Electrochemical measurement of the DNA bases adenine and guanine at surfactant-free graphene modified electrodes†
Edward P.
Randviir
and
Craig E.
Banks
*
Faculty of Science and Engineering, School of Chemistry and the Environment, Division of Chemistry and Environmental Science, Manchester Metropolitan University, Chester Street, Manchester, M1 5GD, Lancashire, UK. E-mail: c.banks@mmu.ac.uk; Fax: ++(0)1612476831; Tel: ++(0)1612471196 Web: www.craigbanksresearch.com
First published on 24th May 2012
Abstract
The electrochemical oxidation of adenine and guanine is studied in aqueous media using commercially available, high purity graphene which is free from surfactants and has not been chemically modified in any way, and is contrasted with edge plane pyrolytic graphite (EPPG), basal plane pyrolytic graphite (BPPG), and graphite modified electrodes. In terms of graphene modified electrodes towards the electrochemical oxidation of adenine, the observed voltammetric response is reduced, in terms of the peak height with the electrochemical oxidation potential occurring at higher oxidation potentials than the underlying BPPG electrode. In comparison, control experiments utilising graphite modified electrodes display an improvement in the voltammetric signal and reduced oxidation potentials are observed compared to the bare BPPG underlying electrode. Such a response in addition to that observed at EPPG and BPPG confirms that the density of edge plane sites are critical, which is in strong agreement with current literature reports. The reduced response at the graphene modified electrode is thus due to graphene having a low density of electroactive (edge plane) sites, given its unique structure. In the case of the electrochemical oxidation of guanine, graphene modified electrodes interestingly exhibit a lower voltammetric response in terms of peak height compared to the underlying electrode, but interestingly exhibit a reduction in the oxidation potential compared to the underlying BPPG electrode. Such a response is a consequence of the unique structure of graphene since it has a large basal plane composition for the adsorption of guanine which, according to literature reports, adsorbs readily on basal plane sites. However, graphene unfortunately has a low density of edge plane sites which accounts for the reduced voltammetric response. Additionally, pH dependence studies performed on both adenine and guanine utilising graphene modified BPPG electrodes reveal an equal number of protons and electrons transferred, suggesting graphene does not change the electrochemical mechanism prior to the chemically irreversible step compared to that observed at graphitic electrodes. Critically, the electrode surface modification with graphene is found to be analytically unacceptable; this coupled with the reduction in the overall electrode kinetics from graphene's low density of edge plane sites questions its future use in the reliable sensing of DNA bases.
Introduction
Following the reported unique electronic properties of graphene,1 many beneficial reports have emerged describing its unique electrochemical properties which could hold potential applications in a myriad of areas, including super-capacitors,2 batteries,3 fuel cells4 and biosensors for molecules such as ascorbic acid,5,6 uric acid,5,7 dopamine,5,8 acetaminophen,9 and DNA bases.10,11 However going against current literature reports are both Pumera12 and Brownson13 who have independently reported that graphene may not be such a beneficial electrode material as first thought. Pumera et al. reported that single-, few- and multi-layer graphene offers no significant advantage over graphite for the detection of uric acid in terms of sensitivity,12 whilst Brownson13 reports that graphene exhibits slow electron transfer kinetics due to its low proportion of edge plane sites.In terms of the electrochemical detection of DNA, where its fundamental role within biological systems is clearly evident such that fast and efficient methods are required for its analysis, one commonly employed strategy is the label free oxidation of target bases where the signals of guanine and adenine are measured following hybridization. Note however that the reproducible electrochemical detection of DNA bases is an on-going activity. In this pursuit, a variety of electrode substrates have been explored such as mercury,14 metallic15,16 and a variety of carbon electrodes including carbon screen printed electrodes17 and carbon nanotube modified electrodes.18,19 Tomschik et al.14 report adenine and cytosine exhibiting reduction waves at a mercury hanging drop electrode; yet interestingly, guanine exhibits oxidation waves at the same electrode. This work also reports adsorptive components of the waves, as does Compton et al.15,20 whose papers focus upon various types of graphitic electrodes. Screen printed carbon electrodes (SPE) have also been utilised in the sensing of DNA bases by Hart et al.17 who report that screen printed electrodes can be utilised effectively for the electrochemical sensing of both adenine and guanine dissolved in aqueous alkaline solutions.17 In addition to the above examples, carbon nanotube modified graphitic electrodes have been shown to increase the peak currents towards guanine18,19 compared to the bare underlying electrode which is consistent with the nanotubes having a greater density of edge plane sites, coupled with the basal-like walls of the carbon nanotubes, which according to Compton et al. combine to give an improved electrochemical response towards guanine.20
More recently, DNA sensing has been extended to the case of graphene. For instance, Dong et al.10 utilised graphene modified glassy carbon electrodes to detect short oligomers with known base sequences quickly and effectively without the need for chemical labelling or hybridization. Their paper10 elegantly demonstrates that a chemically reduced graphene oxide film immobilized upon a glassy carbon electrode exhibits larger and sharper peak currents compared to both bare glassy carbon and graphite modified glassy carbon electrodes using differential pulse voltammetry (DPV). Another paper by Dubuisson et al.21 reports that epitaxially grown graphene on silica enhances the peak currents compared to that of highly ordered pyrolytic graphite (HOPG); again measured using DPV. Pumera et al.22 have also explored the sensing of DNA bases utilising graphene, reporting that graphene modified electrodes exhibit an improved electrochemical response over electrodes such as GC, EPPG, multi-walled carbon nanotubes and graphite modified glassy carbon electrodes. It has been reported that for adenine, the density of edge plane sites are critical with an edge plane pyrolytic graphite (EPPG) electrode exhibiting the highest peak current.15 Conversely for guanine, there are two dominating and controlling factors: the density of edge plane sites required for electrochemical oxidation; and the density of basal plane sites at which guanine may adsorb.20 In addition to this, Husale et al. have reported that ssDNA selectively adsorbs onto mechanically exfoliated graphene via a π–π stacking mechanism as determined by AFM.23 It has been demonstrated that graphene is anisotropic with respect to electron transfer such that its side resembles that of basal plane graphite (slow electron transfer) while its edge resembles that of edge plane graphite (fast electron transfer).13,24 As a consequence of the structure of graphene we intuitively expect the electrochemical oxidation of guanine to be of use, or even beneficial, given the high proportion of basal sites available for adsorption of the DNA base. As discussed above, the literature reports focusing on the application of DNA sensing utilising graphene suggest that it is indeed a beneficial electrode material for the range of graphene materials studied above.
In this paper we explore the electrochemical oxidation of adenine and guanine using commercially available graphene which is free from surfactants and to the best of our knowledge, has not previously been explored towards these highly important target analytes. We find surprisingly that guanine exhibits a reduction in peak potential upon the introduction of surfactant-free graphene on a basal plane pyrolytic graphite (BPPG) surface compared to that of bare BPPG; while there appears to be no significant benefit of using graphene modified electrodes for the electrochemical detection of adenine compared to graphitic electrode substrates and graphite modified electrodes.13,24 Additionally, in the case of graphene modified electrodes towards the sensing of adenine and guanine, the observed high % relative standard deviation (% RSD) values compared to unmodified electrode substrates potentially limit the use of graphene modified electrodes for the reliable electrochemical sensing of DNA.
Experimental
All chemicals used, unless otherwise stated, were of analytical grade and were used as received without any further purification and were obtained from Sigma-Aldrich. All solutions were prepared with deionised water of resistivity not less than 18.2 MΩ cm and were rigorously degassed with high purity, oxygen free nitrogen prior to electrochemical measurements.Voltammetric measurements were carried out using a Palmsens (Palm Instruments BV, The Netherlands) potentiostat. All measurements were conducted using a three electrode system. The EPPG working electrode (Le Carbone, Ltd. Sussex, U.K) was machined into a 4.9 mm diameter, with the disc face parallel with the edge plane as required from a slab of HOPG (highest grade available: SPI-1, equivalent to Union Carbide's ZYA grade, with a lateral grain size, Lα of 1–10 μm and 0.4 ± 0.1° mosaic spread); alternatively, the BPPG working electrode (Le Carbone, Ltd. Sussex, U.K) was machined as above however with the disc face parallel to the basal plane as required. A platinum wire and a saturated calomel electrode (SCE) were used as the auxiliary and reference electrodes, respectively.
The graphene was commercially obtained from ‘Graphene Supermarket’25 (Reading, MA, USA), and are known as ‘Pristine Graphene Monolayer Flakes’, comprising entirely of pristine graphene platelets dispersed in ethanol (solution was 10 μg per 10 mL) that have not been oxidised, reduced, or chemically modified in any way and are free from surfactants. The graphene was synthesized via the substrate-free gas-phase method, as previously reported,26–28 and were sonicated in ethanol to form a homogeneous suspension before being dispatched.28 The graphene has an average flake thickness of 0.35 nm (1 monolayer) with an average particle (lateral) size of 550 nm (150–3000 nm). XPS chemical analyses were performed with a VG-Microtech Multilab electron spectrometer and revealed the material to comprise of 95.04% atomic carbon and 4.96% atomic oxygen; the low O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio indicates near true graphene (see ESI† for further information regarding the commercially received graphene). The graphite utilised was 99.99% synthetic graphite powder obtained from Sigma-Aldrich, with a particle diameter of < 150 μm. The graphite particles were suspended in a solution of 50
C ratio indicates near true graphene (see ESI† for further information regarding the commercially received graphene). The graphite utilised was 99.99% synthetic graphite powder obtained from Sigma-Aldrich, with a particle diameter of < 150 μm. The graphite particles were suspended in a solution of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 water and ethanol to a concentration of 10 μg per 10 mL; the same concentration as the commercially received graphene solution. The electrodes were modified with graphene/graphite by pipetting aliquots of the relevant amount of solution on the surface of the electrode, followed by drying in an oven at a temperature of 50 °C for 5 min. The electrodes were cooled to room temperature before experiments were run.
50 water and ethanol to a concentration of 10 μg per 10 mL; the same concentration as the commercially received graphene solution. The electrodes were modified with graphene/graphite by pipetting aliquots of the relevant amount of solution on the surface of the electrode, followed by drying in an oven at a temperature of 50 °C for 5 min. The electrodes were cooled to room temperature before experiments were run.
The adenine and guanine solutions were prepared according to previous literature reports.15,20 A 0.5 mM stock solution of adenine was prepared in pH 7.2 PBS (50 mM KH2PO4, 50 mM K2HPO4 and 0.1 M KCl) and kept below 5 °C in the dark. The guanine solution was prepared by saturating pH 7.2 PBS (50 mM KH2PO4, 50 mM K2HPO4 and 0.1 M KCl) with guanine and subjected to vigorous stirring using a vortex for 2 min. The solution was cooled below 5 °C then filtered to remove excess guanine. UV/Vis spectrophotometry confirmed the guanine concentration to be ∼19 μM (using data from UV/Vis Atlas29).
Results and discussion
Electrochemical oxidation of adenine
We first consider the electrochemical oxidation of 0.1 mM adenine in pH 7.2 PBS using EPPG and BPPG electrodes. Fig. 1A displays typical cyclic voltammetric profiles where a large sharp peak is observed at + 0.95 V (vs. SCE) at the EPPG electrode, while at the BPPG electrode the voltammetric peak occurs at + 1.03 V (vs. SCE). Both electrochemical responses are in excellent agreement with independent literature reports15,30 where it has been reported that the density of edge plane sites upon the electrode surface greatly influenced the electrochemical response of adenine.15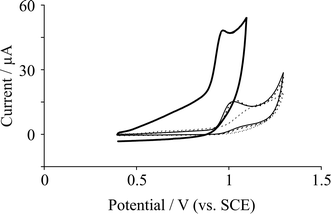 | ||
| Fig. 1 Cyclic voltammetric response arising from the electrochemical oxidation of 0.1 mM adenine in pH 7.2 PBS at various electrode substrates: EPPG (solid line); BPPG (dashed line); 10 ng surfactant-free graphene modified BPPG (dotted line); and 10 ng graphite modified BPPG (thin solid line). Scan rate: 50 mV s−1. | ||
Attention was turned to exploring the electrochemical response of adenine utilising the commercially received graphene. Fig. 1A depicts a typical voltammetric profile from the immobilisation of graphene upon a BPPG electrode where in comparison to the supporting underlying BPPG electrode, a shift to higher oxidation potentials is observed for the case of graphene to ca. +1.2 V (vs. SCE) and also a vast broadening of the peak is observed. For comparative purposes, the response of a graphite modified BPPG electrode towards the electrochemical oxidation of adenine was explored, where as shown in Fig. 1, a small reduction in the oxidation potential from introducing graphite is evident compared to graphene and BPPG. It is known that the electrochemical oxidation of adenine is highly dependent on the density of edge plane like sites/defects15 and thus the underlying BPPG electrode has a low edge plane site density and therefore a response at higher oxidation potentials is observed compared to EPPG. It therefore comes as no surprise that the oxidation potentials are reduced upon the introduction of graphite to the BPPG electrode surface, since the former has a high density of edge plane sites.
The effect of pH on the electrochemical oxidation of adenine was next explored. Using 10 ng graphene immobilised upon a BPPG electrode, the effect of changing the solution pH upon the voltammetric response was investigated. Fig. 2 depicts the observed cyclic voltammetric response with analysis of the peak potential corresponding to the electrochemical oxidation of adenine as a function of solution pH displayed inset in Fig. 2. A linear shift in the peak potential with respect to increasing pH is found to exhibit a gradient of 66 mV, suggesting an equal proton and electron transfer. This is in strong agreement with independent literature reports conducted utilising bare EPPG electrodes, which yielded a gradient of 58 mV.15 The electrochemical oxidation of adenine at graphitic electrode substrates is reported to be an overall process involving 6 protons and 6 electrodes with irreversible chemical steps.15 The observed shift in peak potential as a function of pH at the graphene modified electrode suggests that for the oxidation of adenine, the number of protons and electrons transferred prior to the chemically irreversible step is equal, which is identical to that observed on EPPG electrodes,15 suggesting no difference in the electrochemical mechanism observed using graphene.
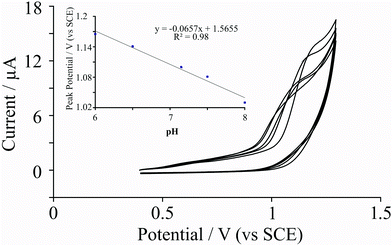 | ||
| Fig. 2 Cyclic voltammetric responses arising from the electrochemical oxidation of 0.1 mM adenine with respect to changes in pH using a 10 ng surfactant-free graphene modified BPPG electrode. Inset, analysis of observed peak potential as a function of the solution pH which yields a linear response with a gradient corresponding to ∼66 mV. Scan rate: 50 mV s−1. | ||
Fig. 3 depicts the effect of graphene coverage supported upon a BPPG electrode and for comparative purposes, the effect of increasing amounts of graphite. As depicted in Fig. 3A, additions of graphene result in the voltammetric oxidation peak shifting to higher potentials, where, upon the modification of 20 ng graphene, the oxidation peak is observed at +1.2 V (vs. SCE) as opposed to +1.03 V (vs. SCE) observed at bare BPPG in Fig. 1. Analysis of the voltammetric peak current, as shown in Fig. 3B, changes upon the addition of graphene and graphite on the electrode surface where the peak current decreases from ca. 9 μA at a bare BPPG electrode to ca. 1 μA at a BPPG electrode modified with 20 ng graphene. The % RSD (N = 5) observed at the bare BPPG was found to be 22%, inferring that the detection of adenine even with a non-graphene modified BPPG electrode is analytically unacceptable. The % RSD (N = 5) for the modifications with graphene are as follows: 7.5% for 5 ng modification; 19% for 10 ng modification; and 32% for 20 ng modification. Interestingly, the addition of graphite upon a BPPG surface enhances the observed average peak currents; which is consistent with the increase in edge plane density upon the electrode surface (see earlier). The peaks are sharper and less broad for the graphite modified electrodes, which we attribute to the larger amount of edge plane sites at the electrode surface which graphite offers compared to bare BPPG. In terms of sensitivity, graphite modified electrodes yield very good analytical reproducibility, as shown in Fig. 3B, and surprisingly the % RSD measurements (N = 5) taken for graphite are far lower than bare BPPG: 22% for bare BPPG; 4.5% for 5 ng; 6% for 10 ng; and 3.5% for 20 ng. In summary, the graphite modified BPPG electrode was found to be the most useful electrode for the effective electrochemical detection of adenine.
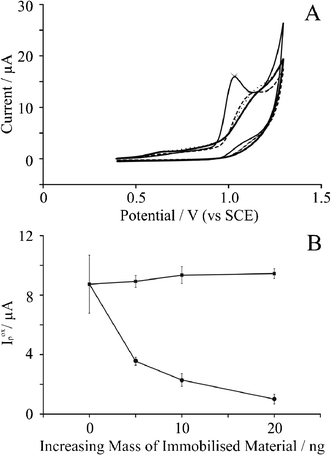 | ||
| Fig. 3 A: Cyclic voltammetric responses resulting from the electrochemical oxidation of 0.1 mM adenine in pH 7.2 PBS at (thin solid line) bare BPPG; (dashed line) BPPG modified with 5 ng surfactant-free graphene; (dotted line) BPPG modified with 10 ng surfactant-free graphene; and (thick solid line) BPPG modified with 20 ng surfactant-free graphene. All scan rates are 50 mV s−1. B: Observed voltammetric peak current as a function of the increasing amount of immobilised material for (squares) graphite modified BPPG and (circles) graphene modified BPPG. The error bars quoted are the standard deviations calculated (N = 5). | ||
It is known that graphene is anisotropic with respect to electron transfer such that basal sites exhibit slow electron transfer while its edge plane sites exhibit fast electron transfer,13,24 and it has been proposed that graphene stacks upon a BPPG surface in a non-covalent π–π fashion.13 Additionally it has been reported that adenine is significantly influenced by the density of edge plane sites,15 that is, solely its electronic structure rather than contribution from oxygenated species. Thus it is likely that the immobilised graphene orientates parallel to the underlying electrode surface in a manner in which the basal plane sides of the graphene are exposed, rather than the edge plane sides, consequently meaning that the overall percentage of edge plane sites on the modified electrode surface are low,13 which accounts for the poor voltammetry observed in the case of graphene modified electrodes. Interestingly this response is quite different to that observed by Goh and Pumera31 who report that graphene modified electrodes exhibit an improved response compared to the underlying graphitic electrode substrate. The graphene used in that study is fabricated using surfactants where the latter might contribute to the observed response, rather than the graphene itself as de-convoluted in other cases.32 It is likely that the surfactant changes the orientation of the immobilised graphene such that more edge plane sites are exposed32,33 or from the fact that they are in “Zone II” as described by Brownson et al.13 where multi-layers of graphene have immobilised such that the voltammetric response is due to graphene acting akin to that of graphite; we note that coverage studies in Goh and Pumera's paper31 are clearly lacking.
Electrochemical oxidation of guanine
The electrochemical oxidation of 19 μM guanine in pH 7.2 PBS was next considered using EPPG and BPPG electrodes which were immersed in guanine solution for one minute prior to measurement, as recommended in previous studies.20 Typical voltammetric responses obtained for the electrochemical oxidation of guanine at various electrode substrates are displayed in Fig. 4A where the resultant potential due to the oxidation of guanine is +0.73 V (vs. SCE) for bare BPPG. The observed potential at a bare BPPG electrode is in strong agreement with Compton et al.20 Upon increasing the accumulation time to 30 minutes, the peak potential with a BPPG electrode remained the same, while the peak potential resulting from the electrochemical oxidation of guanine with a graphene modified BPPG decreased by 15 mV. However, the peak current resulting from the electrochemical oxidation of guanine increased with bare BPPG, while with a graphene modified BPPG electrode the peak current decreased.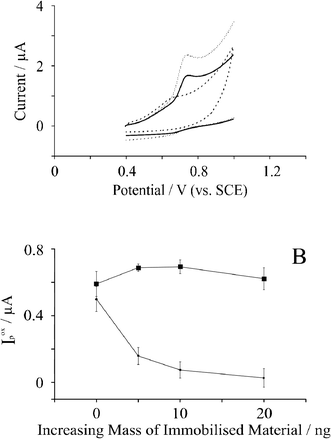 | ||
| Fig. 4 A: Cyclic voltammetric response resulting from the electrochemical oxidation of 19 μM guanine in pH 7.2 PBS utilising bare BPPG (solid line); BPPG modified with 5 ng surfactant-free graphene (dashed line); and BPPG modified with 5 ng graphite (dotted line). B: Observed voltammetric peak current (Ip) as a function of the increasing mass of immobilised material for (circles) graphite modified BPPG; and (squares) graphene modified BPPG. The error bars quoted are the standard deviations calculated (N = 5). | ||
Attention was then turned to exploring the electrochemical response of guanine at graphene modified electrodes. Fig. 4A also compares a graphene modified electrode with a bare BPPG electrode and careful inspection reveals a reduction in the oxidation potential from +0.73 V (vs. SCE) observed at a BPPG electrode to +0.64 V (vs. SCE) following the modification with 5 ng surfactant-free graphene; note however that the voltammetric peak is greatly reduced. Fig. 4B shows the effect of increasing the amounts of graphene where a reduction in the voltammetric peak height is evident. Note that it was found that the voltammetric potential did not change. Authoritative work by Compton20 has demonstrated that there are two dominating and controlling factors present contributing to the electrochemical response of guanine upon graphitic surfaces: one being the proportion of basal sites for guanine adsorption; and the second being the edge plane sites available for electrochemical oxidation.20 We believe that this is the case for graphene on the electrode surface, such that the large proportion of basal sites promotes adsorption of guanine but unfortunately the proportion of edge plane sites required for electron transfer is low, due to graphene's unique structure, giving the voltammetry observed in Fig. 4.
The effect of pH on the electrochemical oxidation of guanine was next explored. Using 10 ng graphene immobilized upon a BPPG electrode, the effect of changing the solution pH upon the voltammetric potential corresponding to the electrochemical oxidation of guanine was investigated. Fig. 5 depicts the observed cyclic voltammetric responses with analysis of peak potential versus solution pH displayed as an inset to Fig. 5. A linear shift in peak potential with respect to increasing pH is evident, exhibiting a gradient of 55 mV, suggesting an equal proton and electron transfer; this value is in excellent agreement with independent reports by Hart et al.17 and Compton et al.20 The effect of scan rate was also explored using the graphene modified electrode where a plot of peak current as a function of the square-root of scan rate was constructed where a vaguely linear response was observed at slow scan rates with deviation at higher scan rates. Note that in our case, the reproducibility exhibited by graphene is a key issue that likely inhibits reliable mechanistic information to be derived in the case of adenine and guanine.
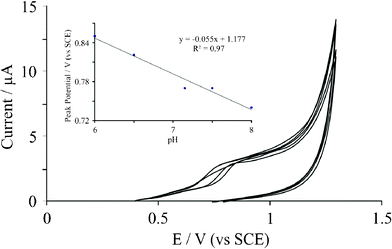 | ||
| Fig. 5 Cyclic voltammetric responses resulting from the electrochemical oxidation of 19 μM guanine with respect to changes in the solution pH. Inset: observed peak potential as a function of solution pH which yields a linear response with a gradient corresponding to ∼55 mV. Scan rate: 50 mV s−1. | ||
In addition to the reduction in overpotentials, the peak currents decrease about ten-fold from ca. 0.5 μA at a bare BPPG electrode to ca. 0.05 μA at a 20 ng surfactant-free graphene modified BPPG electrode, and are presented in Fig. 4B. Noticeably for graphite, the opposite trend is observed. As shown in Fig. 4B, the observed error bars are larger than for adenine, and the % RSD values (bare BPPG 13%; 5 ng modification 16%; 10 ng modification 34%; 20 ng modification 38%) are far larger than what would be analytically acceptable (typically less than 5%). As is also seen for adenine, the peak current observed increases with increasing amount of graphite immobilised on the electrode surface. The % RSD measurements yield largely unacceptable reproducibility: 13% for bare BPPG; 3% for 5 ng graphite; 6% for 10 ng graphite; and 10.5% for 20 ng graphite. We note that in previous literature utilising graphene containing surfactant that the reproducibility of the electrode modification towards adenine and guanine was not reported quantitatively as % RSD values31 and thus comparisons of sensitivity utilising surfactant containing graphene cannot be made.
Conclusions
This article has investigated, for the first time, the use of commercially available surfactant free graphene towards the electrochemical detection of the DNA bases adenine and guanine. It has been shown that in the case of adenine, the introduction of graphene on a BPPG electrode surface increases the peak potential observed in the electrochemical oxidation compared to the peak observed at a bare BPPG electrode and additionally reduces the magnitude of the voltammetric peak current compared to the bare underlying BPPG electrode. Additionally for adenine, we show that the electron transfer mechanism for the electrochemical oxidation of adenine remains unchanged upon the introduction of graphene to a bare BPPG surface.In stark contrast to the findings for adenine, we find that the introduction of graphene on a BPPG surfaces reduces the observed overpotential resulting from the electrochemical oxidation of guanine compared to that observed at a bare BPPG electrode. We attribute this to the large amount of basal sites that graphene offers for the effective adsorption of guanine towards the electrode surface. Conversely, the peak currents are found to decrease upon the addition of graphene at a BPPG electrode surface which leads us to conclude that graphene offers very little in the way of edge plane sites which are reported to be required for the efficient electron transfer at graphitic electrode surfaces. Since pristine graphene has been utilised, that is, graphene with no defects, vacancies, edge plane sites, dangling bonds etc. improvements in the electroanalytical signal could be obtained using graphene with many defects across the basal plane surface but yet enough basal sites for sufficient adsorption to occur.
In comparison to recent a literature report22 using double, few and multi-layer graphene modified electrodes towards the sensing of adenine and guanine, it was observed that, for guanine, the analytical sensitivity (slope of a response versus concentration plot) was found to occur in the order: few layer > double layer > mutli-layer. We surmise in that study, the adsorption of guanine upon the basal plane sites of graphene also occurs, where the amount of edge plane sites giving rise to the observed (analytical) order such that double layer graphene exhibits a response which is improved by the few layer graphene, where there is a greater proportion of edge plane sites available over the former giving rise to an improved analytical response. When multi-layer graphene is introduced, the orientation is such that the available edge plane sites are greatly reduced giving rise to the worse response. We note that the graphene used in that study contains surfactants which will dominate the orientation/assembly of the graphene.
In the case of our surfactant free commercially available graphene, critically, a poor level of reproducibility is observed when using graphene modified electrodes towards the electrochemical sensing of adenine and guanine but interestingly an acceptable level of reproducibility is observed when using graphite modified electrodes. The use of this particular type of graphene is questionable as an electrode material for the sensing of DNA bases; such observations will benefit those constructing sensors for the electrochemical detection of DNA damage.
References
- K. S. Novoselov, A. M. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubunos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS.
- M. D. Stoller, S. J. Park, Y. W. Zhu, J. H. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498 CrossRef CAS.
- E. Yoo, J. Kim, E. Hosono, H. Zhou, T. Kudo and I. Honma, Nano Lett., 2008, 8, 2277 CrossRef CAS.
- B. Seger and P. V. Kamat, J. Phys. Chem. C, 2009, 113, 7990 CAS.
- D. A. C. Brownson, M. Gomez-Mingót and C. E. Banks, Phys. Chem. Chem. Phys., 2011, 13, 20284 RSC.
- F. Li, J. Li, Y. Feng, L. Yang and Z. Du, Sens. Actuators, B, 2011, 157, 110 CrossRef CAS.
- Y. Xue, H. Zhao, Z. Wu, X. Li, Y. He and Z. Yuan, Biosens. Bioelectron., 2011, 29, 102 CrossRef CAS.
- P. Si, H. Chen, P. Kannan and D. H. Kim, Analyst, 2011, 136, 5134 RSC.
- X. Kang, J. Wang, H. Wu, J. Liu, I. A. Aksay and Y. Lin, Talanta, 2010, 81, 754 CrossRef CAS.
- M. Zhou, Y. Zhai and S. Dong, Anal. Chem., 2009, 81, 5603 CrossRef CAS.
- V. C. Diculescu, A. C. Paquim and A. M. O. Brett, Sensors, 2005, 5, 377 CrossRef CAS.
- M. S. Goh and M. Pumera, Anal. Chem., 2010, 82, 8367 CrossRef CAS.
- D. A. C. Brownson, L. J. Munro, D. K. Kampouris and C. E. Banks, RSC Adv., 2011, 1, 978 RSC.
- M. Tomschik, F. Jelen, L. Havran, L. Trnková, P. E. Nielsen and E. Paleek, J. Electroanal. Chem., 1999, 476, 71 CrossRef CAS.
- L. M. Gonçalves, C. Batchelor-McAuley, A. A. Barros and R. G. Compton, J. Phys. Chem., 2010, 114, 14213 Search PubMed.
- E. E. Ferapontova and E. Dominguez, Electroanalysis, 2003, 15, 629 CrossRef CAS.
- K. C. Honeychurch, M. R. O'Donovan and J. P. Hart, Biosens. Bioelectron., 2007, 22, 2057 CrossRef CAS.
- J. Wang, A. Kawde and M. Musameh, Analyst, 2003, 128, 912 RSC.
- A. Erdem, P. Papakonstantinou and H. Murphy, Anal. Chem., 2006, 78, 6656 CrossRef CAS.
- Q. Li, C. Batchelor-McAuley and R. G. Compton, J. Phys. Chem., 2010, 114, 7423 CAS.
- E. Dubuisson, Z. Yang and K. P. Loh, Anal. Chem., 2011, 83, 2452 CrossRef CAS.
- A. Ambrosi and M. Pumera, Phys. Chem. Chem. Phys., 2010, 12, 8943 RSC.
- S. Husale, S. Sahoo, A. Radenovic, F. Traversi, P. Annibale and A. Kis, Langmuir, 2010, 26, 18078 CrossRef CAS.
- D. K. Kampouris and C. E. Banks, Chem. Commun., 2010, 46, 8986 RSC.
- https://graphene-supermarket.com/ , date of access 04/01/12.
- A. Dato, V. Radmilovic, Z. Lee, J. Phillips and M. Frenklach, Nano Lett., 2008, 8, 2012 CrossRef CAS.
- A. Dato, Z. Lee, K. J. Jeon, R. Erni, V. Radmilovic, T. J. Richardson and M. Frenklach, Chem. Commun., 2009, 6095 RSC.
- Z. Lee, K. J. Jeon, A. Dato, R. Erni, T. J. Richardson, M. Frenklach and V. Radmilovic, Nano Lett., 2009, 9, 3365 CrossRef CAS.
- H. Perkampus, UV-Vis Atlas of organic compounds: Part 2 Spectra D1/1 M-19, Weinheim, New YorkVCH, 1992 Search PubMed.
- K. Ito, K. Hashimoto and Y. Ishimori, Kobunshi Ronbunshu, 1994, 51, 173S CrossRef.
- M. S. Goh and M. Pumera, Anal. Chim. Acta, 2012, 711, 29 CrossRef CAS.
- D. A. C. Brownson, J. P. Metters, D. K. Kampouris and C. E. Banks, Electroanalysis, 2011, 23, 894 CrossRef CAS.
- D. A. C. Brownson and C. E. Banks, Analyst, 2011, 136, 2084 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20173a |
| This journal is © The Royal Society of Chemistry 2012 |
