Precursor-directed formation of hollow Co3O4 nanospheres exhibiting superior lithium storage properties†
Jianmin
Ma
and
Arumugam
Manthiram
*
Electrochemical Energy Laboratory & Materials Science and Engineering Program, The University of Texas at Austin, Austin, TX 78712, United States. E-mail: rmanth@mail.utexas.edu
First published on 14th February 2012
Abstract
Hollow Co3O4 nanospheres, assembled by nanoplatelets, have been obtained by annealing urchin-like hollow Co(CO3)0.5(OH)·0.11H2O nanospheres synthesized at room temperature. The as-obtained hollow Co3O4 nanospheres exhibit greatly enhanced lithium storage properties as anode materials for lithium-ion batteries.
Lithium-ion batteries are being considered as one of the viable alternative technologies for transportation due to their high energy density and power capability.1,2 However, the commercial graphite anodes may pose serious safety concerns associated with lithium plating under the conditions of fast charge.3 In this regard, Co3O4 anodes are appealing as they operate at higher voltages and offer a much higher capacity (890 mAh g−1) than graphite (372 mAh g−1).4 However, metal oxide anodes usually suffer from poor capacity retention because of the large volume changes occurring during charge–discharge cycles.5–7 To overcome this problem, two effective strategies have been developed: one strategy is to prepare composite electrodes with other materials, such as carbon,8 carbon nanotubes,9 graphene,4,10,11 and conductive polymers.12 Another strategy is to prepare unique structures, such as hollow structures,13,14 mesoporous structures,15,16 nanowire arrays,7,17 and thin films,18,19 Particularly, the hollow structures have a higher surface area, shorter path length for lithium-ion transport, and more freedom for volume change, allowing better reaction kinetics at the electrode surface.20
Hollow nanostructures have recently attracted intense attention owing to their importance in many fields.21 Some techniques, such as hard or soft templates,22,23 sacrificial templates, and template-free methods,24,25 have been widely employed to obtain hollow structures. Among the various methods, the template-free route is relatively simple and attractive for directing the growth of hollow structures. In this communication, we present a facile route to fabricate hollow Co3O4 nanospheres by annealing urchin-like hollow Co(CO3)0.5(OH)·0.11H2O nanospheres synthesized at room temperature without any template. This synthesis route is simpler compared to the other methods employed for Co3O4 hollow structures in the literature.26–29 Interestingly, the as-obtained hollow Co3O4 nanospheres assembled by nanoplatelets display a high capacity due to their hollow structure.
Co3O4 nanomaterials are usually prepared via thermal decomposition of the precursors, such as Co(OH)2 and CoCO3. Recently, Co(CO3)0.5(OH)·0.11H2O nanobelts have been synthesized under solvothermal conditions and converted to Co3O4 nanobelts via thermal treatment at 350 °C for 4 h. Herein, we obtain urchin-like hollow Co(CO3)0.5(OH)·0.11H2O nanospheres by stirring aqueous Co(NO3)2 and NH4HCO3 solutions at room temperature. Although the product is not well-crystalline, all the diffraction peaks in Fig. 1a correspond to orthorhombic Co(CO3)0.5(OH)·0.11H2O (JCPDS card No. 8–0083). Thermogravimetric analysis (TGA) of the sample (Fig. S1†) indicates a weight loss of 27.1%, which is close to the expected weight loss of 25.6% corresponding to the conversion of the Co(CO3)0.5(OH)·0.11H2O precursor to Co3O4 by the removal of the hydroxyl and carbonate groups.30 The slight discrepancy in the weight loss could be related to adsorbed water or other species, deviation of the precursor composition or the product Co3O4 composition from the ideal formula. The hollow Co(CO3)0.5(OH)·0.11H2O nanospheres were further characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The SEM image in Fig. 2a reveals that the product is composed of many nanospheres. The magnified SEM image in Fig. 2b further reveals that the nanospheres are aggregated and hollow. The TEM image in Fig. 2c clearly reveals that the hollow structure of the Co(CO3)0.5(OH)·0.11H2O nanospheres is composed of nanorods, similar to the urchin-like structure. The magnified TEM image in Fig. 2d reveals the diameter of the nanorods in the hollow nanospheres to be ∼2 nm. To the best of our knowledge, such a special structure of Co(CO3)0.5(OH)·0.11H2O has not been reported before. The formation of the hollow nanosphere structure of Co(CO3)0.5(OH)·0.11H2O may involve a complex process as the intermediate product formed with a reaction time of 3 h appears to consist of solid nanospheres (Fig. S2†).
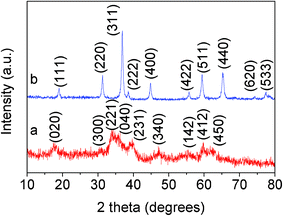 | ||
| Fig. 1 XRD patterns of the (a) Co(CO3)0.5(OH)·0.11H2O precursor and (b) Co3O4. | ||
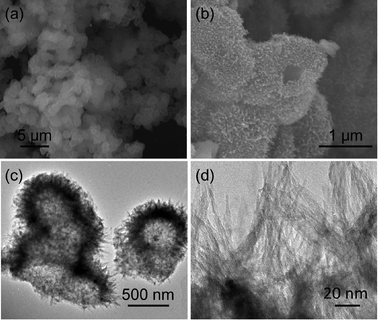 | ||
| Fig. 2 (a and b) SEM images and (c and d) TEM images of the urchin-like hollow Co(CO3)0.5(OH)·0.11H2O nanospheres. | ||
The Co3O4 nanospheres were obtained by thermal treatment of the Co(CO3)0.5(OH)·0.11H2O nanosphere precursor at 300 °C for 4 h. All the diffraction peaks in Fig. 1b could be attributed to Co3O4 (JCPDS card No. 43–1003). The aggregation of nanospheres is more obvious in the SEM image of Co3O4 in Fig. 3a compared to that of the Co(CO3)0.5(OH)·0.11H2O precursor. The TEM image in Fig. 3b further confirms that the nanospheres are fused together after annealing the precursor at high temperature. The magnified TEM images (Fig. 3c and d) reveal that the aggregated Co3O4 hollow nanospheres are composed of a number of nanoplates with a side length of ∼10 nm. In addition, the lattice fringes indicate that they are well-crystalline. Interestingly, this process is largely different from the topochemical conversion,16,30 which is mainly due to the smaller size of the nanorods of the Co(CO3)0.5(OH)·0.11H2O precursor. This indicates that the high temperature annealing makes the nanorods of the Co(CO3)0.5(OH)·0.11H2O precursor transform into the Co3O4 nanoplates via recrystallization. Based on the above facts, we can conclude that the annealing process has two effects: facilitating the decomposition of the precursor into Co3O4 and the recrystallization of the primary nanostructures from nanorods to nanoplates. Interestingly, Co3O4 can still keep the hollow structure, derived from the urchin-like hollow Co(CO3)0.5(OH)·0.11H2O nanospheres. Such a unique hollow structure with aggregated nanoplates is attractive for lithium storage due to its higher surface area, shorter path length for Li+ transport, and more freedom to accommodate volume change.
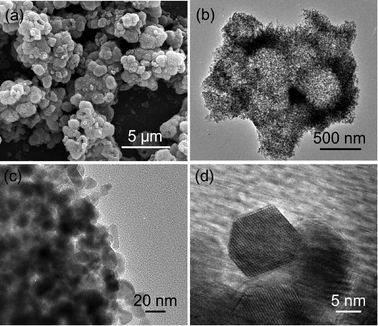 | ||
| Fig. 3 (a) SEM image, (b and c) TEM images, and (d) HRTEM image of the hollow Co3O4 nanospheres assembled by nanoplatelets. | ||
To study the electrochemical properties of the hollow Co3O4 nanospheres assembled by nanoplatelets, we assessed the Li insertion–extraction behaviour of the as-obtained sample. Fig. 4 shows the first discharge–charge profiles of the hollow Co3O4 nanospheres assembled by nanoplatelets with a cutoff voltage of 0.01 V at a current density of 90 mA g−1. The first discharge curve in Fig. 4 displays the characteristic features of nanostructured Co3O4 with two well-defined voltage plateaus at around 1.2 and 1.0 V, corresponding to the reduction of Co3+ to Co2+ and Co2+ to Co, respectively.30 It exhibits a high initial capacity of 1175 mAh g−1 with an irreversible capacity loss of 285 mAh g−1, which is normally attributed to the irreversible reactions occurring during the first discharge–charge process.31,32
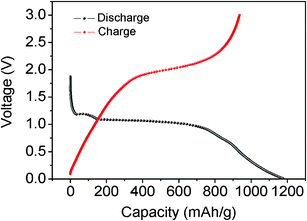 | ||
| Fig. 4 Initial electrochemical Li intercalation–deintercalation curves of the hollow Co3O4 nanospheres assembled by nanoplatelets. | ||
Fig. 5 further compares the cycle life of the hollow Co3O4 nanospheres assembled by the nanoplatelets. The capacity of the hollow Co3O4 nanospheres remains stable up to about 50 cycles and then begins to fade. After 50 cycles, it still maintains as high as 786 mAh g−1. More importantly, the Coulomb efficiency remains close to 100% after the first cycle as seen in Fig. 5. The results demonstrate that the hollow Co3O4 nanospheres exhibit good lithium storage properties with a high lithium storage capacity and comparable cycling performance with those reported in the literature for other Co3O4 nanostructures.33,34 The superior performance may be attributed to the special structure with the thin nanosheet subunits providing a fast and efficient transport of lithium ions, as well as the distinct hollow interior allowing the material to effectively buffer the stress induced during the charge–discharge process. In addition, it should be noted that the reversibility of the hollow Co3O4 nanospheres presented here is better than those of NiO and Fe2O3 nanomaterials.5,31
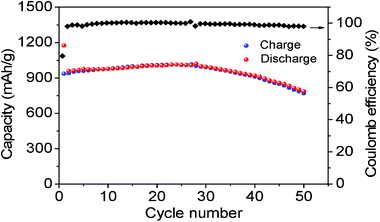 | ||
| Fig. 5 Cyclic performance and Coulomb efficiency of hollow Co3O4 nanospheres assembled by nanoplatelets. | ||
In summary, we have developed a facile solution-phase route for the preparation of urchin-like hollow Co(CO3)0.5(OH)·0.11H2O nanospheres. The urchin-like hollow Co(CO3)0.5(OH)·0.11H2O nanospheres could readily be converted into hollow Co3O4 nanospheres assembled by nanoplatelets by annealing at 300 °C. The hollow Co3O4 nanospheres exhibit high capacity and Coulombic efficiency with good cyclability, making them attractive anodes for lithium-ion batteries.
This work was supported as part of the program “Understanding Charge Separation and Transfer at Interfaces in Energy Materials (EFRC: CST)”, an Energy Frontier Research Center funded by the U.S. Department of Energy Office of Science, Office of Basic Energy Sciences, under Award Number DE-SC0001091.
References
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS.
- X. Zhang, P. N. Ross Jr., R. Kostecki, F. Kong, S. Sloop, J. B. Kerr, K. Striebel, E. J. Cairns and F. McLarnon, J. Electrochem. Soc., 2001, 148, A463 CrossRef CAS.
- B. Wang, J. S. Chen, H. B. Wu, Z. Y. Wang and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 17146 CrossRef CAS.
- Z. S. Wu, W. C. Ren, L. Wen, L. B. Gao, J. P. Zhao, Z. P. Chen, G. M. Zhou, F. Li and H. M. Cheng, ACS Nano, 2010, 4, 3187 CrossRef CAS.
- J. M. Ma, J. Q. Yang, L. F. Jiao, Y. H. Mao, T. H. Wang, X. C. Duan, J. B. Lian and W. J. Zheng, CrystEngComm, 2012, 14, 453 RSC.
- C. M. Ban, Z. C. Wu, D. T. Gillaspie, L. Chen, Y. F. Yan, J. L. Blackburn and A. C. Dillon, Adv. Mater., 2010, 22, E145 CrossRef CAS.
- Y. G. Li, B. Tan and Y. Y. Wu, Nano Lett., 2008, 8, 265 CrossRef CAS.
- J. Liu, W. Li and A. Manthiram, Chem. Commun., 2010, 46, 1437 RSC.
- P. Wu, N. Du, H. Zhang, J. X. Yu and D. R. Yang, J. Phys. Chem. C, 2010, 114, 22535 CAS.
- H. L. Wang, L. F. Cui, Y. Yang, H. S. Casalongue, J. T. Robinson, Y. Y. Liang, Y. Cui and H. J. Dai, J. Am. Chem. Soc., 2010, 132, 13978 CrossRef CAS.
- S. Q. Chen and Y. Wang, J. Mater. Chem., 2010, 20, 9735 RSC.
- Q. G. Shao, W. M. Chen, Z. H. Wang, L. Qie, L. X. Yuan, W. X. Zhang, X. L. Hua and Y. H. Huang, Electrochem. Commun., 2011, 13, 1431 CrossRef CAS.
- B. Wang, J. S. Chen, H. B. Wu, Z. Y. Wang and X. W. Lou, J. Am. Chem. Soc., 2011, 133, 17146 CrossRef CAS.
- X. Wang, X. L. Wu, Y. G. Guo, Y. T. Zhong, X. Q. Cao, Y. Ma and J. N. Yao, Adv. Funct. Mater., 2010, 20, 1680 CrossRef CAS.
- Y. Wang, C. M. Yang, W. Schmidt, B. Spliethoff, E. Bill and F. Schüth, Adv. Mater., 2005, 17, 53 CrossRef CAS.
- Y. Wang, H. Xia, L. Lu and J. Y. Lin, ACS Nano, 2010, 4, 1425 CrossRef CAS.
- Y. G. Li, B. Tan and Y. Y. Wu, J. Am. Chem. Soc., 2006, 128, 14258 CrossRef CAS.
- Y. Sun, X. Y. Feng and C. H. Chen, J. Power Sources, 2011, 196, 784 CrossRef CAS.
- H. C. Liu and S. K. Yen, J. Power Sources, 2007, 166, 478 CrossRef CAS.
- J. Liu and D. F. Xue, Nanoscale Res. Lett., 2010, 5, 1525 CrossRef CAS.
- X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS.
- F. Tao, M. Guan, Y. Jiang, J. Zhu, Z. Xu and Z. Xue, Adv. Mater., 2006, 18, 2161 CrossRef CAS.
- Z. C. Wu, K. Yu, S. D. Zhang and Y. Xie, J. Phys. Chem. C, 2008, 112, 11307 CAS.
- J. Han, Y. Liu and R. Guo, Adv. Funct. Mater., 2008, 19, 1112 CrossRef.
- M. X. Wan, Adv. Mater., 2008, 20, 2926 CrossRef CAS.
- T. He, D. R. Chen, X. L. Jiao, Y. Y. Xu and Y. X. Gu, Langmuir, 2004, 20, 8404 CrossRef CAS.
- T. He, D. R. Chen, X. L. Jiao and Y. L.Wang, Adv. Mater., 2006, 18, 1078 CrossRef CAS.
- N. Du, H. Zhang, B. D. Chen, J. B. Wu, X. Y. Ma, Z. H. Liu, Y. Q. Zhang, D. R. Yang, X. H. Huang and J. P. Tu, Adv. Mater., 2007, 19, 4505 CrossRef CAS.
- X. H. Xia, J. P. Tu, X. L. Wang, C. D. Guamd and X. B. Zhao, Chem. Commun., 2011, 47, 5786 RSC.
- C. C. Li, X. M. Yin, Q. H. Li, L. B. Chen and T. H. Wang, Chem. Eur. J., 2011, 17, 1596 CrossRef CAS.
- J. M. Ma, J. B. Lian, X. C. Duan, X. D. Liu and W. J. Zheng, J. Phys. Chem. C, 2010, 114, 10671 CAS.
- X. W. Lou, D. Deng, J. Y. Lee, J. Feng and L. A. Archer, Adv. Mater., 2008, 20, 258 CrossRef CAS.
- Y. Q. Fan, H. B. Shao, J. M. Wang, L. Liu, J. Q. Zhang and C. N. Cao, Chem. Commun., 2011, 47, 3469 RSC.
- M. W. Xu, F. Wang, M. S. Zhao, S. Yang and X. P. Song, Electrochim. Acta, 2011, 56, 4876 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20092a |
| This journal is © The Royal Society of Chemistry 2012 |
