–Cr2O3 binary oxide should have no surface acidity.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21106071), the New Teachers' Fund for Doctor Stations Sponsored by the Ministry of Education of China (No. 20113221120004), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Research Subject of Environmental Protection Department of Jiangsu Province of China (NO. 201016).References
- K. Tanabe, T. Sumiyoshi, K. Shibata, T. Kiyoura and J. Kitagawa, Bull. Chem. Soc. Jpn., 1974, 47, 1064–1066 CrossRef CAS.
- P. K. Doolin, S. Alerasool, D. J. Zalewski and J.F. Hoffman, Catal. Lett., 1994, 25, 209–223 CrossRef CAS.
- J. A. Anderson, C. Fergusson, I. Rodrfguez-Ramos and A. Guerrero-Ruiz, J. Catal., 2000, 192, 344–354 CrossRef CAS.
- G. H. Jing, J. H. Li, D. Yang and J. M. Hao, Appl. Catal., B, 2009, 91, 123–134 CrossRef CAS.
- C. Lahousse, A. Aboulayt, F. Maugé, J. Bachelier and J. C. Lavalley, J. Mol. Catal., 1993, 84, 283–297 CrossRef CAS.
- C. Lahousse, F. Mauge, J. Bacheiier and J. C. Lavalley, J. Chem. Soc., Faraday Trans., 1995, 91, 2907–2912 RSC.
- J. A. Montoya, J. M. Dominguez, J. Navarrete, I. Shifter, T. Viveros, D. Chadwick and K. Zheng, J. Sol-Gel Sci. Technol., 1994, 2, 431–435 CrossRef CAS.
- J. H. Zhang, X. L. Zhou and J. A. Wang, J. Mol. Catal. A: Chem., 2006, 247, 222–226 CrossRef CAS.
- B. Zhou and K. T. Chuang, J. Chem. Soc., Faraday Trans., 1991, 87, 3695–3702 RSC.
- H. Golinska, P. Decyk, M. Ziolek, J. Kujawa and E. Filipek, Catal. Today, 2009, 142, 175–180 CrossRef CAS.
- E. S. Ribeiro, S. L. P. Dias, Y. Gushikem and L. T. Kubota, Electrochim. Acta, 2004, 49, 829–834 CrossRef CAS.
- G. Guiu and P. Grange, J. Catal., 1995, 156, 132–138 CrossRef CAS.
- W. S. Cardoso, M. S. P. Francisco, A. M. S. Lucho and Y. Gushikem, Solid State Ionics, 2004, 167, 165–173 CrossRef CAS.
- A. Satsuma, Y. Kamiya, Y. Westi and T. Hattori, Appl. Catal., A, 2000, 194–195, 253–263 CrossRef CAS.
- C. Flego, L. Carluccio, C. Rizzo and C. Perego, Catal. Commun., 2001, 2, 43–48 CrossRef CAS.
- P. Salas, J. G. Hernandez, E. Lopez-Salinas, I. Schifter, M. E. Llanos, J. Navarrete, J. Morales and D.F. Mexico, J. Porous Mater., 1996, 3, 241–245 CrossRef CAS.
- N. V. Economidis, D. A. Peña and P. G. Smirniotis, Appl. Catal., B, 1999, 23, 123–134 CrossRef CAS.
- G. Connell and J. A. Dumesic, J. Catal., 1987, 105, 285–298 CrossRef CAS.
- K. Yamashita, M. Hirano, K. Okumura and M. Niwa, Catal. Today, 2006, 118, 385–391 CrossRef CAS.
- F. Domka, A. Basii-Iska, W. Przystajko and R. Fiedorow, Surf. Technol., 1984, 21, 101–108 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |