A microfluidic platform for osmotic fragility test of red blood cells†
Lei
Li
ab,
Jing
Su
c,
Jing
Li
d,
Fei
Peng
ef,
Hongkai
Wu
*bc,
Datian
Ye
*ef and
Hongda
Chen
a
aState Key Laboratory of Integrated Optoelectronics, Institute of Semiconductors, Chinese Academy of Sciences, Beijing, 100083, China
bWPI Advanced Institute for Materials Research, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, 980-8577, Japan. E-mail: chhuwu@ust.hk
cDepartment of Chemistry, The Hong Kong University of Science & Technology, Hong Kong, China
dDepartment of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, 100191, China
eDepartment of Biomedical Engineering, Medical School, Tsinghua University, Beijing, 100084, China. E-mail: yedt6386@sz.tsinghua.edu.cn
fResearch Center of Biomedical Engineering, Graduate School at Shenzhen, Tsinghua University, Shenzhen, 518055, China
First published on 30th May 2012
Abstract
An osmotic fragility test is a useful way to determine the extent of red blood cell (RBC) hemolysis resulting from osmotic stress. A microfluidic chip-based system for measuring the osmotic fragility of RBCs has been developed. The chip was made from a Y-shaped polydimethylsiloxane (PDMS) microchannel sealed to a glass cover plate. Fresh rabbit blood diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 with an isotonic solution and pure water respectively were introduced into the long serpentine channel using two syringe pumps. Hypotonic saline solutions with three different NaCl concentrations were prepared on a chip and images of RBCs at different locations in the channel were captured. The extent of hemolysis was estimated by comparing the cell numbers in the images using an automatic image processing program. Different degrees of hemolysis (no hemolysis, partial hemolysis, and complete hemolysis) can be estimated with this platform. This device provides a promising screening platform for diseases marked by RBC abnormalities with great simplicity, high speed and minimal requirement of blood samples.
10 with an isotonic solution and pure water respectively were introduced into the long serpentine channel using two syringe pumps. Hypotonic saline solutions with three different NaCl concentrations were prepared on a chip and images of RBCs at different locations in the channel were captured. The extent of hemolysis was estimated by comparing the cell numbers in the images using an automatic image processing program. Different degrees of hemolysis (no hemolysis, partial hemolysis, and complete hemolysis) can be estimated with this platform. This device provides a promising screening platform for diseases marked by RBC abnormalities with great simplicity, high speed and minimal requirement of blood samples.
Introduction
The red blood cell osmotic fragility test is based on measuring the resistance of RBCs to lysis as a function of decreasing NaCl concentration. Certain RBC abnormalities can be detected by measuring their osmotic fragility; increased osmotic fragility typically occurs in hereditary spherocytosis and decreased osmotic fragility is a characteristic of thalassemia. Measuring the osmotic fragility of RBCs can thus yield relevant information for diagnostic purposes and fundamental research. The conventional measurement method1 uses multiple test tubes for the preparation of a series of hypotonic solutions, to which a small amount of fresh blood is added. The resultant hemolysis is measured either visually or by a photometer. Though several improved methods have been reported,2–5 most clinical laboratories still use the conventional method, which is manual and laborious. Therefore, new methods and apparatus are of great interest for a rapid, easy, and automatic osmotic fragility test.Microfluidic chip-based systems provide a possible solution that requires a relatively small amount of blood (as little as several μl). The micrometer channel dimensions of the chips are ideally suited for the introduction, manipulation, reaction, separation and detection of a small number of blood cells. Several integrated, miniature and portable microchip-based blood test devices have been previously demonstrated.6–11 A typical example is the blood typing chip,12 which successfully determines human blood groups within 3 min using only a 3 μl blood sample.
In this paper, we propose a promising microfluidic chip-based system for a rapid and automatic osmotic fragility test that requires only several microlitres of blood. Hypotonic solutions with different NaCl concentrations were generated on a single chip with a long serpentine channel. Different degrees of hemolysis (no hemolysis, part hemolysis, and complete hemolysis) were estimated by image processing and cell counting processes. One test at a single concentration will be completed in several minutes. The RBCs can be directly observed in the channel and the results can be recorded and automatically processed by a computer. The chip is highly portable and inexpensive, and thus has the potential for point-of-care (POC) medical diagnosis.
Materials and methods
Microfluidic chip design and fabrication
Fig. 1a shows a schematic diagram of the microfluidic chip used in this paper. There are two inlets to allow the blood sample and pure water to be infused into the channel. After a confluent channel there is a long squarewave serpentine channel in which the liquids mix. The serpentine channel has 40 repeated squarewave structures, as shown in the magnified part in Fig. 1. The straight channel of the structure is 4 mm long and 100 μm wide and the corner is 100 μm long and 100 μm wide. The numbers “1”, “2”, “3”, and “4” were marked beside the channels to show the location of the 10th, 20th, 30th and 40th squarewave structure. The total length of the serpentine channel is about 33 cm. After the serpentine channel, there is an outlet for the liquid flowing from the chip. The depth of all of the channels is 60 μm. The dimension of the chip is about 1 cm × 2 cm. | ||
| Fig. 1 (a) Schematic diagram of the microfluidic chip design; (b) Photograph of the chip with a coin on the right side for size comparison. | ||
The chip was made from a polydimethylsiloxane (PDMS) substrate and a glass cover plate because both materials are biocompatible, optically transparent, have low toxicity, and are cheap and easy to fabricate. The microchannel structure was fabricated in PDMS using rapid prototyping and replica molding techniques.13 Briefly, the negative relief of PDMS was formed by curing the prepolymer (Sylgard 184, Dow Corning, USA) on a silanized Si master that had a positive relief of the channels formed in photoresist (SU-8 2100, MicroChem, USA) on its surface. Holes were drilled at the inlet and outlet locations with a blunted and beveled syringe needle. Finally, an oxygen plasma treatment was performed by a Plasma Cleaner (PDC-32G, Harrick Scientific Products, Inc.) to bond the PDMS replica to a clean slide glass and form the complete microfluidic chip. The picture of the chip is shown in Fig. 1b.
Preparation of reagents and blood samples
Healthy New Zealand white rabbits (male) were purchased from the Laboratory Animal Center, Guangzhou University of Chinese Medicine, China. All experiments involving the rabbits were approved by the Institutional Animal Care and Use Committee (IACUC) of Tsinghua University, and performed in compliance with the relevant laws and institutional guidelines. Sodium heparin was purchased from Beijing Dingguo Biotechnology Co. Ltd., China. Ultra-pure water used for the preparation of solutions was produced by a Milli-Q water system (Millipore, Bedford, MA, USA). Blood was drawn from the peripheral ear vein of the rabbits using a 1 ml syringe and was collected in an EP tube. The syringe was previously wet with sodium heparin (1000 U ml−1) in phosphate-buffered saline (PBS). PBS, an isotonic solution (0.9% NaCl concentration), was used to dilute the blood. In this study, the dilution rate was 10 fold. The concentration of RBCs at this dilution ratio allowed for measurement and ensured that the channel was not blocked by cells or cruors. Considering that this dilute blood sample was lacking large serum components, bovine serum albumin (BSA, Sigma) was added at a concentration of 40 mg ml−1 since the content of albumin is 41±3 mg ml−1 in normal rabbit blood.14Experimental setup
The schematic diagram and the photo of the experimental setup are shown in Fig. 2a and 2b. The microfluidic chip was mounted onto the stage of a trinocular inverted microscope (MOTIC AE31, MOTIC, China) with a digital imaging system (Moticam2206) and software (Motic Images Plus 2.0). The captured images were 800 μm × 600 μm in size, and were displayed and stored in the computer. The resolution of the images is 800 pixels by 600 pixels.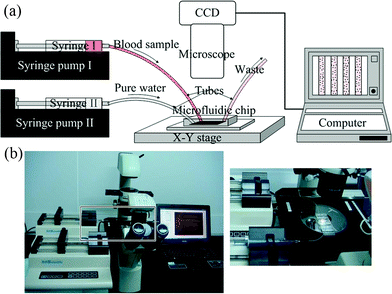 | ||
| Fig. 2 Schematic diagram (a) and the photograph (b) of the experimental setup. | ||
The blood sample and pure water were introduced using two 1 ml syringes controlled by two separate syringe pumps (KDS200, KD Scientific, USA). Two flexible plastic tubes, connected to the two syringes were inserted into the PDMS layer at the inlets for the introduction of liquids. At the end of the channel, there was an additional tube for liquid flowing out of the chip.
Experimental procedure
100 μl of a 1-in-10 dilution of whole blood in an isotonic solution and 100 μl of pure water were drawn into two syringes, the syringes were then mounted onto two syringe pumps. The combined flow rate of blood and water was maintained at 1 μl min−1. At this flow rate, the cells can pass through the chip (from inlet to outlet) in only 2 min, since the total volume of the channels is about 2.0 μl (calculated from the channel dimensions).Three typical NaCl concentrations, 0.60%, 0.45%, and 0.30%, were created by varying the flow rates of the blood sample and water. The three groups for the flow rates of the water and the blood sample were: (I) 0.33 μl min−1 and 0.66 μl min−1 respectively; (II) both 0.5 μl min−1; and (III) 0.66 μl min−1 and 0.33 μl min−1, respectively. After the cells had passed through the entire channel, the pumps were stopped and images of the cells in the channel were taken in sequence. For every group, the image of the Y-shaped junction location was taken first, and then the straight channels of the 10th, 20th, 30th and 40th squarewave structure were taken sequentially to show the change of the cells in the hypotonic saline solution. All experiments were carried out more than three times.
Image processing
The cell numbers in the 10th, 20th, 30th and 40th squarewave structure were counted automatically. Three programs were written using Matlab 7.0 software (The MathWorks, Inc.). Every recorded image (except the Y-shaped junction location) included part of four straight channels (Fig. 4b and c, and ESI† Fig. 1a). Firstly, eight channel walls for the four channels were found and then a 90 μm × 90 μm area inside the channel was randomly cropped by running the programs “crop.m” and “findedge.m” (ESI† Fig. 1b and 1c). Five 90 μm × 90 μm areas were randomly cropped in every image for statistical analysis. Secondly, the cell number in each 90 μm × 90 μm image, with resolution of 90 pixels by 90 pixels, was automatically calculated by running the program “cellcount.m”. The process is briefly described as follows: (1) convert the image to a binary image based on threshold11 (ESI† Fig. 2b); (2) eliminate spike noise using morphological operations in combination with a cell size consideration (ESI† Fig. 2c); (3) segment the image into individual areas by marker-controlled watershed segmentation12 (ESI† Fig. 2d); (4) finally, the number of cells can be counted as the number of the connected objects. All the scripts of the three programs were given in the ESI.†Results
The Y-shaped junction location
The images at the Y-shaped junction location for the three flow rate groups (Fig. 3) show that (1) the interfaces of the two liquids were clear; (2) the width ratios of the two liquids in the channel were in accordance with the flow rate ratios, which demonstrated that the volume of liquids infused in the channel could be controlled by varying the flow rates of the syringe pumps. | ||
| Fig. 3 Images at the Y-shaped junction for three flow rate regions, (a)–(c) correspond to the groups (I)–(III), the flow rates of the water and the blood sample were: (I) 0.33 μl min−1 and 0.66 μl min−1, respectively; (II) both 0.5 μl min−1; and (III) 0.66 μl min−1 and 0.33 μl min−1, respectively. | ||
Mix process
The images of the straight channel regions of the 1st, 10th, and the 20th squarewave structure in group (I) are shown in Fig. 4. Every image included part of the four straight channels. The interface of the two liquids was clear in the straight channel region of the 1st structure, and as the liquids flowed in the serpentine channel, the interface gradually disappeared and the cells became more uniformly distributed in the channel. These images show that the channel was long enough to facilitate complete mixing during the flow process. | ||
| Fig. 4 Microscopic images of the straight channel regions in group (I). (a)–(c) correspond to the 1st, 10th, and 20th squarewave structure, respectively. The image dimensions are 800 μm × 600 μm. The width of the channels is 100 μm. | ||
Cells in the channel at the three flow rate groups
The cells at different locations in the channel for the three groups were acquired by running the programs “crop.m” and “findedge.m”, and are shown in Fig. 5. Panels (a1)–(a4) were cropped from the images of the 10th, 20th, 30th and the 40th squarewave structure of group (I); panels (b1)–(b4) were from the 10th, 20th, 30th and the 40th squarewave structure of group (II); and panels (c1)–(c4) were from the 10th, 20th, 30th and the 40th squarewave structure of group (III), respectively. In Fig. 5, the change in cell numbers can be seen in groups (I), (II), and (III). The number of cells in each cropped area was counted by running the program “cellcount.m”. The false/pseudo identifications were 0–2 cells per image and the missed/unidentified RBCs were 0–3 cells per image. The proportion of mis-identified cells is very low and does not affect the judgement of the change in cell numbers. | ||
| Fig. 5 Panels (a1)–(a4) were from group (I); panels (b1)–(b4) were from group (II); and panels (c1)–(c4) were from group (III). The subscripts 1, 2, 3, 4 indicate that the image was cropped from the images of the 10th, 20th, 30th and the 40th squarewave structure, respectively. The dimension of each image is 90 μm × 90 μm. | ||
The mean cell number in the five randomly cropped areas was used as the cell number per unit at that location in one experiment. The ratio of cell number per unit at the 20th, 30th and the 40th squarewave structure location to the cell number per unit at the 10th squarewave structure location was calculated and plotted in Fig. 6(a), in which the change in the number of cells was clearly represented. Data are shown as the mean ± S.D. values, which came from both image processing and sampling. In group (I), the NaCl concentration of the mix solution was 0.60%, the number of cells hardly changed over the course of the channel. In group (II), the NaCl concentration of the mix solution was 0.45%, and the number of cells gradually decreased as the cells moved along the channel. However, some cells were still intact at the end. In group (III), the NaCl concentration was 0.30% and almost all cells had burst by the end of the channel. The same blood sample was also tested using the conventional multiple tubes method. Hemolysis began in the 0.45% tube and completed in the 0.35% tube. The results of the multiple tube method at the three typical NaCl concentrations were show in Fig. 6(b), in which no hemolysis, part hemolysis and complete hemolysis occurred in the 0.60%, 0.45% and 0.30% tubes, respectively.
 | ||
| Fig. 6 (a) Plot of the ratios of cell number per unit at the 20th, 30th and the 40th squarewave structure location versus the cell number per unit at the 10th squarewave structure location. The NaCl concentrations in the mix solution were 0.60%, 0.45%, and 0.30% in groups (I), (II), and (III), respectively. (b) The results of the conventional multiple tube method. The on-chip osmotic fragility test method and the traditional multiple tubes method demonstrated correlation at the three typical NaCl concentrations: no hemolysis (0.60%), part hemolysis (0.45%), and complete hemolysis (0.30%). | ||
Discussion
The on-chip osmotic fragility test method and the traditional multiple tubes method are based on the same principle and demonstrated correlation at three typical NaCl concentrations; no hemolysis (0.60%), part hemolysis (0.45%), and complete hemolysis (0.30%). The S.D. of the on-chip method is comparable to that of the conventional method.15 The multiple-tube method requires the preparation of a series of hypotonic solutions with NaCl concentrations ranging from 0.1% to 0.9%, to which a small amount of fresh blood is added. After centrifugation and absorbance readings at 540 nm, the percentage of hemolysis is calculated for each solution and plotted against the NaCl concentration. The resulting osmotic fragility curve is then compared with that obtained using normal controls. This process has to be done manually, errors can easily be introduced, and it is not practical for use as a screening procedure.In contrast, the on-chip method is particularly suited for use as a screening procedure for inherited diseases with RBC membrane disorders, such as thalassemia, since this method is both rapid and automatic. The NaCl solution preparation process occurs simultaneously with the mixing process of the blood sample and water in the microfluidic channel. Moreover, one test at a single concentration will be completed in several minutes, because it only takes 2 min for the cells to travel in the channel from the inlet to the outlet when the combined flow rate of blood and water was maintained at 1 μl min−1.
In principle, the on-chip method permits the preparation of a hypotonic solution with an arbitrary NaCl content ranging from 0 to 0.9% by adjusting the input flow rates of the blood sample and water. For a clinical blood sample, osmotic fragility is considered to be high if hemolysis occurs in NaCl concentrations >0.5% and is considered to be low if hemolysis does not complete in a 0.30% NaCl solution. For using as a screening procedure, there is no need to prepare many concentrations. In this paper, three critical concentrations (0.60%, 0.45% and 0.30%) were prepared and different degrees of hemolysis were tested.
After setting up the equipment (the chip, syringe pumps, microscope, camera, and image processing software), one only needs to load the blood sample, run the pumps and record the images. The on-chip method allows RBCs to be observed in the channel, which is more intuitive and may provide better information than the conventional multiple tubes method. The change in number of cells can be simply estimated by eye in most cases. Moreover, the images and videos of the experimental process and the results can be saved in computer files, which permit repeated viewing and further analysis. The programs “crop.m”, “findedge.m” and “cellcount.m” were specifically written for this study. If experiments are performed and images are recorded with other imaging equipment, one can easily adjust the cell count programs based on MATLAB software and its Image Processing Toolbox.
The design of the chip is flexible and versatile. The length, width and height of the channel can be easily changed and the process can be completed with multiple channels working in parallel. Chaotic advective mixers16 could also be used to accelerate the mixing. A microfluidic gradient generator17–19 may be useful to form a continuous concentration profile of the NaCl solution in one channel. However, it is non-trivial to introduce blood samples into the gradient without perturbing the gradient or affecting the cells before they are located at the desired spot in the channel. Moreover, the on-chip system offers great potential for integration with other miniaturized devices20,21 that aim for blood cell separation, DNA purification, and detection of intracellular constituents. The chips are small and are very cheap to manufacture so that they offer the options of both portability and disposability. Furthermore, the amount of blood needed for the sample is largely reduced to the microlitre level.
Conclusions
We have demonstrated a microfluidic chip-based platform for measuring the osmotic fragility of RBCs. Different degrees of hemolysis, i.e. no hemolysis, part hemolysis, and complete hemolysis can be detected by using this platform and the results show coherence with the conventional multiple tube method. Our chip-based method offers the advantages of fast analysis, convenient automatization and easy visualization. Moreover, the chip is portable and cost effective with great potential for point-of-care (POC) medical diagnosis.Acknowledgements
The work performed was supported by the National Basic Research Program of China (No. 2011CB933102 and No. 2011CB933203), the National Natural Science Foundation of China (No. 61078074 and No. 61178082), the China Postdoctoral Science Foundation (No.20090460498), Hong Kong RGC (No. GRF 605210), and the Shenzhen Bureau of Science Technology & Information grant (No. 041201005). We thank Prof. Haishu Ding and Dr. Lan Huang for the useful discussions of this work. We also thank Dr. Kehong Yuan, Dr. Shu Feng and Jiying Zou for the helpful discussions of the image process processing.References
- A. K. Parpart, P. B. Lorenz, E. R. Parpart, J. R. Gregg and A. M. Chase, J. Clin. Invest., 1947, 26, 636–640 CrossRef CAS.
- D. Danon, J. Clin. Pathol., 1963, 16, 377–382 CrossRef CAS.
- D. Danon, E. H. Frei, Y. F. Frei and Y. Lipkin, IEEE Trans Biomed Eng, 1963, 10, 24–28 CAS.
- J. Didelon, W. C. Blondel, P. Mazeron, S. Muller, T. Gigout, M. Gentils, G. Cauchois and J. F. Stoltz, Clin Hemorheol Microcirc, 2000, 23, 31–42 CAS.
- Y. Ito, P. Carmeci and R. Steele, Am. J. Hematol., 1977, 2, 403–412 CrossRef CAS.
- A. W. Browne, L. Ramasamy, T. P. Cripe and C. H. Ahn, Lab Chip, 2011, 11, 2440–2446 RSC.
- I. K. Dimov, L. Basabe-Desmonts, J. L. Garcia-Cordero, B. M. Ross, A. J. Ricco and L. P. Lee, Lab Chip, 2011, 11, 845–850 RSC.
- C. van Berkel, J. D. Gwyer, S. Deane, N. Green, J. Holloway, V. Hollis and H. Morgan, Lab Chip, 2011, 11, 1249–1255 RSC.
- B. S. Lee, J. N. Lee, J. M. Park, J. G. Lee, S. Kim, Y. K. Cho and C. Ko, Lab Chip, 2009, 9, 1548–1555 RSC.
- H. Y. Tan, W. K. Loke, Y. T. Tan and N. T. Nguyen, Lab Chip, 2008, 8, 885–891 RSC.
- J. P. Shelby, J. White, K. Ganesan, P. K. Rathod and D. T. Chiu, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 14618–14622 CrossRef CAS.
- D. S. Kim, S. H. Lee, C. H. Ahn, J. Y. Lee and T. H. Kwon, Lab Chip, 2006, 6, 794–802 RSC.
- J. C. McDonald, D. C. Duffy, J. R. Anderson, D. T. Chiu, H. K. Wu, O. J. A. Schueller and G. M. Whitesides, Electrophoresis, 2000, 21, 27–40 CrossRef CAS.
- Y. Mizoguchi, T. Matsuoka, H. Mizuguchi, T. Endoh, R. Kamata, K. Fukuda, T. Ishikawa and Y. Asano, Laboratory Animals, 2010, 44, 33–39 CrossRef CAS.
- R. H. Orcutt, T. S. Thurmond and K. E. Ferslew, J. Pharmacol. Toxicol. Methods, 1995, 34, 169–174 CrossRef CAS.
- A. D. Stroock, S. K. W. Dertinger, A. Ajdari, I. Mezic, H. A. Stone and G. M. Whitesides, Science, 2002, 295, 647–651 CrossRef CAS.
- N. L. Jeon, S. K. W. Dertinger, D. T. Chiu, I. S. Choi, A. D. Stroock and G. M. Whitesides, Langmuir, 2000, 16, 8311–8316 CrossRef CAS.
- S. K. W. Dertinger, D. T. Chiu, N. L. Jeon and G. M. Whitesides, Anal. Chem., 2001, 73, 1240–1246 CrossRef CAS.
- X. Jiang, J. M. Ng, A. D. Stroock, S. K. Dertinger and G. M. Whitesides, J. Am. Chem. Soc., 2003, 125, 5294–5295 CrossRef CAS.
- X. Chen, D. F. Cui, C. C. Liu, H. Li and J. Chen, Anal. Chim. Acta, 2007, 584, 237–243 CrossRef CAS.
- J. Gao, X. F. Yin and Z. L. Fang, Lab Chip, 2004, 4, 47–52 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra20051a |
| This journal is © The Royal Society of Chemistry 2012 |
