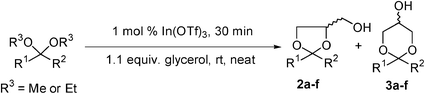
Metal triflate catalysed acetal exchange reactions of glycerol under solvent-free conditions
Brendan M.
Smith
,
Tomasz M.
Kubczyk
and
Andrew E.
Graham
*
Department of Science and Sport, University of Glamorgan, Pontypridd, CF37 4AT, UK. E-mail: AEGraham@Glamorgan.ac.uk
First published on 21st February 2012
Abstract
Catalytic quantities of indium(III) triflate (In(OTf)3) efficiently promote transacetalisation reactions of glycerol with acyclic acetals to generate the corresponding cyclic acetals under solvent-free reaction conditions. The protocol is rapid and employs very mild reaction conditions. Unreactive ketones, such as acetophenone or benzophenone, undergo efficient acetalisation in the presence of In(OTf)3 and trimethyl orthoformate (TMOF) to give high conversions of the corresponding glycerol acetals.
Introduction
Glycerol is produced as a by-product in the transesterification of vegetable oils and animal fats in biodiesel production, or by fermentation of biomass,1 and while a number of established uses for this glycol exists, the large quantities of glycerol produced during biodiesel manufacture in particular cannot be absorbed by these existing markets. Glycerol is therefore an ideal candidate as a renewable and highly flexible feedstock chemical, given its wide availability and biosustainable production, its rich chemical functionality, and its low toxicity and biodegradability. It is therefore unsurprising that processes for the valorisation of glycerol have attracted considerable recent interest.2 The generation of acetals and ketals, derived from glycerol is an emerging area of importance that has attracted considerable recent attention, given the potential of these materials as fuel additives and surfactants.3 Approaches employing transacetalisation protocols are an attractive route to access these materials, since the exchange process produces methanol as the only by-product of the reaction. In contrast, acetalisation reactions employing carbonyl compounds produce quantities of water that can have a deleterious effect on catalysts and the overall position of the equilibrium. These limitations have been partially addressed by use of co-solvents for azeotropic removal of water, however, the solvents typically employed have a considerable environmental impact, and typically require high temperatures and extended reaction times.4 An additional benefit of employing acetals in acetal exchange reactions is that they are stable to oxidation, and therefore contain no traces of the corresponding carboxylic acids which have been identified as promoters of acetalisation and which require the use of inert atmospheres and anhydrous conditions.We recently disclosed that indium(III) triflate (In(OTf)3) is a highly efficient Lewis acid catalyst for the formation of cyclic acetals and ketals from acyclic acetals in a rapid and very clean transacetalisation process.5 These reactions do not require prolonged reaction times, high temperatures, azeotropic removal of water or employ a large excess of one of the reagents,6 and produces a product that requires minimal purification. Furthermore, we have demonstrated that this protocol can be extended to reactions under solvent-free conditions without any impact on overall yields or rates, further enhancing the efficiency and environmental profile of this transformation (Scheme 1).7
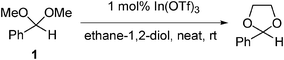 | ||
| Scheme 1 Indium triflate mediated acetal exchange reaction under solvent-free conditions. | ||
We have now extended our work in this area to consider the use of metal triflate salts as catalysts for transacetalisation reactions involving glycerol to demonstrate their application in processes for the valorisation of glycerol.
Results and discussion
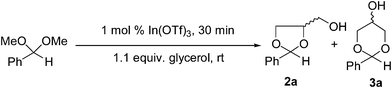
Initial studies considered the reactions of benzaldehyde dimethyl acetal (BDMA) 1 with glycerol under our original solvent-free reaction conditions employing low loadings of the In(OTf)3 catalyst. We were gratified to observe that 1 underwent a highly efficient exchange reaction to provide a mixture of the cis-and trans-cyclic dioxolane 2a and cis-and trans-1,3-dioxane products 3a with excellent overall conversions (Table 1, entry 1). The final product distribution of these reactions is determined by a temperature-dependent acid catalysed equilibration in a two-stage reaction mechanism.4a,8 In the first, kinetically controlled stage, formation of the dioxolane product is rapid and favoured over formation of the dioxane product. In the second stage, the catalyst slowly interconverts the dioxolane product into the thermodynamically more stable dioxane material giving ratios favouring this product. The final product distribution is therefore determined by a number of factors, including reaction temperature, reaction time and the catalyst employed, however, high conversions typically lead to the formation of product ratios of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a in the region of 40
3a in the region of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60. In our case, In(OTf)3 catalysed transacetalisation reactions of 1 produced product distributions comparable to previous literature reports, however, these conversions were achieved under much milder reaction conditions and in considerably shorter reaction times. Heating the reaction mixtures or extending reaction times led to only minor changes in the product ratios of 2a and 3a.
60. In our case, In(OTf)3 catalysed transacetalisation reactions of 1 produced product distributions comparable to previous literature reports, however, these conversions were achieved under much milder reaction conditions and in considerably shorter reaction times. Heating the reaction mixtures or extending reaction times led to only minor changes in the product ratios of 2a and 3a.
| Entry | Acetal | Product | Conversion (%)a | Product selectivityb ((trans)-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (cis)-2 (cis)-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (trans)-3 (trans)-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (cis)-3) (cis)-3) |
Ratio (%) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
|---|---|---|---|---|---|
| a Determined by 1H analysis of the crude reaction mixture. b Determined by 1H and GC-MS analysis of the crude reaction mixture. c 88% isolated yield. d 84% isolated yield. | |||||
| 1 |

|
2a and 3a R1 = Ph R2 = H | > 95 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62 |
| 2 |

|
2b and 3b R1 = Pent R2 = H | 85 | 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.7 4.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
| 3 |
|
2c R1 = R2 = CH3 | > 95c | — | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 4 |

|
2d R1 = R2 = (CH2)5 | > 95d | — | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Reactions of dimethyl acetals derived from aliphatic aldehydes, such as hexane dimethyl acetal (entry 2), also proceeded readily giving high conversions with product mixtures favouring the expected dioxolane product 2b. This is again explained by the two stage reaction mechanism. However, in this case the isomerisation of the dioxolane 2b to the dioxane 3b is slower than in the case of 1, due to the reduced stability of the intermediate cation formed during the isomerisation process. Reactions of acetals derived from symmetrical ketones, such as dimethoxy propane and cyclohexanone diethyl acetal (entries 3 and 4), proceeded under kinetic control and gave high conversions to the expected dioxolane products 2c and 2d with excellent selectivity. These products required minimal purification, typically involving only removal of the catalyst and unreacted glycerol, and products were obtained in excellent isolated yields.
With effective conditions established for the transacetalisation process, we next considered the use of exchange reactions employing glycerol/water mixtures. The use of glycerol/water mixtures in acetalisation or transacetalisation protocols is a highly desirable goal, given the abundance of this cheaper source of glycerol generated during biodiesel production. Few reports have addressed the effect of water on the rate of acetalisation of carbonyl compounds in glycerol valorisation procedures,4e due in part to its detrimental effect on the acetalisation catalysts typically employed, and the facile hydrolysis of acetals under aqueous conditions. We reasoned that if the rate of transacetalisation of 1 to 2a and 3a was more rapid than the competing hydrolysis reaction of either the starting material or the cyclic product acetals, then it may permit the use of glycerol solution derived from alternative sources in transacetalisation protocols. We were mindful that cyclic acetal products could be generated by acetalisation reactions of benzaldehyde generated by hydrolysis of 1 with glycerol, and with this in mind we initially investigated the reactions of benzaldehyde under our standard transacetalisation conditions. Reactions employing benzaldehyde in place of 1 gave similar product profiles with good overall conversion to dioxolane and dioxane products 2a and 3a. However, these conversions dropped to approximately 40% when a 10% glycerol/water mixture was employed, and remained at his level even after extended reaction times (Fig. 1a). Reactions employing 30% glycerol/water mixtures displayed even poorer conversions with approximately 25% of acetal products being produced in these cases. Importantly, reactions of 1 gave significantly higher conversions to 2a and 3a with 10% glycerol/water mixtures than benzaldehyde. These conversions were reduced to 50% and 40%, respectively, for the 20% and 30% glycerol/water mixtures (Fig. 1b). The balance of material in these reactions is accounted for by benzaldehyde, formed by hydrolysis of 1, and small quantities of unreacted 1. In all of these cases the overall ratios of cis- and trans-2a and cis- and trans3a were similar to those observed previously. Whilst these conversions are significantly reduced in comparison to reactions under anhydrous conditions, these experiments do suggest that the use of glycerol/water mixtures in transacetalisation procedures is an achievable goal. Studies in this area are currently underway in our laboratory to further optimise these transformations.
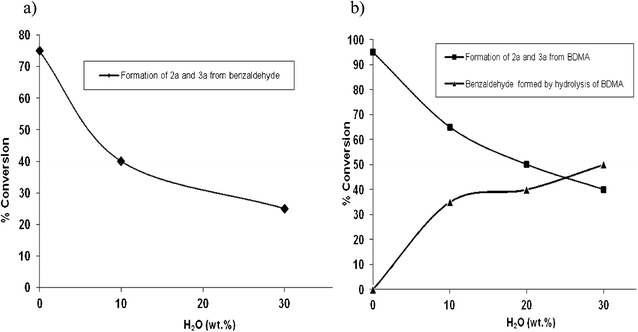 | ||
| Fig. 1 In(OTf)3 mediated acetal formation employing glycerol/water mixtures: (a) from benzaldehyde and (b) from BDMA. | ||
We next considered the reactions of 1 with glycerol in a range of solvents to determine whether the final product composition is significantly influenced by choice of solvent, and whether these distributions are significantly different from that obtained under solvent-free conditions. Solvent effects have been identified as critical in determining dioxolane and dioxane ring formation by controlling the degree of inter- and intramolecular hydrogen-bonding. In non-polar solvents, intermolecular hydrogen bonding is diminished and intramolecular hydrogen bonding is dominant, favouring the formation of the dioxane products.9 We therefore undertook a series of reactions in a range of polar and non-polar solvents under our standard reaction conditions to determine whether product distribution could be controlled simply by changing the physical properties of the reaction medium. In all cases, the cyclic acetal products were produced with good to excellent conversions. However, product distributions broadly similar to those observed under solvent-free conditions were observed (Table 2). This outcome may be explained either by concentration effects which would require the use of more dilute reaction mixtures, by the presence of quantities of methanol, produced during the reaction, adversely affecting the equilibrium or by rapid equilibration under the reaction conditions which may require the use of low reaction temperatures or decreased quantities of catalyst.
| Entry | Solvent | Conversiona (%) | Product selectivityb ((trans)-2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (cis)-2b (cis)-2b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (trans)-3a (trans)-3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (cis)-3b) (cis)-3b) |
Ratio (%) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
|---|---|---|---|---|
| a Determined by 1H analysis of the crude reaction mixture. b Determined by 1H and GC-MS analysis of the crude reaction mixture. | ||||
| 1 | Neat | >95 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62 |
| 2 | Hexane | 85 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61 61 |
| 3 | DCM | >95 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55 |
| 4 | Methanol | 65 | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 59 |
Finally, we considered the reactions of deactivated aromatic ketones with glycerol. Unreactive ketones, such as acetophenone, generally provide poor isolated yields of acyclic or cyclic acetal products due to the establishment of an equilibrium which favours the carbonyl compound.10 Reactions of ketones which are both sterically and electronically deactivated, such as benzophenone, are even less rewarding. In order to achieve acceptable yields, it is necessary to employ extended reaction times using solvents such as benzene or toluene with azeotropic removal of water, or to employ protocols that use reagents with high environmental impact.10a,d Indeed, the few reported instances where the reaction of glycerol with acetophenone has been studied reported disappointing overall conversions, and the reaction with benzophenone provided no cyclic acetal products at all.4c We recently developed a modified In(OTf)3 mediated transacetalisation protocol to successfully produce dioxane and dioxolane products from unreactive ketones under very mild reaction conditions5a and we were intrigued as to whether this procedure could be extended to access the corresponding products from glycerol. Our approach employs the diol in the presence of trimethyl orthoformate (TMOF), and it is envisaged that the reaction proceeds through an initial acetalisation reaction that generates the acyclic dimethyl acetal, followed by a subsequent acetal exchange reaction to give the cyclic acetal product. Such tandem reaction protocols have attracted considerable recent interest due to the improvements in efficiency, and the benefits of producing reactive or unstable species in situ.11 We were highly gratified to observe that, under these modified conditions, acetophenone underwent rapid reaction in the presence of glycerol, giving high conversions to the dioxolane products (trans)- and (cis)-2e (Scheme 2), with quantities of the corresponding dioxane products being identified only by GC-MS analysis. Reactions employing benzophenone were even more impressive and proceeded smoothly to give high conversions to the dioxolane product 2f in an extremely clean and facile reaction.
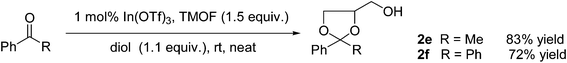 | ||
| Scheme 2 Indium triflate mediated tandem acetalisation-acetal exchange reactions. | ||
To complete these studies, we carried out a short study to identify other metal triflates that are effective promoters of transacetalisation, and to determine whether the nature of the catalyst has a significant effect on product distributions. Metal triflate catalysed reactions are currently of significant interest in synthetic chemistry, where they have found wide use as highly effective, water-stable, reuseable Lewis acids in a range of transformations.12 Of the catalysts studied, only copper(II) triflate (Cu(OTf)2) showed significant activity under solvent free conditions (Table 3, entry 4), giving a product distribution similar to In(OTf)3 albeit employing higher catalyst loadings.
| Entry | Catalyst | Loading (mol%) | Conversiona (%) | Product Selectivityb ((trans)-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (cis)-3 (cis)-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (trans)-2 (trans)-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (cis)-2) (cis)-2) |
Ratio (%) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
|---|---|---|---|---|---|
| a Determined by 1H analysis of the crude reaction mixture. b Determined by 1H and GC-MS analysis of the crude reaction mixture. | |||||
| 1 | In(OTf)3 | 1% | >98 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62 |
| 2 | Yb(OTf)3 | 5% | 23 | — | — |
| 3 | Gd(OTf)3 | 5% | 15 | — | — |
| 4 | Cu(OTf)2 | 5% | 85 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62 |
Conclusions
In conclusion, we have demonstrated that In(OTf)3 is a highly efficient promoter of the transacetalisation reactions of acyclic acetals with glycerol under extremely mild, solvent-free reaction conditions. This protocol employs low catalyst loadings and gives excellent conversions to dioxane and dioxolane products. Product ratios are similar to previous literature reports, favouring formation of the dioxane product. However, high conversions are achieved in reaction times that are considerably shorter than previous literature examples. We have demonstrated that good conversions of acetal products can be achieved in transacetalisation reactions of glycerol/water mixtures, and that these reactions proceed more rapidly than the corresponding reactions of aldehydes suggesting that it may be possible to employ glycerol solutions derived from alternative sources. In the case of acetals derived from symmetrical ketones, excellent isolated yields of dioxolane products are produced with high selectivity, and require only minimal purification to provide high isolated yields. Other metal triflate salts show moderate activities in transacetalisation reactions. However, only Cu(OTf)2 displayed significant activity for the reaction of BDMA with glycerol and required the use of higher catalyst loadings. We have further extended our studies to demonstrate that cyclic acetals can be efficiently generated from unreactive ketones, such as acetophenone and benzophenone, employing a tandem acetalisation-acetal exchange protocol under solvent-free conditions. This protocol employs TMOF in the presence of glycerol, and gives dioxolane products with high conversions without the requirement for high temperatures, high catalyst loadings, azeotropic removal of water, the use of toxic reagents or extended reaction times.Experimental section
Typical experimental procedure for the In(OTf)3 catalysed acetal exchange reaction under solvent-free conditions
In(OTf)3 (5.6 mg, 1 mol%) was added to a mixture of cyclohexanone diethyl acetal (172 mg, 1.00 mmol) and glycerol (101 mg, 1.10 mmol) and the reaction mixture was stirred at room temperature for 30 min. The reaction mixture was then passed through a short plug of silica which was washed with methanol (2 × 2 mL) and the solvent removed under reduced pressure to give the product 1,4-dioxaspiro[4,5]decane-2-methanol as a clear oil;7c1H NMR (CDCl3; 270 MHz) δ = 4.20–4.10 (1H, m), 3.95 (1H, dd, J = 6 and 8 Hz), 3.75 (1H, dd J = 6 and 8 Hz), 3.65 (1H, dd J = 5 and 11 Hz), 3.55 (1H, dd, J = 5 and 11 Hz), 2.40 (1H, br s), 1.75–1.25 (10H, m); 13C NMR (CDCl3; 67.5 MHz) δ = 109.9, 75.7, 65.4, 63.1 36.3, 34.7, 25.0, 23.9 and 23.7; νmax(film)/cm−1 (neat) 3448, 1449, 1281, 1163, 1099, 911, and 735; MS (EI) m/z 172.Typical experimental procedure for tandem acetalisation–acetal exchange under solvent-free conditions
In(OTf)3 (5.6 mg, 1 mol%) was added to a mixture of finely ground benzophenone (182 mg, 1.00 mmol), trimethyl orthoformate (159 mg, 1.50 mmol) and glycerol (101 mg, 1.10 mmol) and the reaction mixture stirred at room temperature for 2 h. The reaction mixture was then passed through a short plug of silica which was washed with hexane (2 × 2 mL) and then methanol (2 × 2 mL) and the solvent removed under reduced pressure to give the product (2,2-diphenyl-[1,3]-dioxolane-4-yl)methanol as a clear oil; 1H NMR (CDCl3; 270 MHz) δ = 7.60–7.45 (4H, m), 7.40–7.25 (6H, m), 4.35–4.25 (1H, m), 4.05–3.90 (2H, m), 3.75 (1H, dd J = 4 and 12 Hz), 3.65 (1H, dd J = 5 and 12 Hz), 1.95 (1H, br s); 13C NMR (CDCl3; 67.5 MHz) δ = 141.9, 128.3, 128.2, 128.1, 126.2, 125.9, 109.8, 76.8, 66.2 and 63.0; νmax(film)/cm−1 (neat) 3479, 1449, 1319, 1278, 1177, 1029, 912 and 640; MS (EI) m/z 256.Acknowledgements
The authors thank the Engineering and Physical Sciences Research Council (EPSRC) and the Royal Society of Chemistry for financial support, and the EPSRC National Mass Spectrometry Service, Swansea University.References
- (a) L. R. Morrison, Glycerol, Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., New York, 2001 Search PubMed; (b) Z. Z. J. Wang, J. Zhuge, H. Fang and B. A. Prior, Biotechnol. Adv., 2001, 19, 201–223 CrossRef CAS.
