Recent advances in steroidal supramolecular gels
Hana
Svobodová
,
Virpi
Noponen
,
Erkki
Kolehmainen
and
Elina
Sievänen
*
Department of Chemistry, P.O. Box 35, FI-40014 University of Jyväskylä, Finland. E-mail: elina.i.sievanen@jyu.fi; Fax: +358 14 260 2501; Tel: +358 40 805 3710
First published on 3rd February 2012
Abstract
During the last decade or two the interest towards small molecules capable of self-assembly leading to gelation has increased intensively. The investigation of these supramolecular gels aims not only at understanding the fundamental processes underlying gel formation but also at development of new materials with a myriad of applications. Steroids are widely-spread natural products with a large and rigid steroidal nucleus combined with derivatizable functional groups leading to an adjustable polarity profile, which makes them attractive building blocks when designing novel low molecular weight gelators. Due to their unique properties, steroid-based supramolecular gels may find use in applications ranging from materials science and nanoelectronics to their application as reaction media or as sensing and responsive materials. Moreover, biomaterials based on steroidal gels may find use in biomedicine, drug delivery, regenerative medicine, and tissue engineering. This article summarizes the most recent advances in the field of steroidal supramolecular gels in terms of steroid-derived hydro- and organogels, metallogels, two-component gels, and stimuli-responsive gels. Furthermore, the potential applications of the systems are discussed.
 Hana Svobodová | Hana Svobodová was born in 1984 in Trebic, Czech Republic. She received her MSc degree in chemistry in 2008 from the Institute of Chemical Technology, Prague, Czech Republic, under the supervision of Professor Zdeněk Wimmer. Currently, she works as a PhD student in organic chemistry at the University of Jyväskylä, Finland, under the supervision of Professor Erkki Kolehmainen. She is interested in the design and synthesis of steroid-based molecules toward functional assemblies and materials. |
 Virpi Noponen | Virpi Noponen was born in 1980 in Mikkeli, Finland. She received her MSc degree in organic chemistry in 2005 from the University of Jyväskylä, Finland. Currently, she is finalizing her PhD project on amides of bile acids and biologically important small molecules under the supervision of Academy Research Fellow, Adjunct Professor Elina Sievänen. The emphasis of her PhD research is placed on the properties and applications of the prepared molecules, particularly in the fields of supramolecular gels and metal nanoparticles. |
 Erkki Kolehmainen | Erkki Kolehmainen was born in 1947 in Hankasalmi, Finland. He received his MSc degree in physical chemistry in 1973 from the University of Jyväskylä and his PhD degree in organic chemistry in 1990 from the University of Kuopio. At present Erkki Kolehmainen is working as a Professor Emeritus in the Department of Chemistry, University of Jyväskylä. His research interests are focused on the structures and dynamics of biologically interesting organic molecules (bile acids and other steroids as well as nitrogen heterocycles and their tautomerism) studied by liquid and solid state NMR spectroscopy, X-ray crystallography, and thermal analysis. |
 Elina Sievänen | Elina Sievänen (née Virtanen) was born in 1977 in Jyväskylä, Finland. She received her PhD degree in organic chemistry in 2003 from the University of Jyväskylä, under the supervision of Professor Erkki Kolehmainen. In 2004 she visited the National Centre of Biomolecular Research in Masaryk University, Brno, Czech Republic, as a postdoctoral student. In 2007 Elina Sievänen was appointed as an Adjunct Professor in organic chemistry at the University of Jyväskylä, where she currently works as an Academy Research Fellow. Her research interests focus on all aspects of steroid-based functional materials, including supramolecular gels, metal nanoparticles, and composite materials. |
Introduction
The definition of a gel is not straightforward, albeit gels are generally considered to be viscoelastic solid-like materials comprised of an elastic cross-linked network and a solvent. A three-dimensional network of entangled fibers is formed by aggregation of gelator molecules, which with a large surface area entraps and attaches the solvent molecules, resulting in an increase in viscosity.1Polymeric gels have been known for centuries and their applications in different fields, such as food, medicine, materials science, cosmetics, pharmacology, sanitation, and environmental clean-up, are renowned. Since they are formed by covalent cross-linking, they cannot be re-dissolved and are thermally irreversible. In contrast to traditional polymeric gels, supramolecular gels organize by self-assembly processes based on non-covalent interactions, including hydrogen bonding, π–π stacking, electrostatic, van der Waals, and dipole–dipole interactions as well as metal coordination. They can be transformed to a fluid (sol) by heating and are thermally reversible.1 Applications in numerous fields ranging from materials science and nanoelectronics to drug delivery and biomedicine are envisaged for supramolecular gels2–6 as a result of the intense investigation within the field during the last decade or two.
The rational design of new molecules capable of self-assembly leading to gelation has proven complicated, due to the challenging specification of conditions and structural requirements for a gelator. Structural features known to promote one-dimensional aggregation include amidic, carbamate, urea, or oxalamide groups combined with aliphatic or aromatic molecules with a large surface area, and many efficient gelators of organic solvents (organogelators) as well as water (hydrogelators) have actually been prepared by their structural combination.7
Steroids are natural products comprised of a tetracyclic ring system, to which functional groups are attached. Cholesterol typifies the fundamental structure of steroids. It is a constituent of the cell membranes and thus found in all animal tissues, where it maintains membrane fluidity, microdomain structure, and permeability. Further modifications of the stereochemistry and oxidation states of the fused rings, the side chain, as well as the functional groups of cholesterol lead to a wide variety of biologically important molecules. These include steroidal saponins, cardioactive glycosides, bile acids, and steroid hormones. Phytosterols are characterized by the presence of additional one- or two-carbon substituents attached at C-24 of the side chain, and may vary with respect of the oxidation states of the rings and/or the side chain. The widespread plant sterols, campesterol and sitosterol, respectively, are 24-methyl and 24-ethyl derivatives of cholesterol, whereas stigmasterol contains additional unsaturation of the side chain.8
The physical and chemical nature of steroids is well-known. Particularly cholesterol and the bile acids (Fig. 1) have risen as attractive starting materials for organic syntheses, due to their easily derivatized functional groups, availability, and low cost.9–11 Furthermore, the overall polarity profile of these compounds can be tailored and the self-assembling characteristics adjusted, which makes them potential components of supramolecular gels.
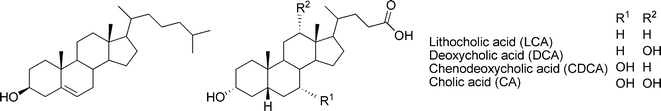 | ||
| Fig. 1 Structures of cholesterol (left) and four of the most common bile acids (right). | ||
In this article we summarize the most recent advances in the field of steroidal supramolecular gels. First, the latest achievements in the areas of steroid-based hydro- and organogels are discussed, after which the attention is focused on steroidal metallogels, two-component gels, and stimuli-responsive gels. Moreover, the potential applications of these gels are highlighted.
Hydrogels
The earliest reports on the pH-dependent gel formation of bile salts in water date back to the early 20th century.12,13 In the late 1950s it was discovered by Blow and Rich14 that under appropriate conditions sodium deoxycholate aggregated in solution forming a gelatinous helical complex of macromolecular dimensions. Forty years later, the use of sodium deoxycholate hydrogel for drug delivery applications was investigated.15 However, the number of hydrogel-forming steroidal derivatives other than bile acids/salts and their conjugates is very limited. Moreover, the majority of the recently published articles presenting bile acid-based hydrogelators seem to originate from the laboratories of Maitra and his co-workers.A tripodal cholamide 1 (Fig. 2) has turned out to be a supergelator (the minimum gel concentration is as low as 0.15 mM) of aqueous fluids. The “best” gels, meaning transparent and thermally stable, have been obtained in acetic acid–water systems ranging from 0.01 to 30% AcOH in water, depending on the gelator concentration. A variety of physical techniques, including cryo-TEM, CD, steady-state fluorescence, time-resolved fluorescence, and dynamic light-scattering, have been employed in order to understand the structure and dynamics of the gel. The studies have revealed that the molecules of 1 aggregate in an asymmetric manner to yield an entangled network structure of nanofibers having a relatively immobilized aqueous compartment. The kinetic data has indicated that this relates to the progressive ordering of the solvent molecules around the network of nanoscale fibers.16
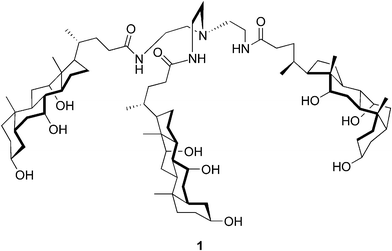 | ||
| Fig. 2 Structure of the tripodal cholamide 1. | ||
The above-mentioned gel systems have been further studied by the small-angle neutron scattering and rheometry techniques. The self-assembled systems have turned out to exhibit particular scattering and flow properties unusual in the class of molecular gels. Based on the results obtained, the authors were able to propose an aggregation model, in which three gelator molecules per cross-section of fibrillar aggregates are packed in a tail-to-tail fashion. Moreover, it has been observed that the rheological phenomenology of the solutions and gels of 1 is similar to that of DNA solutions.17 The gel formed by the tripodal cholamide has been exploited in preparing gold nanoparticle–organogel hybrid materials. A bile acid-based thiol derivative has been used as a capping agent in forming the steroidal gold nanoparticles (AuNPs). The hybrid material has been prepared by dissolving the NPs and the gelator compound first in acetic acid, and then diluting the system with water. The formed dispersion has been shown to transform into gel in 4–5 h. The steroid-derived gel has proven to be an excellent medium for stabilizing the structurally analogous AuNPs.18
A series of cationic analogues of bile salts have been reported as potent hydrogelators. Some of the compounds have been shown to form thermoreversible gels in water in the presence of NaCl. One of the compounds (2, Fig. 3) has been shown to form a stable gel in pure water at a concentration above 0.8%. According to the investigations, the use of NaCl with bile salts is essential for better gelation of aqueous solvents, since NaCl provides an ionic environment in addition to the salting out effect. It has been recognized that the concentration of NaCl has a significant influence on the gel strength of the studied bile salts. However, salt concentrations above 4 M were found to prevent the gelation process. Gelation was shown to require at least 0.1 M NaCl solution, and to obtain optically transparent hydrogels, 0.5–1 M NaCl solutions were the optimum. Gels have been obtained also in 1 M aqueous solutions of NaBr, Na2SO4, Na2CO3, NaN3, NaNO3, KCl, LiCl, and BaCl2. Some of the compounds (3–5, Fig. 3) were shown to form a gel in the presence of organic co-solvents, such as MeOH, EtOH, DMF, and DMSO (up to 20% of organic co-solvent).19
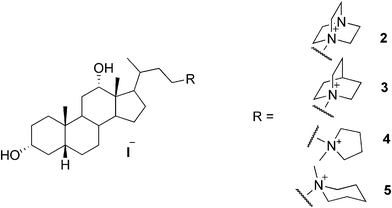 | ||
| Fig. 3 Selected examples of cationic bile salts as hydrogelators. | ||
Maitra and co-workers were the first to design and synthesize a bile acid analogue with a neutral hydrophilic side chain (6, Fig. 4) capable of self-assembly promoting gel formation. The amide conjugate of deoxycholic acid and 2-amino-2-hydroxymethyl-1,3-propanediol was shown to be insoluble in water, but in the presence of varying amounts of organic solvents, such as MeOH, EtOH, DMSO, and DMF, it was shown to form stable, thermoreversible, and transparent gels.20 An analogue of 6 (7, Fig. 4), lacking one hydroxyl group in the side chain, was also shown to form gels in aqueous mixtures of MeOH, EtOH, DMSO, and DMF (at 0.2% w/v). However, the gels of 7 in aqueous mixtures of MeOH, EtOH, and acetic acid were unstable and phase-separated to give crystals in 2–24 h. Complementary scattering, diffraction, and microscopy techniques provided a precise structural description of the network architecture and its variation as a function of concentration, aging time, solvent composition, and the type of the gelator. The diameters of the gel fibers of the gels of 6 and 7 were determined. As indicated by the results, the head-to-tail molecular arrangements were shown to be similar in the solid and gel phases.21 The structural and rheological features of a series of hydrogels formed by these gelators were scrutinized further rather recently. The results showed that even a small modification to the bile acid backbone leads to striking variations in the self-association properties (structure, stability, and topography–elasticity–strain fields in the related self-assembled fibrillar networks).22
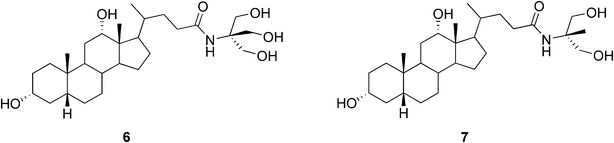 | ||
| Fig. 4 Structures of bile acid-based hydrogelators with neutral hydrophilic side chains. | ||
As an example of the exploitation of bile acid analogue-derived hydrogels as reaction vessels a photodimerization reaction has been investigated. The selectivity of the photodimerization of acenaphthylene was shown to correlate with the rigidity of the gels. However, the selectivity was shown to be lower compared to the reactions reported in other media.23
A series of 23- and 24-phosphonobile salts from cholic, deoxycholic, chenodeoxycholic, ursodeoxycholic, and lithocholic acids (Fig. 5) was prepared and the aggregation properties of the compounds investigated. All of the compounds in the series formed hydrogels at different pH values ranging from 1.7 to 7.5. The pH range at which the gelation occurred turned out to be rather narrow. As suggested by the authors, after further development this property might be useful for drug delivery and other pH-responsive systems. The critical micellar concentrations of 23- and 24-phosphonobile salts were measured using fluorescence and 31P NMR methods, the results of which were in good agreement with each other. 23-Phosphonodeoxycholate 8b was shown to form a reversible thermochromic system, which changes color upon gelation.24
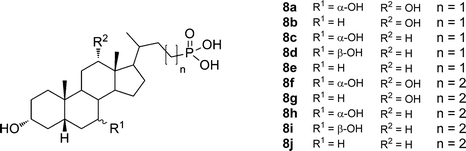 | ||
| Fig. 5 Structures of 23- and 24-phosphonobile acids. | ||
Maitra and co-workers have also introduced novel luminescent materials containing a cholic acid-based europium hydrogel, in which the lanthanide ions were an integral part of the gel matrix, and were essential for the self-assembly process.25 The sensitization of the system was increased by incorporating a hydrophobic chromophore, pyrene, into the hydrophobic gel fibers (Fig. 6). This approach provides a very simple way of making new lanthanide-derived responsive soft materials, whose luminescent properties can be modulated by incorporating suitable hydrophobic chromophores. The applicability of this idea was shown in another work, in which hydrogels based on cholic acid and different lanthanide ions were prepared.26
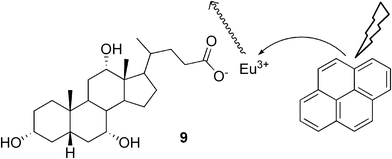 | ||
| Fig. 6 Schematic representation of energy transfer in the sensitization process in a gel system created from europium cholate and pyrene. | ||
Very recently, a novel method for the modification of known sodium deoxycholate hydrogels, their application as templates for nanomaterial synthesis, as well as their potential applications in biotechnology and drug delivery have been demonstrated. The gel crystallinity and rigidity have been shown to be enhanced in the presence of increasing concentrations of tris(hydroxymethyl)aminomethane (TRIS). The tunable hydrogel microstructures obtained under various conditions have been successfully utilized as templates to synthesize cyanine-based fluorescent nanoGUMBOS (nanoparticles from a group of uniform materials based on organic salts). The gel microstructures have been shown to direct the size as well as the molecular self-assembly of the nanomaterials, thereby tuning their spectral properties. Moreover, the release profiles of the hydrogel systems have been studied in order to evaluate their utilization for drug delivery purposes.27
The few reported hydrogelators based on cholesterol are represented by the ammonium salts of the organic diacid monoamides of cholesteryl glycinate (Fig. 7). The gels are formed only after excessive bubbling of ammonia gas through the mixture of water and the neutral cholesterol-derivative. Introducing ammonia into the system of, for example, 10a/water by slow bubbling resulted in a turbid solution. The system was shown to form a turbid, stable gel within 5 min by heating and cooling, or by ultrasonic treatment. The formed salts do not only gelate water, but also apolar solvents, making them typical ambidextrous gelators.28
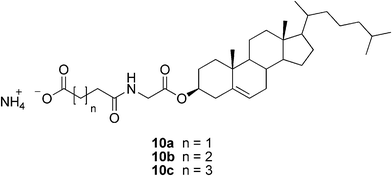 | ||
| Fig. 7 Structures of cholesterol-based salts capable of hydrogelation. | ||
A very recent and interesting report on steroidal hydrogelators described the use of dexamethasone as a part of a larger molecular hydrogelator system composed of two complementary anti-cancer drugs, namely Taxol and 10-hydroxycamptothecin (HCPT).29 This co-delivery hydrogel system might be introduced in the cavity after surgical tumor removal for the long-term release of anti-cancer drugs.
Organogels
Cholesterol-based organogels
The first rational synthesis of cholesterol-based low molecular weight organogelators was reported by Weiss and co-workers in the 90s.30,31 Since then, this class of compounds has been under intensive investigation. Cholesterol-based gelators were comprehensively reviewed by Žinić, Vögtle, and Fages in 2005,32 which is why in this review we concentrate on the most recent achievements in the field.Weiss and co-workers showed that molecular systems comprised of an aromatic moiety (A) connected to a steroidal group (S) via a functionalized linker (L) could display an effective, and in some cases predictable, gelation ability. Later on, new generations of steroidal gelators having dimeric A(LS)2- or LS2-type structures have appeared (Fig. 8).
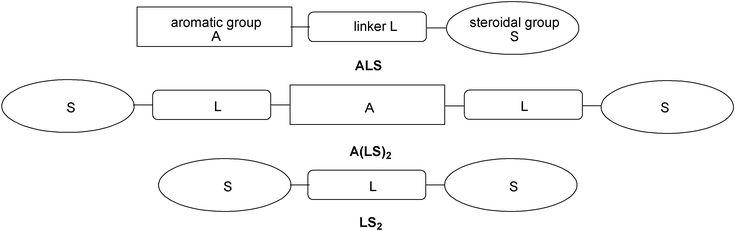 | ||
| Fig. 8 ALS, A(LS)2, and LS2 architectures of cholesterol-based gelators. | ||
Recently, Weiss and co-workers reported pioneering research aimed at investigating the kinetics of organogel formation.33,34 A combination of four techniques – circular dichroism (CD), small-angle neutron scattering (SANS), rheology, and fluorescence spectroscopy – that probe the different aspects of the aggregation process was used to follow the kinetics of the gelation of two n-alkanes by 5α-cholestan-3β-yl N-(2-naphthyl)carbamate (CNC). The gelation was shown to occur via instantaneous nucleation and one-dimensional growth, which was further supported by optical microscopy studies. The data collected indicated, that the initial nucleation – being largely dependent on the nature of the gelator, the degree of supersaturation, and heterogeneities within the system – determines the morphology of the final gel.33 Moreover, Weiss and co-workers extracted the fractal dimensions of the self-assembled fibrous networks (SAFINs) of the molecular organogel systems from the kinetic data for the first time. In addition, they showed that by varying the incubation temperature and the concentrations of the solutions containing CNC in n-alkane, the compound can be directed to form one out of two types of SAFINs corresponding to two crystal forms.34
Professor Shinkai directs another group actively involved in the research of steroidal gelators, particularly those capable of acting as templates for the creation of novel inorganic materials. In 2001 Shinkai and Jung reviewed their studies on crown-appended cholesterol gelators. The superstructures of the prepared gels could be transcribed to a silica gel affording novel inorganic materials with controlled morphologies.35 Furthermore, the gel structure of the dimeric azobenzene-appended cholesterol organogel (compound 11, Fig. 9) was shown to successfully transcribe into the silica nanotubes yielding monodisperse inner helical hollows of the silica.36 The self-assembled compound 12 (Fig. 9) was shown to exhibit a tubular structure, whereas compounds 14 and 15 formed spherical structures in acetonitrile. Thus the authors concluded that the balance between the solvophilic and solvophobic groups is important for the formation of the tubular structure by self-assembly in organic solvents. The self-assembled compound 12 was further used as a template as well as a catalyst for sol–gel polymerization of inorganic precursors to produce novel double-walled tubular structures of transition-metal oxides (TiO2, Ta2O5, and ZrO2). Moreover, this sol–gel transcription was able to give binary transition-metal oxide nanotubes of TiO2/ZrO2.37
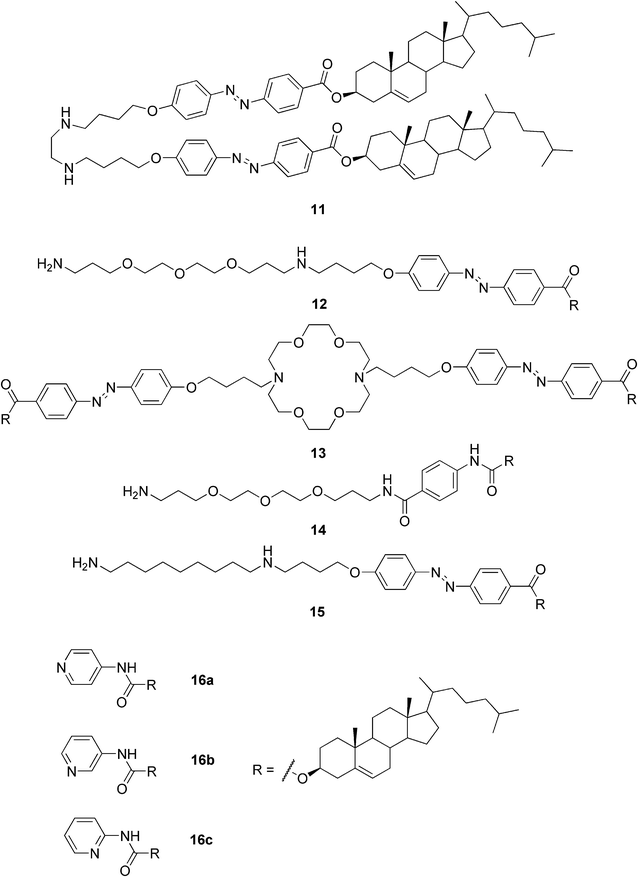 | ||
| Fig. 9 Representative examples of cholesterol-based gelators reported by Shinkai and co-workers. | ||
The Shinkai group also designed and prepared pyridine-containing cholesterol-based gelators (16a–c, Fig. 9). Compound 16a proved to be an efficient gelator capable of forming gels in 16 solvents out of the 19 studied. Three of the solvents were gelated at a gelator concentration of 0.5 wt%, making compound 16a a supergelator in those solvents. Even a solid solvent, naphthalene, was stabilized by compounds 16a and 16c. The authors showed that the morphologies of the systems strongly depended on the process of solvent removal from the gel state. By using metal–ligand interactions, for example between Ag(I) and the ligands, the tuning of the morphologies and stabilities of the gels was enabled. Moreover, the gel fibers were decorated by fluorescent molecules, like tetraphenylporphyrin Zn(II), which had photopolymerizable units at the end of the tether groups. The modified fibers were then characterized by UV-VIS absorption spectroscopy, confocal laser scanning microscopy, and TEM. Attachment of the fluorescent molecules to the gel fibers and/or tubes might find application in photochemical and electrochemical devices.38
An additional example of exploiting a steroidal organogel as a template for creating inorganic materials has been reported by Lu and co-workers. CuS nanofibers with tunable helical pitches were observed to form using an organogel of a dicholesterol derivative as a template. It was shown that the morphologies of the inorganic nanofibers could be controlled by the binding sites between the inorganic precursor and the organogel.39 Another interesting example of the applications of organogels composed of cholesterol derivatives has been reported very recently. An all-organic steroid-D–π–A modular design was shown to lead to ferroelectricity in nano-architectures constituted of organogels.40
Also Fang et al. have put a lot of effort into designing and preparing new cholesterol-based gelators along with extensively studying the formed gels. Quite recently, four novel conjugates of cholesterol and linear glucose were designed and prepared. The compounds differed from each other by the length of the linking diaminoalkyl moiety. The gelation behavior of the compounds was examined in 26 solvents at a concentration of 2.5% (w/v). All of the studied compounds showed gelation properties in both protic and aprotic solvents, and it was shown that the number of gelated solvents and the possibility of forming transparent gels increased with increasing the length of the linker chain. Three of the compounds started gelating xylene already at concentrations below 0.03% (w/v), making them possible supergelators. More interestingly, the above-mentioned compounds can be used for preparing supramolecular gel films by injecting a hot xylene solution of the gelator into a film mold and then cooling the system to room temperature. These films are stable in the wet state, and can even be slightly stretched as shown in Fig. 10.41
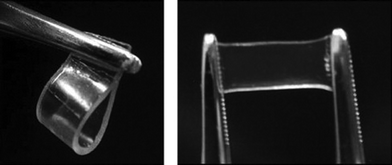 | ||
| Fig. 10 Photographs of the gel films of a conjugate of cholesterol and linear glucose in xylene.41 Reproduced by permission of Elsevier. | ||
Also, dimeric cholesterol-based compounds of the A(LS)2-type were prepared and their gelation properties extensively studied by Fang et al. They prepared a series of three gelators differing by the positions of the linkers in the benzene ring. Compounds bearing the m- and p-substituents, were shown to be more efficient gelators than the compound possessing o-substitution. Interestingly, the xylene gel of the compound with m-substitution was shown to form spontaneously at room temperature. Shaking of the gel was shown to result in a phase transition from the gel state to solution. Moreover, the gel was shown to recover when the shaking was stopped. This thixotropic behavior of the gel was further proved by rheological studies.42 Later, an analogous series of A(LS)2-type gelators was prepared and the gelation properties of the compounds investigated as well as compared with the above-mentioned studies. The difference between the two series of compounds lies in the linker moieties of the structures having in the latter case more sites for hydrogen bond formation. Numerous gel systems were shown to form spontaneously at room temperature, and also these gels possessed smart thixotropic properties as revealed by the rheological studies.43
The same group has discovered even more examples of thixotropic gels. Dicholesteryl derivatives with spacers containing two L(D)-alanine residues and 3–6 carbon atoms between them were prepared in order to investigate the effect of the length of the spacer and the chirality of the amino acid residue to the gelation properties of the compounds. Indeed, the above-mentioned features were shown to have profound effects on the gelation properties. The compounds containing D-alanine residues and shorter spacers were shown to be able to gelate more solvents than their analogues with the opposite chirality. For the compounds having longer spacers, however, an opposite result was obtained. At least 11 of the gel systems studied were shown to form gels spontaneously at room temperature. Again, the rheological studies proved the thixotropy of the gels.44 Very recently, yet another series of diacid amides of dicholesteryl L-glycinates as organogelators was reported. The length of the linker connecting the two cholesteryl residues was shown to play a crucial role in the gelation behavior of the compounds and in the nature of the microstructures of the gels.45
As the first example of cholesterol derivatives, compound 17 (Fig. 11) was shown to form a water-in-oil type gel emulsion by agitating the system at room temperature.46 Similar types of compounds, differing only in the L-alanine amino acid being changed to L-phenylalanine, have shown to be versatile organogelators (compounds 18a–b, Fig. 11). Again, many of the gel systems studied were shown to form spontaneously at room temperature. Furthermore, compounds 18a and 18b were capable of selectively gelating xylene or kerosene from their water mixtures. Gels of 18a/xylene were even shown to be mechanically strong enough for separation, thus making compound 18a a strong candidate for the practical separation of xylene from its water mixtures (Fig. 12).47
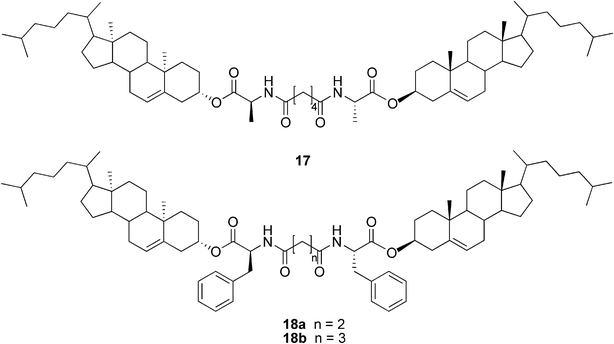 | ||
| Fig. 11 Structures of compounds 17 and 18a–b. | ||
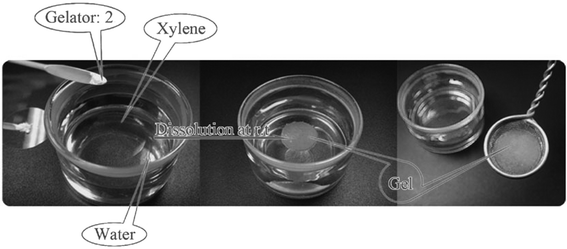 | ||
| Fig. 12 Selective gelation of xylene from its mixture with water (gelator 2 refers to compound 18a).47 Reproduced by permission of the Royal Society of Chemistry (RSC) for the Centre National de la Recherche Scientifique and the RSC. | ||
Yet another group of LS2-type dimeric cholesteryl derivatives, now having a linker with three benzene rings in addition to two amide and two carbamate groups, has been reported by Fang and co-workers. This group of compounds was shown to gelate a wide variety of organic solvents by three different ways. In addition to the traditional heating–cooling cycle, some of the gels were shown to form by mixing at room temperature or by ultrasound treatment. The studies revealed that one of the compounds was a supergelator for DMSO (0.04% w/v), and that the DMSO gel could be prepared by any of the three methods mentioned above. Moreover, the gel possesses excellent mechanical strength and smart thixotropic properties.48
The microstructures of diphenylbutadiene derivatives linked via flexible alkyl chains to one or two cholesterol units have recently been investigated by Das and co-workers. Scanning electron microscopy of the xerogels of the mono-cholesterol derivatives indicated that the molecules self-assemble to form 3D networks consisting of helically twisted fibers. However, the xerogel morphology of the bis-cholesterol derivatives indicated agglomerated spheres. Further investigation of spectroscopic properties has suggested that the morphology of the superstructures formed in these systems may be correlated to the nature of the molecular aggregates formed.49
Yi et al. have recently introduced and extensively studied the organogelation behavior of novel compounds of the ALS-type with a naphthalic unit as the aromatic group. The linker connecting the steroidal and aromatic parts varies from an alkyl chain with a variable number of acyl amino linkages to an alkyl chain with an L-alanine moiety embedded in it. The introduction of a fluorophore (naphthalic group) into the system makes it convenient to study the molecular aggregation, for example, by absorption spectroscopy and confocal scanning microscopy. The aggregation of these compounds is reversibly controlled by ultrasonic radiation, as presented later in this review.50–52
Cholesterol-based perylene derivatives have been synthesized in order to design novel visible-light-harvesting gel systems.53 The studied conjugate 19 (Fig. 13) created gels in mixtures of alcohols and aromatic solvents. It was shown that the organogels could absorb a wide range of light energy through the perylene-stacked assemblies in the gel phase. Interestingly, the gel phase (but not the sol phase) showed CD activity.
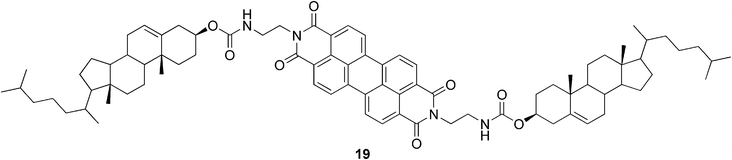 | ||
| Fig. 13 Perylene-appended cholesterol-based gelator. | ||
Although organogelators with tunable emission properties are well-known, so far only a few examples of white-light-emitting gels have been reported. One of the rare illustrations of such systems are organogelators 20 and 21 (Fig. 14).54,55 Compound 20 was found to be a weak gelator for decane, whereas compound 21 created a strong gel in the same solvent. This difference in the gelation behavior was explained as a different packing mode in the gel phase and resulted in the systems having different optical, chiroptical, and morphological properties.56 In order to investigate the light emitting properties of the gels, energy transfer in the presence and absence of a red-light-emitting acceptor 22 was studied.54,55 In the case of moderate self-assembly, a partial energy transfer of a blue-light-emitting donor to a red-light-emitting acceptor was expected and a mixture of blue, green, and red emission being generated, leading to a white-light-emitting gel. On the other hand, a strong self-assembly and complete energy transfer should lead to exclusive red emission of the acceptor. These hypotheses were proven by experiments and it was shown that the self-organization of the donor was crucial for energy transfer, suggesting this to be a way to efficiently control energy migration.
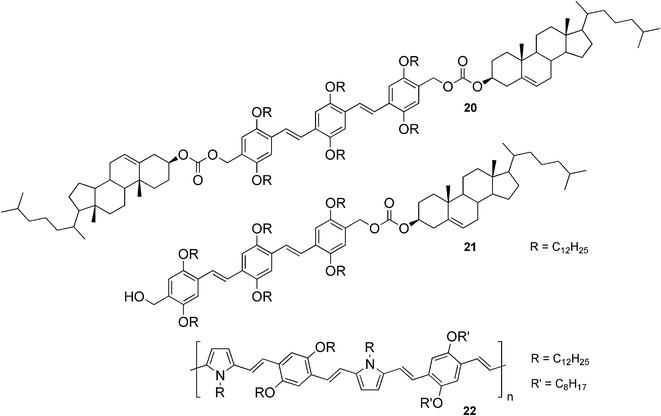 | ||
| Fig. 14 Structures of steroidal organogelators 20 and 21, and the red-light-emitting acceptor 22. | ||
Bile acid-based organogels
One of the earliest reports on bile acid-based organogelators introduced the organogelation properties of N-isopropyl cholamide in 1998.57 Since then, several reports on the organogelation properties of bile acid derivatives have been published. Moreover, a few review articles have touched upon the issue.1,58,59Very recently a new class of efficient gelators of organic and aqueous–organic media, namely perfluoroalkyl bile esters, as first examples of fluorinated gelators derived from the bile acids, were introduced. The target compounds were prepared by attaching the perfluoroalkyl chains of different lengths to the bile acids through two different ester linkages (Fig. 15). Three different bile acid moieties (LCA, DCA, and CA) were exploited in the syntheses. By varying the above-mentioned variables, the gels were obtained in aromatic hydrocarbons, DMSO, and DMSO/DMF–H2O mixtures of different proportions. The most efficient gelators among these compounds formed gels well below 1.0% (w/v) and hence they can be termed supergelators. The effect of the bile acid moiety, the nature of the ester linkage, and the length of the fluoroalkyl side chain on the gelation properties of the compounds, as well as on the properties of the formed gels (thermal stability, mechanical properties, and morphology), were carefully and systematically investigated. Rheological studies demonstrated that the gels behaved as viscoelastic materials, and that the mechanical properties of the studied gels could be modulated by changing either the bile acid moiety or by varying the length of the fluoroalkyl segment. The presence of CO2-philic perfluoroalkyl group is expected to enhance the solubility of the studied compounds in supercritical CO2, thus making these compounds promising candidates for forming aerogels.60
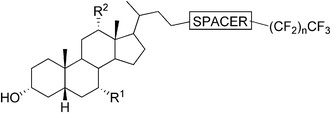 | ||
| Fig. 15 Fluorinated bile acid-based gelators by Maitra et al. In LCA-derivatives R1 = R2 = H; DCA-derivatives R1 = H, R2 = OH; CA-derivatives R1 = R2 = OH. The spacer is either –(CO)–OCH2– (type P) or –O–(CO)– (type Q); n = 1, 6, 8 (with spacer type P) or n = 6, 8, 9, 10, 12 (with spacer type Q). | ||
The simplest esters of bile acids showing the ability to promote self-assembly leading to organogelation were serendipitously discovered by Maitra et al. in 2007. The compounds included allyl and propargyl cholates, and simple alkyl (n-propyl and ethyl) esters of cholic acid. All of the compounds were able to gelate mesitylene, xylene, benzene, and toluene at concentrations below 1.0% (w/v). However, methyl and butyl esters of cholic acid were not able to form gels in any of the solvents tested.61 Recently, further studies utilizing the solid state NMR and X-ray powder diffraction methods revealed a close resemblance in the packing pattern between the gelators and the bulk solid, xerogel, and the gel in its native state. A doublet resonance pattern of 13C signals in the 13C CPMAS NMR spectra were observed for the gelator molecules, whereas the non-gelators were shown to exhibit simple singlet resonances or to result in the formation of inclusion complexes/solvates.62
The gelation abilities of some alkyl esters of cholic acid were studied in 2002. According to these studies, alkyl cholates with chain lengths of 8, 10, and 12 carbon atoms were not able to form gels in any of the solvents tested. However, in apolar solvents they were shown to increase the solubility of the more polar components, like sugars and water. These findings are further discussed in the context of two-component gels later in this article. Besides the alkyl esters, a group of amide and urea derivatives of cholic acid were prepared and the gelation properties of the compounds investigated. These compounds formed transparent gels in aromatic solvents and cycloalkenes. By using a reversed amide bond, it was shown that the direction of the amide bond had a minor influence on the gelation ability of these compounds, while the introduction of an aryl group decreased the gelling capability. Dimers containing two cholic acid units were shown to form organogels in more polar solvents.63
The same research group reported that N-cholyl amino acid alkyl esters acted as organogelators for aromatic solvents and cyclohexene affording stable, transparent, and thermoreversible gels. Compounds with varying amino acid side chains and ester groups were prepared and their gelation properties systematically investigated. In order to study the effect of the bile acid moiety, L-leucine methyl ester conjugates of lithocholic, chenodeoxycholic, and deoxycholic acids were synthesized and their gelation properties studied. No gels formed by these reference compounds were observed.64 Detailed network structures of some of the gels formed by the cholic acid derivatives presented above have been further studied by small-angle neutron scattering (SANS).65
Our group has been interested in preparing conjugates of bile acids and biologically important small molecules linked via an amide bond. Recently, we published the synthesis and detailed gelation studies for a series of three bile acids (LCA, DCA, and CA) conjugated with L-methionine methyl ester. Two of the conjugates, namely lithocholyl and cholyl derivatives 23a and 23b (Fig. 16), respectively, were shown to undergo self-assembly leading to organogelation in certain aromatic solvents. The properties of the formed gels were investigated by the conventional methods typical for molecular gel studies along with 13C CPMAS NMR spectroscopic studies of the native gel. Estimation of the packing similarities/differences in the solid and gel states exploiting solid state NMR spectroscopy together with X-ray diffractometry was performed. Our results suggest that in some cases the mode of packing in the gel or xerogel may differ from that of the single crystal X-ray structure.66
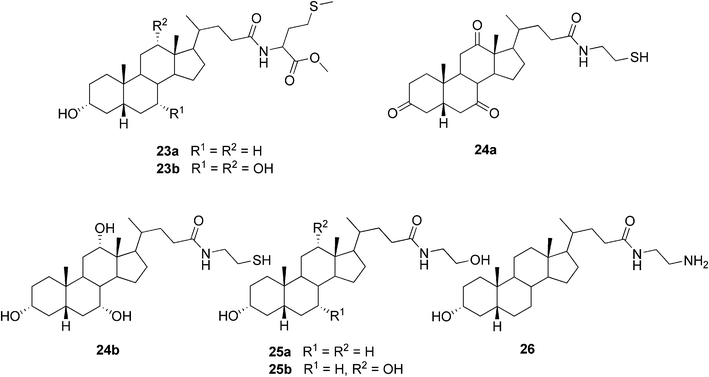 | ||
| Fig. 16 Selected examples of bile acid-based organogelators prepared by us. | ||
In another paper by our group six derivatives of bile acids with a drug molecule cysteamine (2-aminoethanethiol) were prepared. Two of the conjugates (dehydrocholyl and cholyl derivatives 24a and 24b, respectively, Fig. 16) were shown to form organogels. All but one of the solvent systems gelated were aromatic solvents, 1-octanol being the exception in the case of compound 24a.67 Furthermore, two groups of compounds structurally related to the previous cysteamine conjugates of bile acids have been synthesized and their gelation properties extensively studied by us.68,69 First, simple bile acid amides of lithocholic and deoxycholic acids with 2-aminoethanol (25a and 25b, Fig. 16) and 3-aminopropanol were shown to be effective gelators for mainly chlorinated organic solvents or aromatic solvents, depending on the length of the side chain. The compounds were shown to be capable of thickening neutral and acidic water solutions, but accurate gel formation in those conditions has not been detected.68 Second, the ability of a series of amino- and dihydroxyalkyl amides of bile acids to promote gel formation was clarified.69 Out of 396 combinations formed by 11 compounds and 36 different solvents, 22 gel-containing systems (1% w/v) were obtained. Apart from one exception, the compounds capable of gel formation were shown to be lithocholic acid derivatives.
Comparison of the above-obtained results for bile acid amidoalcohols, -amines, and -thiols has enabled the investigation of the effect of the replacement of a single functional group in the side chain on the gelation behavior of these structurally related compounds. Based on the data, the amidoalcohols 25a and 25b were evidently shown to be the most effective gelators being able to gelate the greatest number of solvents. Of the amidoamines studied, only the lithocholyl derivative 26 (Fig. 16) has shown to be able to undergo self-assembly leading to gelation. However, LCA-, DCA-, and CDCA-derived amidothiols were not shown to form any gels, except a partial gel of the lithocholyl derivative in benzene. It is assumed that this difference in the behavior of structurally rather similar compounds relates to the stronger hydrogen bonding capacity of the OH-group compared to the NH2- and SH-groups, leading to stronger intergelator interactions. In the case of the studies on the amino- and dihydroxyalkyl amides of bile acids,69 a correlation between the values of the Kamlet–Taft parameters and solvent preferences for gelators was observed. Moreover, the morphologies of the solid and gel structures studied by SEM were shown to vary from fibers to spherical microscale aggregates, the latter of which are unique among bile acid-based organogels. The gels were also shown to exhibit rather complex behavior, judging from the microscale diversity present in gelating and non-gelating systems.
Yet another recent example from our laboratory represents two alkylamide–phenylurea derivatives of deoxycholic acid with organogelation properties. The monomeric derivative was shown to form gels in CHCl3 and chlorobenzene, whereas the dimeric derivative gelated THF and higher alkanols (1-heptanol, 1-octanol, 1-nonanol, and 1-decanol).70
Organogels composed of other steroids
There are only a few examples of steroidal organogel systems based on steroids other than cholesterol or bile acids. Di Chenna et al. have reported the synthesis of a new pregnane derivative having a silyl ether group at C-3 combined with a 6β,19-oxo bridge (27, Fig. 17). Compound 27 was capable in gelating hydrocarbons and tetraethyl orthosilicate (TEOS) at very low concentrations (<1 wt%). The self-assembled fibrillar network was studied by FTIR, X-ray powder diffraction, CD spectroscopy, and microscopic techniques (SEM, TEM, and AFM). In addition, the ability of compound 27 to gelate TEOS was exploited in the preparation of SiO2 nanotubes. The slow, catalyst-free polymerization of the TEOS gel of 27 resulted in fibers of SiO2. After calcination, the SEM pictures showed hollow, straight fibers with a uniform inner diameter of about 7 nm and an external diameter comparable to that of the non-calcined material. The observations indicated that the organogel structure was successfully transcribed into the silica nanotubes by an exo-templating process.71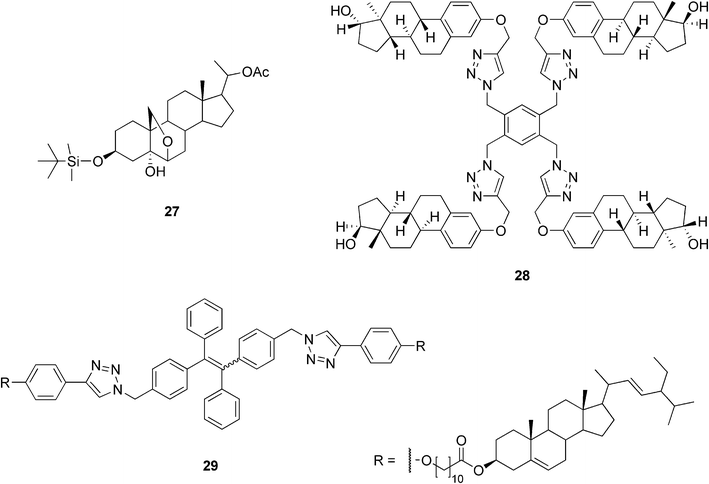 | ||
| Fig. 17 Selected examples of organogelators based on steroids other than cholesterol or bile acids. | ||
An interesting new family of estradiol-based low molecular weight gelators readily accessible using click-chemistry has recently been reported. Extensive structure–property studies for the family of gelators showed that the symmetry of the gelator molecules plays an important role in the gelation ability of the compounds. C2-symmetric dimeric and tetrameric compounds (28, Fig. 17) gelated different organic solvents in the presence of water even at concentrations as low as 0.04 wt%, while their monomeric and C3-symmetric analogues did not behave as gelators.72
Stigmasterol belongs to the group of plant steroids. Its structure resembles that of cholesterol, but differs with respect to the side chain. In our laboratory, three amino acid conjugates of stigmasterol, exploited in preparing stimuli-responsive, acid–base triggered reversible sol–gel transitions, as discussed later, have been prepared.73 Another example of stigmasterol-based gels has been reported by Wimmer and co-workers. The compound consisting of stigmasterol and L-phenylalanine interconnected via short-chained dicarboxylic acyls by ester and amide bonds, respectively, was shown to gelate 1-octanol when cooled in the refrigerator.74
Recently, cholesteryl and stigmasteryl derivatives containing tetraphenylethenes were synthesized.75 Both molecules were almost non-luminescent in a solution, but became highly emissive in an aggregated state. Their photoluminescence spectra were measured in THF/water mixtures and it was found that higher water content induced a stronger light emission. This is probably due to a low water solubility of the compounds, which leads to a higher aggregation of the molecules. The stigmasteryl derivative 29 (Fig. 17) formed a gel in methanol in the presence of a few drops of THF (added due to the low gelator solubility in methanol). Under normal room light illumination, the organogel appeared white, but upon UV irradiation, it emitted an intense blue light, whose intensity was 18 times higher compared to that from a hot methanol solution (before the gel was formed).
Metallogels
One of the most rapidly developing areas of functional materials nowadays is metallogels.76,77 The incorporation of metal ions to gels brings new properties to the system. Examples are catalytic and redox activity, conductivity, luminescence, and magnetism, which significantly increase the attractiveness of these materials for potential applications as catalysts, electronic devices, sensors, etc. Since the ability of a steroidal unit to bind metal ions is limited, the incorporation of a suitable metal binding site is necessary. Metal ions can form part of the covalent structure of the gelator, or most often are bound by weaker coordination interactions. So far an unsolved problem is the reversibility of the gelation process.One of the first examples of steroidal metallogels was reported by the Shinkai group several years ago.78 They prepared a series of porphyrin-based gelators bearing a cholesterol moiety, where Zn(II) ions were irreversibly incorporated into the porphyrin unit, and studied their gelation properties in the presence and absence of [60]fullerene. π–π Stacking, hydrophobic interactions, and van der Waals forces were proven to be the leading forces for the self-assembly, whereas the contribution of metal–ligand interactions to the process was not significant.
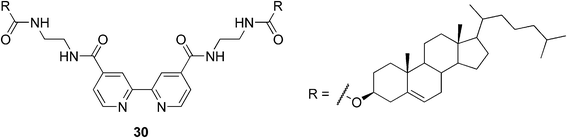
Nowadays the research focuses more on situations, where the metal is incorporated into the system either to decrease or, as it is in most cases, to enhance the gel strength by increasing the density of the gelator network. As an example, Shinkai and co-workers have reported the first redox-responsive metallogel; a Cu(I) complex in which the ligand is a 2,2′-bipyridine derivative bearing two cholesteryl groups (compound 30, Fig. 18).79 They have shown that the temperature stability of the gel is highly dependent on the metal–ligand molecular ratio. As an optimal ratio, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry was determined. This is in good agreement with the expectable stoichiometry of the complex. Moreover they found that a thermochromic sol–gel transition in 1-butyronitrile is accompanied with a color change, from reddish brown to greenish blue, which is untypical for Cu(I) complexes. As the phase-transition behavior could be reversibly repeated the possibility of the air oxidation of Cu(I) to Cu(II) was ruled out. It was concluded that the chromatic change in the complex was induced by the sol–gel phase transition, which is associated with the distortion of the coordination complex in the specific cholesteric gel fibril. They also reported the gel–sol transition of the complex based on the redox stimuli. When the oxidative agent was added to the Cu(I) complex and the mixture was heated, the deep green gel turned into the sol. This gel–sol transition could be reversibly induced by the addition of the oxidizing and reducing reagents, so it is evident that the redox state of the Cu ions plays a critical role in the stability of the gel system.
2 stoichiometry was determined. This is in good agreement with the expectable stoichiometry of the complex. Moreover they found that a thermochromic sol–gel transition in 1-butyronitrile is accompanied with a color change, from reddish brown to greenish blue, which is untypical for Cu(I) complexes. As the phase-transition behavior could be reversibly repeated the possibility of the air oxidation of Cu(I) to Cu(II) was ruled out. It was concluded that the chromatic change in the complex was induced by the sol–gel phase transition, which is associated with the distortion of the coordination complex in the specific cholesteric gel fibril. They also reported the gel–sol transition of the complex based on the redox stimuli. When the oxidative agent was added to the Cu(I) complex and the mixture was heated, the deep green gel turned into the sol. This gel–sol transition could be reversibly induced by the addition of the oxidizing and reducing reagents, so it is evident that the redox state of the Cu ions plays a critical role in the stability of the gel system.
 | ||
| Fig. 18 Steroidal 2,2′-bipyridine ligand for preparation of redox-responsive metallogels. | ||
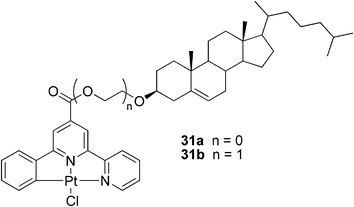
In another study, an enantioselective metallogel for a visual chiral recognition of BINAP, 2′-bis(diphenylphosphino)-1,1′-binaphthyl, was reported.80 Compounds 31a and 31b (Fig. 19) were utilized as steroid-based gelators of the ALS-type. It was shown that π–π stacking of the heteroarene moiety and metal–metal bonding were key contributors to the gelation process, and that the replacing of the chloro ligand in the Pt complex to a bulky ligand could block the assembly process between the heteroaromatic rings inducing a collapse of the gel network. The theory was proven utilizing chiral BINAP as the bulky ligand. The addition of one equivalent of (R)- or (S)-BINAP to the stable gel with subsequent heating to reflux and cooling to room temperature resulted in a collapse of the metallogels. Surprisingly, when the amount of the chiral phosphine was reduced to 0.1 eq., a striking difference in the behavior of the respective gels was observed. Whereas the gel sample containing (S)-BINAP survived the heating and cooling sequence as a robust gel, the gel sample containing the (R)-BINAP enantiomer collapsed. Similar behavior was observed for other chiral phosphine ligands, demonstrating the potential use of the metallogels in a simple protocol for visual chiral recognition.
 | ||
| Fig. 19 Steroidal gelators for visual chiral recognition of BINAP. | ||
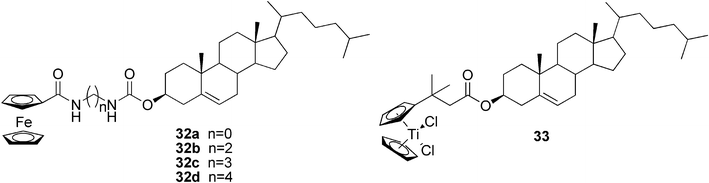
Recent development in the use of metallocene groups in the gelator synthesis was reported by Fang and co-workers. They studied the gelation abilities of cholesterol-appended ferrocene derivatives with glycine81 and diamines as linkers (compounds 32a–d, Fig. 20)82 in various solvents. It was found that the critical gelation concentration of compound 32a in cyclohexane was only 0.09% meaning that it is one of the best up-to-now reported metal-containing gelators. The gel was strong and could be moulded into a film, which was moreover bendable. Furthermore, some unusual redox and mechanical sol–gel phase transition phenomena were observed. These include, for example, a very rare heating-free sol–gel phase transition attained by a combination with a chemical oxidation and reduction reaction of the ferrocenyl residue accompanied by a typical color change from orange yellow to dark green, or a stress-triggered reversible sol–gel phase transition induced either by shaking or sonication.
 | ||
| Fig. 20 Cholesterol-appended ferrocene and titanocene derivatives. | ||
Equivalent to the steroidal ferrocene derivatives are cholesterol-functionalized titanocenes.83,84 They form twisted fiber structures and are able to gelate a variety of solvents of different polarity due to the amphiphilic character of the compounds. For example, the steroidal titanocene complex 33 (Fig. 20) creates a gel at low concentrations, even less than 1 wt%, in toluene, benzene, ethyl acetate, and acetone. It was observed that the formation of the bundles and gels proceeded with some degree of stereoselectivity; the majority of fibers assembled in the left-handed M-helices, which induced supramolecular chirality in the gel network, as confirmed by CD spectroscopy.
One of the rarer examples, where the presence of the metal leads to a weaker gel was reported by Drašar and co-workers.85 The phenantroline-containing ligand 34 (Fig. 21) created a gel on its own in a methanol–water mixture in low concentrations starting from 0.1%. Upon complexation of the gelator with Zn(II) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, the gel formation required higher concentrations and the gel tended to break down over time to a low-viscosity liquid. It was shown that the gel could be reversibly reformed after a rapid heating to 70 °C and subsequent cooling.
1 ratio, the gel formation required higher concentrations and the gel tended to break down over time to a low-viscosity liquid. It was shown that the gel could be reversibly reformed after a rapid heating to 70 °C and subsequent cooling.
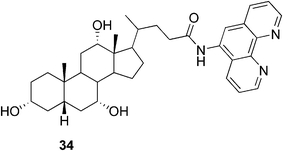 | ||
| Fig. 21 Cholic acid-based phenantroline gelator. | ||
Two-component gels
Two-component gel systems have recently attained ever increasing interest and have developed rapidly. The first attempts in this field were reported in the late 90s. They were mostly based on host–guest complex interactions, which could markedly change the gelation ability of the components as it was shown, for example, in the work of Shinkai and co-workers, who reported a cholesterol-based barbital receptor86 and cholesterol-based crown-ammonium pseudo-rotaxane complexes.87 Nowadays the field is more oriented to gel systems, whose gelation abilities can be finely tuned by adding other molecules to the system. Still mostly hydrogen bonds are utilized for the designing purposes due to their strength and directionality. In the “true” two-component gel systems, both components are essential for gel formation. However, several other examples, where one or both components can create a gel on their own but together induce formation of either a stronger gel or a gel with significantly different properties can be found as well.In general, complicated multi-step syntheses leading to gelator molecules are not needed, because the gel formation can be reached by simple mixing of suitable ready-to-use components, as was reported, for example, by Bhattacharya and co-workers.88 They demonstrated that a supramolecular gel was formed in aqueous solution using simple bile acids, such as lithocholic acid, in the presence of various dimeric and oligomeric amines. By carefully choosing the amines, the gelation properties of the mixtures could be modulated. Furthermore, varying the molar ratio of the two components provided an additional level of controlling the properties of the materials. Spectroscopic studies confirmed that the carboxylate and ammonium residues of the two components are involved in the salt (ion pair) formation, which promotes further self-assembly reinforced by a continuous hydrogen-bonded network leading to gel formation.
Another example of a simple two-component gel system was published by Raghavan and co-workers.89 They reported sodium deoxycholate-assisted gel formation of a twin-tailed anionic surfactant, sodium bis(2-ethylhexyl) sulfosuccinate, in nonpolar solvents. The surfactant 35 (Fig. 22) is widely known to form spherical reverse micelles in a range of organic solvents. After adding the bile salt 36, however, the situation was dramatically changed. It was observed that addition of even a trace of sodium deoxycholate to the micellar solution of the surfactant led to the formation of a transparent gel. It was proposed that the bile salt forms hydrogen bonds with the surfactant headgroups, transforming some of the spherical surfactant micelles into semiflexible filaments, which can entrap solvent molecules and create a gel structure.
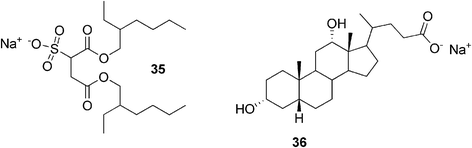 | ||
| Fig. 22 Moieties of a simple two-component gel system: anionic surfactant and sodium deoxycholate. | ||
A uracil-appended cholesteryl gelator 37 (Fig. 23) has been observed to form stable gels in polar organic solvents.90,91 Upon addition of the complementary polyadenylic acid 38, the gel system was not only stabilized, but the gel created a helical structure. Based on the obtained results, it was concluded that the self-assembly mode of nucleobase-appended organogelators could be controlled by the addition of complementary polynucleotides and that the single-stranded polynucleotide templates could force the gelator molecules to adopt the highly ordered structure through the complementary hydrogen-bonding interactions.
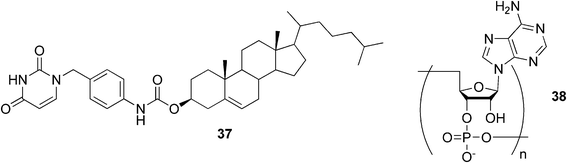 | ||
| Fig. 23 A uracil-appended cholesteryl gelator and a complementary polyadenylic acid. | ||
Jiang and co-workers have synthesized an ALS-type cholesterol-based molecule (compound 39, Fig. 24), which by itself displayed no gelation ability.92 Interestingly, when 3-cholesteryloxycarbonylpropanoic acid (compound 40) was added as a counterpart to the system, a gel was formed. The hydrogen-bonded complex (Fig. 24) displayed strong gelation ability in alcohols and aromatic solvents, and typical mesomorphic behavior of thermotropic liquid crystals.
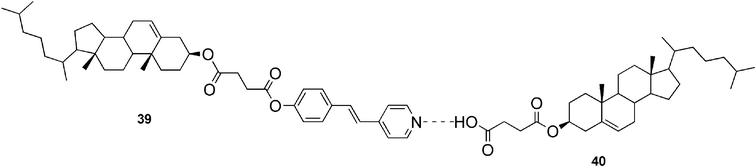 | ||
| Fig. 24 Hydrogen-bonded complex formed by 39 and 40. | ||
The construction of two-component gels is possible also without utilizing hydrogen bonds as driving forces for the self-assembly process. It was found that cholesterol-based dinitrobenzoyl esters (Fig. 25) could gelate various organic solvents in the presence of a wide range of polyaromatic hydrocarbons, such as anthracene and its derivatives, due to donor–acceptor interactions and π–π stacking.93 It was shown that both components were essential for gel formation, since the monomeric precursors themselves did not gelate any of the tested solvents.
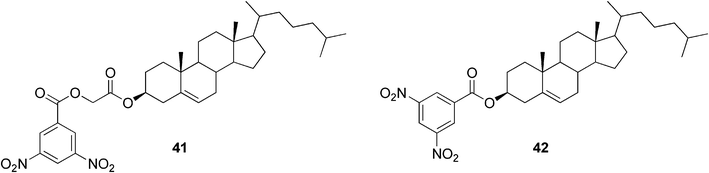 | ||
| Fig. 25 Cholesterol-based dinitrobenzoyl esters. | ||
Maitra and co-workers observed that the gelation ability of bile acid–pyrene derivatives was improved after adding a charge transfer agent, 2,4,7-trinitrofluorenone, to the system.94,95 Additionally, it was shown that the gelation properties could be finely tuned by inserting different functional groups to the bile acid side chain.
Since the series of alkyl cholates 43a–c (Fig. 26) prepared by Marcelis et al. did not gelate any of the tested solvents, they were then tested for use for improving the solubility of selected carbohydrates (isomannide 44 and isosorbide 45) in organic solvents, such as hexane or octane.63 Surprisingly, after mixing the components and a heating–cooling cycle, the solution turned into a thermoreversible opaque gel. It was found that the amount of carbohydrate added determined the gel stability. The optimum ratio of both components was close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The lower concentration of alkyl cholate compared to the carbohydrate led to an incomplete dissolution of the latter.
1. The lower concentration of alkyl cholate compared to the carbohydrate led to an incomplete dissolution of the latter.
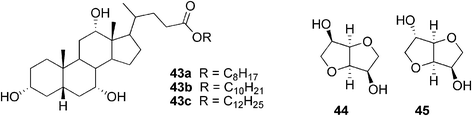 | ||
| Fig. 26 Selected alkyl cholates 43a–c and carbohydrates (44, 45) used for forming two-component gels. | ||
In order to solubilize carbon nanotubes in water sodium deoxycholate and single-walled carbon nanotubes were mixed.96 After ultrasonic treatment, a hydrogel was formed. The hydrogel exhibited excellent viscoelastic properties, e.g. it could be extended 50-fold along the direction of additional stretching force. Moreover, it could be used as a “solid” ink in preparing nanowires and nanopatterns. The conductivity of the nanowires was studied in order to examine the potential applications of the material in electronics.
Combining a cholesterol-based gelator 46 (Fig. 27) with Zn(II)–phthalocyanine-containing complementary steroidal structures 47 or 48 led to the formation of thermoreversible organogels.97 The chosen cholesterol-based gelator 46 is known to gelate alkanes and alkanols, but not aromatic solvents. Nevertheless, stable photoactive gels in various aromatic solvents were obtained, when the compound and a Zn(II)–phthalocyanine component were mixed in a molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Unfortunately, the prepared gels were stable only for a few weeks. In order to improve their temporal and thermal stabilities, the gels were prepared in the presence of tiny amounts (5 mol%) of suitable complementary diacetylene 49, and diazides 50 and 51, appropriate for “click”-reactions and able to, at least partially, integrate into the gel structures. All of the prepared cross-linked gels displayed better thermoreversibility and stability over time.
1. Unfortunately, the prepared gels were stable only for a few weeks. In order to improve their temporal and thermal stabilities, the gels were prepared in the presence of tiny amounts (5 mol%) of suitable complementary diacetylene 49, and diazides 50 and 51, appropriate for “click”-reactions and able to, at least partially, integrate into the gel structures. All of the prepared cross-linked gels displayed better thermoreversibility and stability over time.
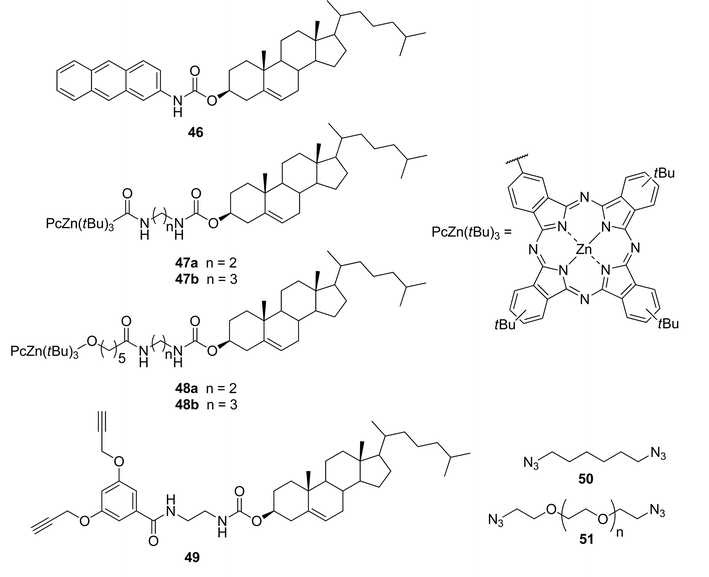 | ||
| Fig. 27 Moieties of two-component gel systems. | ||
Stimuli-responsive gels
Although there still is a deep interest towards the synthesis of new gelators in general, in the last few years more and more attention has been given to the design of responsive soft materials.3,5,98,99 These materials are characterized by a change in their properties in response to a specific physical (temperature, mechanical stress, light, etc.) or chemical (pH, ions, oxidation state, etc.) stimulus. The most common response of the systems is a transition from solution to gel (or vice versa). Other responses include changes in chemical or physical properties, such as conductivity, color, or light emission. Stimuli-responsive gels have a great potential for designing and constructing new functional materials, such as sensors, actuators, molecular devices, etc.1,2Physical stimuli
Recently, it was reported that ultrasound could act as a trigger for the instant gelation of organic solvents by certain cholesterol-based compounds.50–52 The use of ultrasonic irradiation in order to reversibly control aggregation of asymmetric cholesterol-based fluorescent gelators was studied. According to the results, it was suggested that the sonication-switch phenomenon could only happen in a compound with two hydrogen bonding sites, through a competition of intra- and intermolecular hydrogen bonds, as well as hydrophobic interactions. The location of the hydrogen bonds (determined by the lengths of the alkyl chains) had a strong effect on the solubility and gelling properties of the compounds. The organogelator 52 (Fig. 28) formed gels only upon ultrasonic treatment, while the other external stimuli, such as quick heating or cooling, did not initiate aggregation. Compounds 53a–c and 54, for one, could form gels either upon sonication or upon a heating–cooling cycle (though higher concentrations were needed for a successful gel formation). Interestingly, differences in the morphology of the xerogels were observed. Generally, the morphology was strongly dependent on the solvents and external stimuli. However, some trends could be observed; in most cases a thermal process afforded hollow spherical motifs, while ultrasound irradiation resulted in the spontaneous formation of the intermolecular hydrogen bonds and thus aggregation-induced helical motifs.
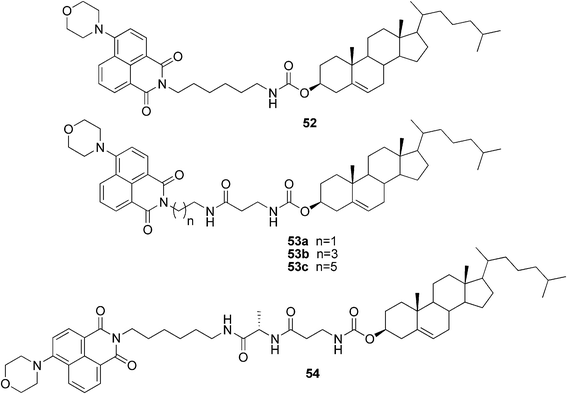 | ||
| Fig. 28 Ultrasound-responsive cholesterol-based fluorescent gelators. | ||
 | ||
| Scheme 1 Cis–trans photoisomerization of the azobenzene moiety of a cholesterol derivative. | ||
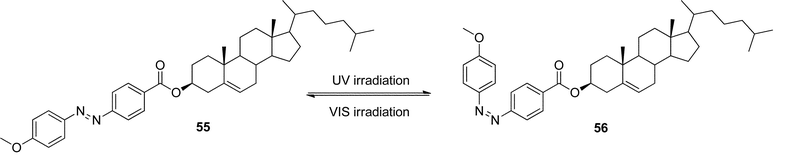
Tian and co-workers synthesized a gelator 57 (Scheme 2) containing bisthienylethene-bridged fluorescent naphthalimide units bearing two cholesteryl groups.101 It was shown that the color of the gel system can be turned from yellow to red after absorption of UV irradiation and back after absorption of visible light (Scheme 2). The cycle could be repeated more than 10 times and the gel phase remained stable at room temperature. A difference in luminescence spectra between these two photochromic states was observed. In addition, the system was sensitive to fluoride ions in solution. This makes it a promising fluorescent molecular switch activated by fluoride ions and protons (Fig. 29).
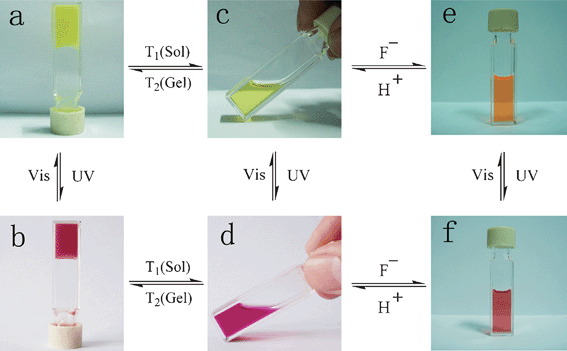 | ||
| Fig. 29 Multiple switching images of bisthienylethene-bridged cholesteryl derivative under the cooperative effects of light, temperature, fluoride anions, and protons. (a) Gel of 57; (b) gel of 58; (c) sol of 57; (d) sol of 58; (e) sol of 57 + F−; (f) sol of 58 + F−.101 Reproduced by permission of the Royal Society of Chemistry. | ||
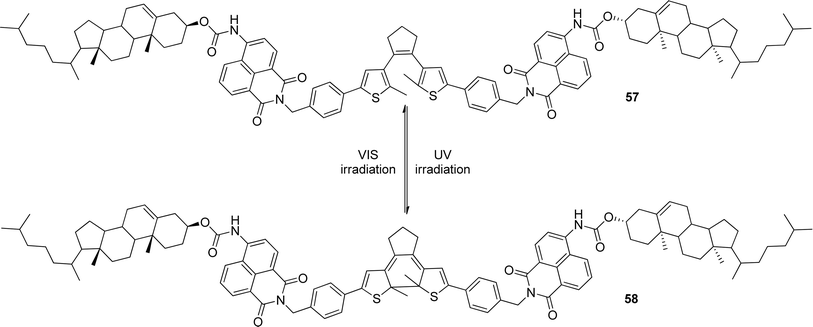 | ||
| Scheme 2 Structure and photochromic process of bisthienylethene-bridged cholesteryl derivative. | ||
Chemical stimuli
The Shinkai group showed that a 1,10-phenanthroline-appended cholesterol-based gelator 59 (Fig. 30) created gels in alcohols, polar aprotic solvents, organic acids, and triethylamine.103 In fluorescence measurements, most gels afforded an emission maximum at 394 nm (purple emission), whereas only the acetic acid gel afforded an emission maximum at 522 nm (yellow emission). Interestingly, upon addition of acetic acid to the gel of 59/1-propanol, yellow emission was observed suggesting that the protonation of the 1,10-phenantroline nitrogen in the gel state has a big influence on the fluorescence properties of the system. The fluorescence intensity of 59·H+ became particularly strong in the gel, presumably due to the energy transfer from neutral 59* to protonated 59·H+ and the restriction of the 59·H+ molecular motion in the gel phase.
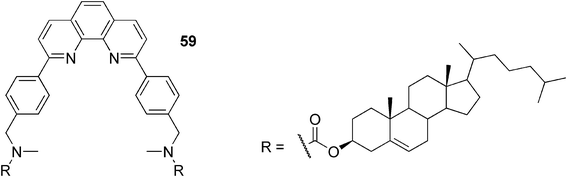 | ||
| Fig. 30 Proton-sensitive fluorescent 1,10-phenanthroline-appended cholesterol-based gelator. | ||
Fang and co-workers prepared three ammonium salts of diacid monoamides of cholesteryl glycinate (compounds 10a–c, Fig. 7) and studied their gelation behavior aiming at controlling it.28 It was found that the acids formed only weak gels, but neutralization with ammonia significantly enhanced their gelation ability. The amino salt of the succinic acid derivative behaved as an ambidextrous gelator (gelating both apolar solvents and water) suggesting two different ways of self-assembly, hydrophobic surface-mediated in apolar and hydrophilic surface-mediated in polar solvents. More interestingly, some of the alkyl alcohols and water could be gelatinized at room temperature simply by bubbling ammonia through the system. This could be applied in the design of ammonia sensors.
Maitra and co-workers observed two bile acid-derived molecules containing basic amino groups (Fig. 31) to act as efficient gelators for organic and aqueous solvents.104 The organogelator 60 was found to be a non-gelator in its neutral form, whereas, as its iodide salt, it formed a strong gel in 1,2-dichlorobenzene and chlorobenzene at very low concentrations (0.05% w/v). To illustrate the acid–base switching of the gel, a simple experiment was performed. Upon exposure to ammonia vapour, the gel transformed to a solution. Upon exposure to HI vapour, on the other hand, the gel was re-formed. However, for the hydrogel derived from compound 61, the situation was reversed. The neutral amine formed a gel in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO/water (0.5% w/v), whereas exposure to HI vapour disrupted the gel framework.
1 DMSO/water (0.5% w/v), whereas exposure to HI vapour disrupted the gel framework.
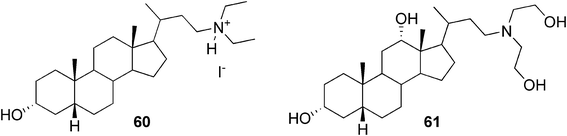 | ||
| Fig. 31 Bile acid-based pH-responsive gelators. | ||
As mentioned before, quinuclidine-grafted cationic bile salt 3 (Fig. 3) has been found to form a hydrogel.105 For this system, the influence of electrolytes and counter-ions on the rheological properties of the gel was investigated. The concentration of the electrolyte was shown to produce a dramatic effect on the gel stability. Increase in the added salt content led to weaker gels. Moreover, the fibrillar network of the conjugate with iodide as a counter-ion appeared to be more sensitive to the added salt compared to the conjugates with other counter-ions.
Similarly to the pH-responsive systems, there is a rapidly growing interest in the incorporation of anions into gels and towards their use in tuning and switching the gel behavior. Examples of the anion-triggered gel–sol transitions as well as of the opposite situations, where the presence of an anion leads to a gel formation, are known.
Lee and co-workers, for example, have reported a fluoride-responsive gel system.106 They synthesized an organogelator 62 (Fig. 32) containing a cholesterol unit with a fluorophore, 2-(2-hydroxyphenyl)benzoxazole, linked via an amide group. The compound created fluorescent supramolecular gels in mixtures of cyclohexanone and cyclohexane or cyclohexane and p-xylene. Based on the role of intramolecular hydrogen bonds in the fluorophore, they suggested that addition of fluoride ions, which are known to interact with the N–H bond in the amide group or to deprotonate it, could have an impact on the gelation process. Upon addition of tetrabutyl ammonium fluoride, they observed a rapid transition from an opaque gel to a homogenous solution with an altered fluorescence color. Moreover, the change was irreversible. They suggested that the gel–sol transition was caused by the change in inter- and intramolecular hydrogen bonding patterns due to newly established interactions by the added fluoride anions.
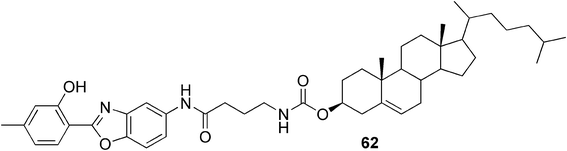 | ||
| Fig. 32 A fluoride-responsive organogelator. | ||
On the contrary, Pandey and co-workers reported a bile acid-based imidazolium anion-receptor 63 (Fig. 33), which formed a stable gel in a CHCl3/DMSO mixture only in the presence of anions.107 They observed that among the tested anions (F−, Cl−, Br−, AcO−, H2PO4−, and HSO4−) only the HSO4− ion was effective in inducing the gel formation. The system could thus be used for a selective naked-eye detection of hydrogen sulfate ions.
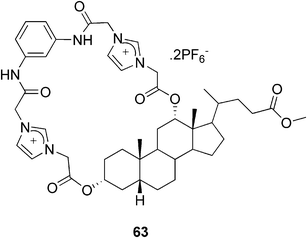 | ||
| Fig. 33 A bile acid-based imidazolium anion-receptor. | ||
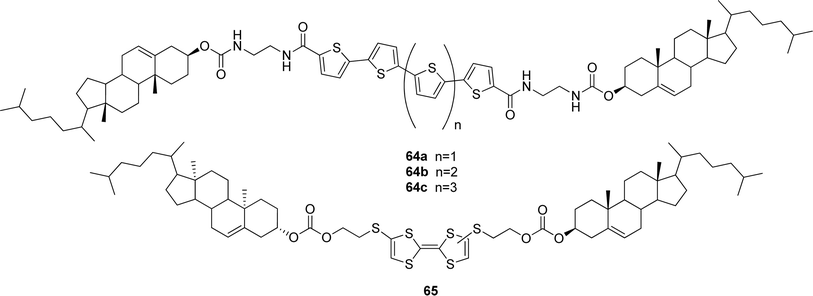 | ||
| Fig. 34 Oligothiophene- (64a–c) and tetrathiafulvalene-appended (65) redox-responsive cholesterol-based gelators. | ||
Recently, the synthesis of a redox active tetrathiafulvalene derivative bearing two cholesteryl units was reported.109 Compound 65 (Fig. 34) was found to create a gel in n-hexane after heating and ultrasonic treatment. It was demonstrated that besides heating, the gel–sol transition occurred upon oxidation of the tetrathiafulvalene unit by I2, making the organogel redox-responsive. The reversibility of the process was not studied.
Another example utilizing redox properties of a tetrathiafulvalene moiety was reported by Stoddart and co-workers.110 They synthesized a rotaxane 67 (Fig. 35) with tetrathiafulvalene and 1,5-dioxynaphthalene recognition units situated in the rod and with cholesterol units as stoppers, and investigated its gelation behavior and switching properties. They demonstrated that rotaxane 67 as well as its precursor 66 formed organogels in a CH2Cl2/MeOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture and could be liquified by adding an oxidizing agent [Fe(ClO4)3]. Moreover, they showed that the cholesterol stoppers were essential for the self-aggregation process and suggested that the same design could be used as a general strategy for introducing gelation properties to mechanically interlocked molecules.
2) mixture and could be liquified by adding an oxidizing agent [Fe(ClO4)3]. Moreover, they showed that the cholesterol stoppers were essential for the self-aggregation process and suggested that the same design could be used as a general strategy for introducing gelation properties to mechanically interlocked molecules.
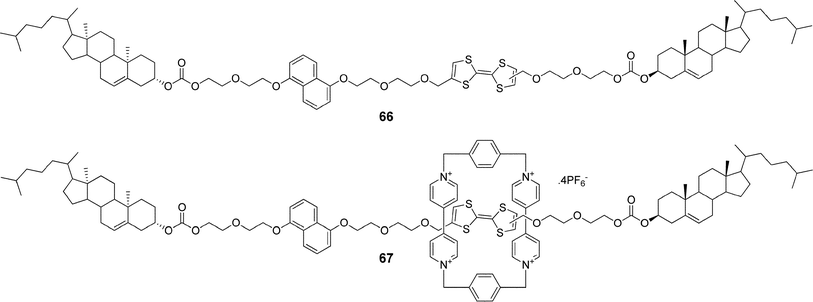 | ||
| Fig. 35 The steroidal rotaxane 67 with tetrathiafulvalene and 1,5-dioxynaphthalene recognition units in the rod and its precursor 66. | ||
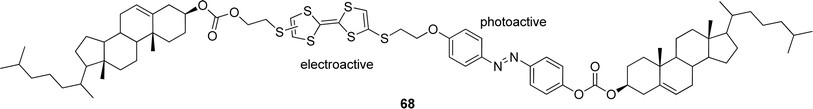 | ||
| Fig. 36 Electro- and photo-responsive steroidal organogelator. | ||
Recently, the cholesterol-based perylene derivative 19 (discussed in the organogel part of this review, Fig. 13) was used in a mixture with oligothiophene-bridged cholesteryl derivatives 64a and 64c (discussed in the redox stimuli part of this review, Fig. 34) to prepare a self-sorting photoactive gel system (Fig. 37).112 The components act as opposite charge carriers of the n-type (electron poor perylene derivative) and p-type (electron rich oligothiophene derivatives), respectively. Because of the structural similarities of the molecules, they could form gels in the same organic solvents, e.g. in chlorobenzene. On the other hand, their molecular lengths are different and also the number of hydrogen bonding sites is different (two for the perylene derivative, and four for the oligothiophene derivatives). Upon mixing the components in chlorobenzene and after a heating and cooling cycle, gel formation was observed. The gelation process was monitored by variable temperature UV-VIS spectroscopy. The absorption spectrum of the mixed gel showed no difference to the combined spectra of the components. This indicates an absence of interactions between the oligothiophene and perylene moieties. Interestingly, also the dissociation temperatures of the gelators in the mixed gel matched with those of the individual ones, suggesting that a self-sorting gel system was formed. A fluorescence measurement of the mixed gel of 19 and 64c showed the same λmax (600 nm) compared to the spectrum of 64c, but the maximum was partly quenched (∼63%), indicating an electron and/or energy transfer between the two components. In another experiment, photo-current measurements were carried out. Upon photo-irradiation of the gel film on a working electrode, an anodic photo-current was generated. This photo-responsive phenomenon could be reversibly repeated many times, and the film was sufficiently robust. Fluorescence spectra showed that both the thiophene and the perylene components acted as photoactive species for the photo-current generation.
 | ||
| Fig. 37 A schematic representation of a self-sorting assembly of oligothiophene (orange) and perylene (red) derivatives (19 and 64c, respectively) with p–n heterojunction nodes (black arrows). | ||
Conclusions and future prospects
The unique chemical and physical properties of steroids combined with their availability and low cost have raised them among significant building blocks when designing new soft materials. This article summarizes the latest developments within the field of steroid-based supramolecular gels, most of which are derived from cholesterol or bile acids – pregnane, estradiol, and stigmasterol being the few exceptions. The myriad of applications of supramolecular gels derived from steroids include their use as templates for syntheses, hybrid materials, sensing and responsive materials, media for selective reactions, and light-harvesting systems. Due to the endogeneity of cholesterol, bile acids, and steroid hormones, materials based on them are promising from the biomedical and pharmacological point of views.Nowadays, physical – or supramolecular – gels belong to one of the most rapidly developing groups of materials. An ever increasing expansion in the field of targeted design of functional and tunable systems is expected in the future. With respect to steroid-based supramolecular gels in particular these might, in our opinion, relate to the exploitation of the chiral gel network as a template in reactions, such as photochemical as well as asymmetric transformations, and as a crystallization medium, not forgetting the wide-ranging applications of stimuli-responsive systems. Biomaterials based on steroidal supramolecular hydrogels may find use in biomedicine, drug delivery, regenerative medicine, and tissue engineering. The ability of the gel forming systems to act in molecular recognition processes will undoubtedly be capitalized upon. Moreover, the amount of hybrid materials containing steroid-based supramolecular gel component(s) for applications in optics, electronics, ionic liquids, mechanics, biology, and catalysis is expected to increase. The use of steroidal components other than bile acids or cholesterol in supramolecular gel systems opens fascinating opportunities. Finally, the move from potential to active applications is to be expected.
Although the research of low molecular weight gelators and supramolecular gels has experienced extensive development during the last decade or so, several topics remain to be addressed. Undoubtedly, one of the most pivotal problems is the lack of sufficient methods for studying the gel systems in detail at the molecular level. The development of these methods will increase our understanding related to the detailed mechanism of the gel formation, ultimately enabling the rational design of gelator molecules combined with the ability to predict the properties of the forming gel systems. Moreover, the practical challenges, like the stability of the supramolecular gels in a prolonged use, enhancement of the mechanical strength of the systems, and their response to a multi-enzyme environment encountered in the human cells if used in biomedical applications are still to be solved.
Acknowledgements
Financial support from the Academy of Finland (projects no. 119616, 218178, and 255648 to E.S.), the University of Jyväskylä (V.N.), and the National Graduate School of Organic Chemistry and Chemical Biology (V.N.; H.S.) are gratefully acknowledged.References
- N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821 RSC.
- A. Hirst, B. Escuder, J. Miravet and D. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002 CrossRef CAS.
- S. Banerjee, R. K. Das and U. Maitra, J. Mater. Chem., 2009, 19, 6649 RSC.
- W. T. Truong, Y. Su, J. T. Meijer, P. Thordarson and F. Braet, Chem.–Asian J., 2011, 6, 30 CrossRef CAS.
- A. Dawn, T. Shiraki, S. Haraguchi, S. Tamaru and S. Shinkai, Chem.–Asian J., 2011, 6, 266 CrossRef CAS.
- J. W. Steed, Chem. Commun., 2011, 47, 1379 RSC.
- F. Fages, F. Vögtle and M. Žinić, Top. Curr. Chem., 2005, 256, 77 CrossRef CAS.
- P. M. Dewick, Medicinal natural products: a biosynthetic approach, Wiley, Chichester, 2009, ch. 5, pp. 187–310 Search PubMed.
- A. P. Davis, Chem. Soc. Rev., 1993, 22, 243 RSC.
- E. Virtanen and E. Kolehmainen, Eur. J. Org. Chem., 2004, 3385 CrossRef CAS.
- S. Mukhopadhyay and U. Maitra, Curr. Sci., 2004, 87, 1666 CAS.
- S. B. Schryver, Proc. R. Soc. London, Ser. B, 1914, 87, 366 CrossRef CAS.
- S. B. Schryver and M. Hewlett, Proc. R. Soc. London, Ser. B, 1916, 89, 361 CrossRef CAS.
- A. Rich and D. M. Blow, Nature, 1958, 182, 423 CrossRef CAS.
- C. Valenta, E. Nowack and A. Bernkop-Schnürch, Int. J. Pharm., 1999, 185, 103 CrossRef CAS.
- S. Mukhopadhyay, U. Maitra, Ira, G. Krishnamoorthy, J. Schmidt and Y. Talmon, J. Am. Chem. Soc., 2004, 126, 15905 CrossRef CAS.
- P. Terech and U. Maitra, J. Phys. Chem. B, 2008, 112, 13483 CrossRef CAS.
- S. Bhat and U. Maitra, Chem. Mater., 2006, 18, 4224 CrossRef CAS.
- S. Bhat and U. Maitra, Tetrahedron, 2007, 63, 7309 CrossRef CAS.
- N. M. Sangeetha, R. Balasubramanian, U. Maitra, S. Ghosh and A. R. Raju, Langmuir, 2002, 18, 7154 CrossRef CAS.
- P. Terech, N. M. Sangeetha and U. Maitra, J. Phys. Chem. B, 2006, 110, 15224 CrossRef CAS.
- P. Terech, S. Dourdain, S. Bhat and U. Maitra, J. Phys. Chem. B, 2009, 113, 8252 CrossRef CAS.
- S. Bhat and U. Maitra, Molecules, 2007, 12, 2181 CrossRef CAS.
- P. Babu, D. Chopra, T. N. Guru Row and U. Maitra, Org. Biomol. Chem., 2005, 3, 3695 CAS.
- S. Bhowmik, S. Banerjee and U. Maitra, Chem. Commun., 2010, 46, 8642 RSC.
- S. Banerjee, R. Kandanelli, S. Bhowmik and U. Maitra, Soft Matter, 2011, 7, 8207 RSC.
- S. Das, S. L. de Rooy, A. N. Jordan, L. Chandler, I. I. Negulescu, B. El-Zahab and I. M. Warner, Langmuir, 2012, 28, 757 CrossRef CAS.
- K. Liu, N. Yan, J. Peng, J. Liu, Q. Zhang and Y. Fang, J. Colloid Interface Sci., 2008, 327, 233 CrossRef CAS.
- L. Mao, H. Wang, M. Tan, L. Ou, D. Kong and Z. Yang, Chem. Commun., 2012, 48, 395 RSC.
- Y. C. Lin and R. G. Weiss, Macromolecules, 1987, 20, 414 CrossRef CAS.
- Y. C. Lin, B. Kachar and R. G. Weiss, J. Am. Chem. Soc., 1989, 111, 5542 CrossRef CAS.
- M. Žinić, F. Vögtle and F. Fages, Top. Curr. Chem., 2005, 256, 39 CrossRef.
- X. Huang, P. Terech, S. R. Raghavan and R. G. Weiss, J. Am. Chem. Soc., 2005, 127, 4336 CrossRef CAS.
- X. Huang, S. R. Raghavan, P. Terech and R. G. Weiss, J. Am. Chem. Soc., 2006, 128, 15341 CrossRef CAS.
- J. H. Jung and S. Shinkai, J. Inclusion Phenom. Macrocyclic Chem., 2001, 41, 53 CrossRef CAS.
- J. H. Jung, S. Shinkai and T. Shimizu, Chem. Mater., 2003, 15, 2141 CrossRef CAS.
- J. H. Jung, T. Shimizu and S. Shinkai, J. Mater. Chem., 2005, 15, 3979 RSC.
- S. Malik, S. Kawano, N. Fujita and S. Shinkai, Tetrahedron, 2007, 63, 7326 CrossRef CAS.
- P. Xue, R. Lu, D. Li, M. Jin, C. Tan, C. Bao, Z. Wang and Y. Zhao, Langmuir, 2004, 20, 11234 CrossRef CAS.
- D. Asthana, A. Kumar, A. Pathak, P. K. Sukul, S. Malik, R. Chatterjee, S. Patnaik, K. Rissanen and P. Mukhopadhyay, Chem. Commun., 2011, 47, 8928 RSC.
- D. Gao, M. Xue, J. Peng, J. Liu, N. Yan, P. He and Y. Fang, Tetrahedron, 2010, 66, 2961 CrossRef CAS.
- M. Xue, K. Liu, J. Peng, Q. Zhang and Y. Fang, J. Colloid Interface Sci., 2008, 327, 94 CrossRef CAS.
- M. Xue, D. Gao, X. Chen, K. Liu and Y. Fang, J. Colloid Interface Sci., 2011, 361, 556 CrossRef CAS.
- J. Peng, K. Liu, J. Liu, Q. Zhang, X. Feng and Y. Fang, Langmuir, 2008, 24, 2992 CrossRef CAS.
- K. Liu, J. Peng, M. Xue, N. Yan, J. Liu and Y. Fang, Sci. China Chem., 2011, 54, 475 CrossRef CAS.
- J. Peng, H. Xia, K. Liu, D. Gao, M. Yang, N. Yan and Y. Fang, J. Colloid Interface Sci., 2009, 336, 780 CrossRef CAS.
- J. Peng, K. Liu, X. Liu, H. Xia, J. Liu and Y. Fang, New J. Chem., 2008, 32, 2218 RSC.
- X. Hou, D. Gao, J. Yan, Y. Ma, K. Liu and Y. Fang, Langmuir, 2011, 27, 12156 CrossRef CAS.
- S. Abraham, R. K. Vijayaraghavan and S. Das, Langmuir, 2009, 25, 8507 CrossRef CAS.
- J. Wu, T. Yi, T. Shu, M. Yu, Z. Zhou, M. Xu, Y. Zhou, H. Zhang, J. Han, F. Li and C. Huang, Angew. Chem., Int. Ed., 2008, 47, 1063 CrossRef CAS.
- J. Wu, T. Yi, Q. Xia, Y. Zou, F. Liu, J. Dong, T. Shu, F. Li and C. Huang, Chem.–Eur. J., 2009, 15, 6234 CrossRef CAS.
- X. Yu, Q. Liu, J. Wu, M. Zhang, X. Cao, S. Zhang, Q. Wang, L. Chen and T. Yi, Chem.–Eur. J., 2010, 16, 9099 CrossRef CAS.
- K. Sugiyasu, N. Fujita and S. Shinkai, Angew. Chem., Int. Ed., 2004, 43, 1229 CrossRef CAS.
- C. Vijayakumar, V. K. Praveen and A. Ajayaghosh, Adv. Mater., 2009, 21, 2059 CrossRef CAS.
- A. Ajayaghosh, V. Praveen, C. Vijayakumar and S. George, Angew. Chem., Int. Ed., 2007, 46, 6260 CrossRef CAS.
- A. Ajayaghosh, C. Vijayakumar, R. Varghese and S. George, Angew. Chem., Int. Ed., 2006, 45, 456 CrossRef CAS.
- Y. Hishikawa, K. Sada, R. Watanabe, M. Miyata and K. Hanabusa, Chem. Lett., 1998, 27, 795 CrossRef.
- P. Babu, N. M. Sangeetha and U. Maitra, Macromol. Symp., 2006, 241, 60 CrossRef CAS.
- Nonappa and U. Maitra, Org. Biomol. Chem., 2008, 6, 657 CAS.
- S. Banerjee, V. M. Vidya, A. J. Savyasachi and U. Maitra, J. Mater. Chem., 2011, 21, 14693 RSC.
- Nonappa and U. Maitra, Soft Matter, 2007, 3, 1428 RSC.
- Nonappa, M. Lahtinen, B. Behera, E. Kolehmainen and U. Maitra, Soft Matter, 2010, 6, 1748 RSC.
- H. M. Willemen, T. Vermonden, A. T. M. Marcelis and E. J. R. Sudhölter, Langmuir, 2002, 18, 7102 CrossRef CAS.
- H. M. Willemen, T. Vermonden, A. T. M. Marcelis and E. J. R. Sudhölter, Eur. J. Org. Chem., 2001, 2329 CrossRef CAS.
- H. M. Willemen, A. T. M. Marcelis, E. J. R. Sudhölter, W. G. Bouwman, B. Demé and P. Terech, Langmuir, 2004, 20, 2075 CrossRef CAS.
- V. Noponen, Nonappa, M. Lahtinen, A. Valkonen, H. Salo, E. Kolehmainen and E. Sievänen, Soft Matter, 2010, 6, 3789 RSC.
- V. Noponen, H. Belt, M. Lahtinen, A. Valkonen, H. Salo, J. Ulrichová, A. Galandáková and E. Sievänen, Steroids, 2012, 77, 193 CrossRef CAS.
- A. Valkonen, M. Lahtinen, E. Virtanen, S. Kaikkonen and E. Kolehmainen, Biosens. Bioelectron., 2004, 20, 1233 CrossRef CAS.
- M. Löfman, J. Koivukorpi, V. Noponen, H. Salo and E. Sievänen, J. Colloid Interface Sci., 2011, 360, 633 CrossRef.
- J. Koivukorpi and E. Kolehmainen, Tetrahedron Lett., 2010, 51, 1199 CrossRef CAS.
- V. C. Edelsztein, G. Burton and P. H. Di Chenna, Tetrahedron, 2010, 66, 2162 CrossRef CAS.
- P. Ramírez-López, M. C. de la Torre, M. Asenjo, J. Ramírez-Castellanos, J. González-Calbet, A. Rodríguez-Gimeno, C. Ramírez de Arellano and M. A. Sierra, Chem. Commun., 2011, 47, 10281 RSC.
- H. Svobodová, Nonappa, Z. Wimmer and E. Kolehmainen, J. Colloid Interface Sci., 2011, 361, 587 CrossRef.
- J. Šusteková, P. Drašar, D. Šaman and Z. Wimmer, Molecules, 2011, 16, 9357 CrossRef.
- Y. Liu, J. W. Y. Lam, F. Mahtab, R. T. K. Kwok and B. Z. Tang, Front. Chem. China, 2010, 5, 325 CrossRef.
- M.-O. M. Piepenbrock, G. O. Lloyd, N. Clarke and J. W. Steed, Chem. Rev., 2010, 110, 1960 CrossRef CAS.
- F. Fages, Angew. Chem., Int. Ed., 2006, 45, 1680 CrossRef CAS.
- T. Ishi-i, R. Iguchi, E. Snip, M. Ikeda and S. Shinkai, Langmuir, 2001, 17, 5825 CrossRef CAS.
- S. Kawano, N. Fujita and S. Shinkai, J. Am. Chem. Soc., 2004, 126, 8592 CrossRef CAS.
- T. Tu, W. Fang, X. Bao, X. Li and K. H. Dötz, Angew. Chem., Int. Ed., 2011, 50, 6601 CrossRef CAS.
- J. Liu, J. Yan, X. Yuan, K. Liu, J. Peng and Y. Fang, J. Colloid Interface Sci., 2008, 318, 397 CrossRef CAS.
- J. Liu, P. He, J. Yan, X. Fang, J. Peng, K. Liu and Y. Fang, Adv. Mater., 2008, 20, 2508 CrossRef CAS.
- A. Gansäuer, I. Winkler, T. Klawonn, R. J. M. Nolte, M. C. Feiters, H. G. Börner, J. Hentschel and K. H. Dötz, Organometallics, 2009, 28, 1377 CrossRef.
- T. Klawonn, A. Gansäuer, I. Winkler, T. Lauterbach, D. Franke, R. J. M. Nolte, M. C. Feiters, H. Börner, J. Hentschel and K. H. Dötz, Chem. Commun., 2007, 1894 RSC.
- M. Dukh, D. Šaman, J. Kroulík, I. Černý, V. Pouzar, V. Král and P. Drašar, Tetrahedron, 2003, 59, 4069 CrossRef CAS.
- K. Inoue, Y. Ono, Y. Kanekiyo, T. Ishi-i, K. Yoshihara and S. Shinkai, J. Org. Chem., 1999, 64, 2933 CrossRef CAS.
- S. Kawano, N. Fujita and S. Shinkai, Chem. Commun., 2003, 1352 RSC.
- A. Pal, H. Basit, S. Sen, V. K. Aswal and S. Bhattacharya, J. Mater. Chem., 2009, 19, 4325 RSC.
- S. Tung, Y. Huang and S. R. Raghavan, Soft Matter, 2008, 4, 1086 RSC.
- M. Numata and S. Shinkai, Chem. Lett., 2003, 32, 308 CrossRef CAS.
- M. Numata, K. Sugiyasu, T. Kishida, S. Haraguchi, N. Fujita, S. M. Park, Y. J. Yun, B. H. Kim and S. Shinkai, Org. Biomol. Chem., 2008, 6, 712 CAS.
- Q. Hou, S. Wang, L. Zang, X. Wang and S. Jiang, J. Colloid Interface Sci., 2009, 338, 463 CrossRef CAS.
- D. Rizkov, J. Gun, O. Lev, R. Sicsic and A. Melman, Langmuir, 2005, 21, 12130 CrossRef CAS.
- S. Bhat, A. Valkonen, J. Koivukorpi, A. Ambika, E. Kolehmainen, U. Maitra and K. Rissanen, J. Chem. Sci., 2011, 123, 379 CrossRef CAS.
- P. Babu, N. M. Sangeetha, P. Vijaykumar, U. Maitra, K. Rissanen and A. R. Raju, Chem.–Eur. J., 2003, 9, 1922 CrossRef CAS.
- Z. Tan, S. Ohara, M. Naito and H. Abe, Adv. Mater., 2011, 23, 4053 CrossRef CAS.
- D. D. Díaz, J. J. Cid, P. Vázquez and T. Torres, Chem.–Eur. J., 2008, 14, 9261 CrossRef.
- J. J. D. de Jong, B. L. Feringa and J. H. van Esch, in Molecular Gels. Materials with Self-Assembled Fibrillar Networks, ed. R. G. Weiss and P. Terech, Springer, Netherlands, 2006, ch. 26, pp. 895–927 Search PubMed.
- X. Yang, G. Zhang and D. Zhang, J. Mater. Chem., 2012, 22, 38 RSC.
- K. Murata, M. Aoki, T. Suzuki, T. Harada, H. Kawabata, T. Komori, F. Ohseto, K. Ueda and S. Shinkai, J. Am. Chem. Soc., 1994, 116, 6664 CrossRef CAS.
- S. Wang, W. Shen, Y. Feng and H. Tian, Chem. Commun., 2006, 1497 RSC.
- Y. Li, K. Liu, J. Liu, J. Peng, X. Feng and Y. Fang, Langmuir, 2006, 22, 7016 CrossRef CAS.
- K. Sugiyasu, N. Fujita, M. Takeuchi, S. Yamada and S. Shinkai, Org. Biomol. Chem., 2003, 1, 895 CAS.
- U. Maitra and A. Chakrabarty, Beilstein J. Org. Chem., 2011, 7, 304 CrossRef CAS.
- P. Terech, S. Dourdain, U. Maitra and S. Bhat, J. Phys. Chem. B, 2009, 113, 4619 CrossRef CAS.
- T. H. Kim, N. Y. Kwon and T. S. Lee, Tetrahedron Lett., 2010, 51, 5596 CrossRef CAS.
- A. Tripathi and P. S. Pandey, Tetrahedron Lett., 2011, 52, 3558 CrossRef CAS.
- S. Kawano, N. Fujita and S. Shinkai, Chem.–Eur. J., 2005, 11, 4735 CrossRef CAS.
- C. Wang, F. Sun, D. Zhang, G. Zhang and D. Zhu, Chin. J. Chem., 2010, 28, 622 CrossRef CAS.
- Y. Zhao, I. Aprahamian, A. Trabolsi, N. Erina and J. F. Stoddart, J. Am. Chem. Soc., 2008, 130, 6348 CrossRef CAS.
- C. Wang, Q. Chen, F. Sun, D. Zhang, G. Zhang, Y. Huang, R. Zhao and D. Zhu, J. Am. Chem. Soc., 2010, 132, 3092 CrossRef CAS.
- K. Sugiyasu, S. Kawano, N. Fujita and S. Shinkai, Chem. Mater., 2008, 20, 2863 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