- (a) C.-H. Zhou, J. N. Beltramini, Y.-X. Fan and G. Q. Lu, Chem. Soc. Rev., 2008, 37, 527–549 RSC; (b) S. Papanikolaoua, S. Fakas, M. Fick, I. Chevalot, M. Galiotou-Panayotou, M. Komaitis, I. Marc and G. Aggelis, Biomass Bioenergy, 2008, 32, 60–71 CrossRef; (c) A. Behr, J. Eilting, K. Irawadi, J. Leschinski and F. Lindner, Green Chem., 2008, 10, 13–30 RSC; (d) M. Pagliaro, R. Cirimuina, H. Kimura, M. Rossi and C. D. Pina, Angew. Chem., Int. Ed., 2007, 46, 4434–4440 CrossRef CAS; (e) B. Katryniok, S. Paul, V. Bellière-Baca, P. Rey and F. Dumeignil, Green Chem., 2010, 12, 2079–2098 RSC.
- (a) J. A. Melero, R. V. Grieken, G. Morales and M. Paniagua, Energy Fuels, 2007, 21, 1782–1791 CrossRef CAS; (b) A. Piasecki, A. Sokolowski, B. Burczyk and U. Kotlewska, J. Am. Oil Chem. Soc., 1997, 74, 33–37 CrossRef CAS.
- (a) J. Deutsch, A. Martin and H. Lieske, J. Catal., 2007, 245, 428–435 CrossRef CAS; (b) S. B. Umbarkar, T. V. Kotagi, A. V. Biradar, R. Pasricha, J. Chanale, M. K. Dongare, A.-S. Mamede, C. Lancelot and E. Payen, J. Mol. Catal. A: Chem., 2009, 310, 150–158 CrossRef CAS; (c) C. Crotti, E. Farnetti and N. Guidolin, Green Chem., 2010, 12, 2225–2231 RSC; (d) C. X. A. da Silva, V. L. C. Gonçalves and C. J. Mota, Green Chem., 2009, 11, 38–41 RSC; (e) V. R. Ruiz, A. Velty, L. L. Santos, A. Leyva-Pérez, M. J. Sabater, S. Iborra and A. Corma, J. Catal., 2010, 271, 351–357 CrossRef CAS.
- (a) B. M. Smith and A. E. Graham, Tetrahedron Lett., 2011, 52, 6281–6283 CrossRef CAS; (b) B. M. Smith and A. E. Graham, Tetrahedron Lett., 2006, 47, 9317–9319 CrossRef CAS.
- (a) G. Sartori, R. Ballini, F. Bigi, G. Bosica, R. Maggi and P. Righi, Chem. Rev., 2004, 104, 199–250 CrossRef CAS; (b) K. Ishihara, Y. Karumi, M. Kubota and H. Yamamoto, Synlett, 1996, 839–841 CrossRef CAS; (c) N. M. Leonard, M. C. Oswald, D. A. Freiberg, B. A. Nattier, R. C. Smith and R. S. Mohan, J. Org. Chem., 2002, 67, 5202–5207 CrossRef CAS; (d) P. Srivastava and R. Srivastava, Catal. Commun., 2008, 9, 645–649 CrossRef CAS; (e) B. Wang, Y. Gu, G. Song, T. Yang, L. Yang and J. Suo, J. Mol. Catal. A: Chem., 2005, 233, 121–126 CrossRef CAS.
- (a) M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol and P. Machado, Chem. Rev., 2009, 109, 4140–4182 CrossRef CAS; (b) P. J. Walsh, H. M. Li and C. A. de Parrodi, Chem. Rev., 2009, 107, 2503–2545 CrossRef; (c) G. W. V. Cave, C. L. Raston and J. L. Scott, Chem. Commun., 2001, 2159–2169 RSC.
- C. Piantadosi, C. E. Anderson, E. A. Brecht and C. L. Yarbro, J. Am. Chem. Soc., 1958, 80, 6613–6617 CrossRef.
- (a) D. M. Clode, Chem. Rev., 1979, 79, 491–513 CrossRef CAS; (b) N. Baggett, J. M. Duxbury and A. B. Foster, Carbohydr. Res., 1966, 2, 216–223 CrossRef CAS.
- (a) B. T. Gregg, K. C. Golden and J. F. Quinn, Tetrahedron, 2008, 64, 3287–3295 CrossRef CAS; (b) B. Karimi and M. Ghoreishi-Nezhad, J. Mol. Catal. A: Chem., 2007, 277, 262–265 CrossRef CAS; (c) S.-J. Ji and L. Wu, J. Mol. Catal. A: Chem., 2003, 202, 41–46 CrossRef CAS; (d) M. W. C. Robinson and A. E. Graham, Tetrahedron Lett., 2007, 48, 4727–4731 CrossRef CAS; (e) S. Ueno, T. Oshima and T. Nagai, J. Org. Chem., 1984, 49, 4060–4063 CrossRef CAS.
- (a) L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS; (b) R. J. K. Taylor, M. Reid, J. Foot and S. A. Raw, Acc. Chem. Res., 2005, 38, 851–869 CrossRef CAS; (c) T. M. Kubczyk, S. M. Williams, J. R. Kean, T. E. Davies, S. H. Taylor and A. E. Graham, Green Chem., 2011, 13, 2320–2325 RSC; (d) M. W. C. Robinson, A. M. Davies, I. Mabbett, S. H. Taylor and A. E. Graham, Org. Biomol. Chem., 2009, 7, 2559–2564 RSC; (e) D. J. Phillips, K. S. Pillinger, L. Wei, A. E. Taylor and A. E. Graham, Chem. Commun., 2006, 2280–2282 RSC; (f) D. J. Phillips, K. S. Pillinger, L. Wei, A. E. Taylor and A. E. Graham, Tetrahedron, 2007, 63, 10528–10533 CrossRef CAS; (g) B. M. Smith and A. E. Graham, Tetrahedron Lett., 2007, 48, 4891–4894 CrossRef CAS; (h) D. J. Phillips and A. E. Graham, Synlett, 2008, 649–652 CAS; (i) M. W. C. Robinson, A. M. Davies, R. Buckle, I. Mabbett, D. C. Apperley, S. H. Taylor and A. E. Graham, J. Mol. Catal. A: Chem., 2009, 314, 10–14 CrossRef CAS; (j) G. H. Harris and A. E. Graham, Tetrahedron Lett., 2010, 51, 6890–6892 CrossRef CAS; (k) M. W. C. Robinson, A. M. Davies, R. Buckle, I. Mabbett, T. E. Davies, D. C. Apperley, S. H. Taylor and A. E. Graham, J. Mol. Catal. A: Chem., 2010, 329, 57–63 CrossRef CAS; (l) D. P. Phillips and A. E. Graham, Synlett, 2010, 769–773 CAS; (m) M. C. Bagley, V. Lin, D. J. Phillips and A. E. Graham, Tetrahedron Lett., 2009, 50, 6823–6825 CrossRef CAS.
- (a) S. Kobayashi, M. Sugiura, H. Kitagawa and W. W. L. Lam, Chem. Rev., 2002, 102, 2227–2302 CrossRef CAS; (b) B. D. G. Williams and M. C. Lawton, Green Chem., 2008, 10, 914–917 RSC; (c) B. M. Smith, E. J. Skellam, S. J. Oxley and A. E. Graham, Org. Biomol. Chem., 2007, 5, 1979–1982 RSC; (d) M. W. C. Robinson, K. S. Pillinger, I. Mabbett, D. A. Timms and A. E. Graham, Tetrahedron, 2010, 66, 8377–8382 CrossRef CAS; (e) B. M. Smith, S. D. Kean, M. F. Wyatt and A. E. Graham, Synlett, 2008, 1953–1956 CAS; (f) M. W. C. Robinson, K. S. Pillinger and A. E. Graham, Tetrahedron Lett., 2006, 47, 5919–5921 CrossRef CAS; (g) C. Torberg, D. D. Hughes, R. Buckle, M. W. C. Robinson, M. C. Bagley and A. E. Graham, Synth. Commun., 2008, 38, 205–211 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |